Improved Drug-Response Prediction Model of APC Mutant Colon Cancer Patient-Derived Organoids for Precision Medicine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Oxaliplatin Resistance Is Induced by the Culture Conditions
2.2. Oxaliplatin Response Is Attenuated by the Culture Conditions in a 2D/3D Model
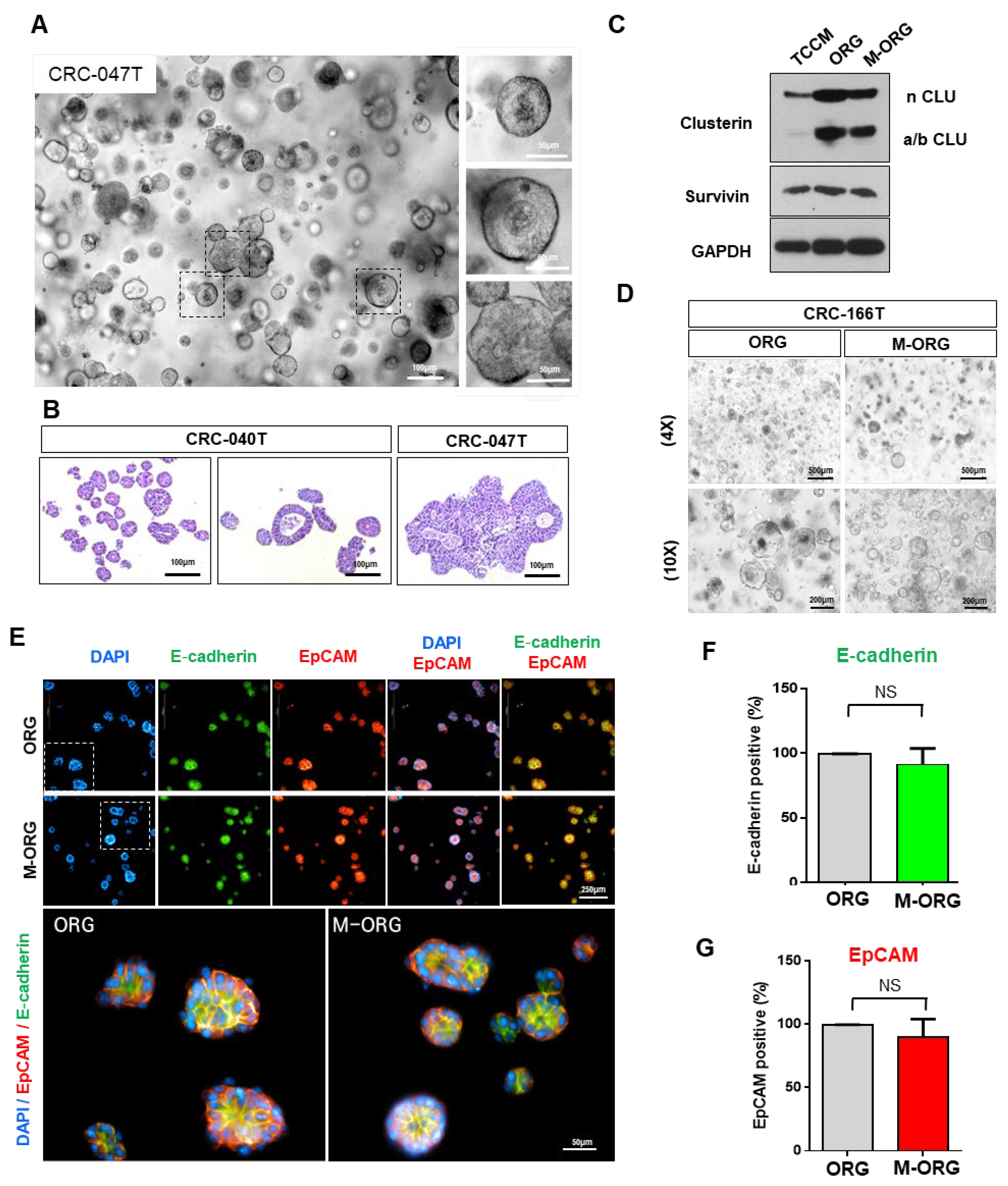
2.3. Oxaliplatin Resistance Is Selectively Increased in Organoid Culture Medium
2.4. Oxaliplatin Response Sensitivity Is Restored by Medium Modification
2.5. Oxaliplatin Response Is Repressed by Clusterin under an Organoid Culture Medium
2.6. Patient-Derived Organoids (PDO) Can Predict Oxaliplatin Response in a Modified Medium
2.7. CRC-PDO Drug Sensitivity Predicts Response to Treatment with Oxaliplatin in the M-ORG Media Condition
3. Discussion
4. Materials and Methods
4.1. Two-Dimensional Cell and 3D Spheroid Culture
4.2. CRC Specimen Processing and Derivate Organoid Culture
4.3. Cell Viability and Proliferation Assay
4.4. Drug Screening
4.5. Plasmids and Lentiviral Transduction
4.6. Antibodies
4.7. Western Blot Assays
4.8. Immunofluorescence Analysis and HCS
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Piedbois, P.; Rougier, P.; Buyse, M.; Pignon, J.; Ryan, L.; Hansen, R.; Zee, B.; Weinerman, B.; Pater, J.; Leichman, C.; et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J. Clin. Oncol. 1998, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Van Cutsem, E.; Diaz-Rubio, E.; Cervantes, A.; Humblet, Y.; Andre, T.; Van Laethem, J.L.; Soulie, P.; Casado, E.; Verslype, C.; et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2007, 25, 5225–5232. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C., 2nd; Scoggins, C.R.; Schreeder, M.; Rilling, W.S.; Laing, C.J.; Tatum, C.M.; Kelly, L.R.; Garcia-Monaco, R.D.; Sharma, V.R.; Crocenzi, T.S.; et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 2015, 121, 3649–3658. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- McRee, A.J.; Goldberg, R.M. Optimal management of metastatic colorectal cancer: Current status. Drugs 2011, 71, 869–884. [Google Scholar] [CrossRef]
- Hou, X.; Du, C.; Lu, L.; Yuan, S.; Zhan, M.; You, P.; Du, H. Opportunities and challenges of patient-derived models in cancer research: Patient-derived xenografts, patient-derived organoid and patient-derived cells. World J. Surg. Oncol. 2022, 20, 37. [Google Scholar] [CrossRef]
- Bae, J.; Choi, Y.S.; Cho, G.; Jang, S.J. The Patient-Derived Cancer Organoids: Promises and Challenges as Platforms for Cancer Discovery. Cancers 2022, 14, 2144. [Google Scholar] [CrossRef]
- Li, Y.; Tang, P.; Cai, S.; Peng, J.; Hua, G. Organoid based personalized medicine: From bench to bedside. Cell Regen. 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Clevers, H.; Shen, X. Promises and Challenges of Organoid-Guided Precision Medicine. Med 2021, 2, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Karlsson, K.; Kuo, C.J. Applications of Organoids for Cancer Biology and Precision Medicine. Nat. Cancer 2020, 1, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Viard, I.; Wehrli, P.; Jornot, L.; Bullani, R.; Vechietti, J.L.; Schifferli, J.A.; Tschopp, J.; French, L.E. Clusterin gene expression mediates resistance to apoptotic cell death induced by heat shock and oxidative stress. J. Investig. Dermatol. 1999, 112, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, J.K.; Edwards, C.A.; Xu, Z.; Taichman, R.; Wang, C.Y. Clusterin inhibits apoptosis by interacting with activated Bax. Nat. Cell Biol. 2005, 7, 909–915. [Google Scholar] [CrossRef]
- Koltai, T. Clusterin: A key player in cancer chemoresistance and its inhibition. OncoTargets Ther. 2014, 7, 447–456. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Y.; Qin, S.; Zhao, W.; Chu, Q.; Wu, K. Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp. Hematol. Oncol. 2018, 7, 30. [Google Scholar] [CrossRef]
- Linnekamp, J.F.; Hooff, S.R.V.; Prasetyanti, P.R.; Kandimalla, R.; Buikhuisen, J.Y.; Fessler, E.; Ramesh, P.; Lee, K.; Bochove, G.G.W.; de Jong, J.H.; et al. Consensus molecular subtypes of colorectal cancer are recapitulated in in vitro and in vivo models. Cell Death Differ. 2018, 25, 616–633. [Google Scholar] [CrossRef]
- van Iersel, L.B.; Koudijs, T.M.; Hoekman, E.J.; Janssen-van Rhijn, C.M.; Vahrmeijer, A.L.; Nortier, J.W.; van de Velde, C.J.; Gelderblom, H.; Kuppen, P.J. In vitro schedule-dependent interaction between melphalan and oxaliplatin in human colorectal cancer cell lines. J. Surg. Res. 2011, 167, 273–278. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Virshup, D.M. Wnt Signaling and Drug Resistance in Cancer. Mol. Pharmacol. 2020, 97, 72–89. [Google Scholar] [CrossRef]
- Yuan, S.; Tao, F.; Zhang, X.; Zhang, Y.; Sun, X.; Wu, D. Role of Wnt/beta-Catenin Signaling in the Chemoresistance Modulation of Colorectal Cancer. Biomed. Res. Int. 2020, 2020, 9390878. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Tewari, S.; Cicco, C.E.; Atamna, W.; Lazarova, D.L. A switch from canonical to noncanonical Wnt signaling mediates drug resistance in colon cancer cells. PLoS ONE 2011, 6, e27308. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Hu, H.C.; Chao, J.I. Oxaliplatin down-regulates survivin by p38 MAP kinase and proteasome in human colon cancer cells. Chem. Biol. Interact. 2010, 188, 535–545. [Google Scholar] [CrossRef]
- Almendro, V.; Ametller, E.; Garcia-Recio, S.; Collazo, O.; Casas, I.; Auge, J.M.; Maurel, J.; Gascon, P. The role of MMP7 and its cross-talk with the FAS/FASL system during the acquisition of chemoresistance to oxaliplatin. PLoS ONE 2009, 4, e4728. [Google Scholar] [CrossRef]
- Ametller, E.; Garcia-Recio, S.; Pastor-Arroyo, E.M.; Callejo, G.; Carbo, N.; Gascon, P.; Almendro, V. Differential regulation of MMP7 in colon cancer cells resistant and sensitive to oxaliplatin-induced cell death. Cancer Biol. Ther. 2011, 11, 4–13. [Google Scholar] [CrossRef]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, C.; Zhang, Y.; Zhang, X.; Zhang, C.; Zhang, P.; Xie, X.; Ren, Z. miR-506 enhances the sensitivity of human colorectal cancer cells to oxaliplatin by suppressing MDR1/P-gp expression. Cell Prolif. 2017, 50, e12341. [Google Scholar] [CrossRef]
- Bu, L.; Baba, H.; Yasuda, T.; Uchihara, T.; Ishimoto, T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020, 111, 3468–3477. [Google Scholar] [CrossRef]
- Wei, L.; Surma, M.; Shi, S.; Lambert-Cheatham, N.; Shi, J. Novel Insights into the Roles of Rho Kinase in Cancer. Arch. Immunol. Ther. Exp. 2016, 64, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.A.; Han, J.; Kim, I.S. Rho-Kinase as a Target for Cancer Therapy and Its Immunotherapeutic Potential. Int. J. Mol. Sci. 2021, 22, 12916. [Google Scholar] [CrossRef] [PubMed]
- Seklic, D.S.; Jovanovic, M.M.; Virijevic, K.D.; Grujic, J.N.; Zivanovic, M.N.; Markovic, S.D. Pseudevernia furfuracea inhibits migration and invasion of colorectal carcinoma cell lines. J. Ethnopharmacol. 2022, 284, 114758. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Sansom, O.J.; Reed, K.R.; Hayes, A.J.; Ireland, H.; Brinkmann, H.; Newton, I.P.; Batlle, E.; Simon-Assmann, P.; Clevers, H.; Nathke, I.S.; et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes. Dev. 2004, 18, 1385–1390. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Qin, Q.; Zheng, T. Clusterin role in hepatocellular carcinoma patients treated with oxaliplatin. Biosci. Rep. 2020, 40, BSR20200071. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.A.; Bentzen, S.M.; Chen, E.X.; Siu, L.L. Surrogate end points for median overall survival in metastatic colorectal cancer: Literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J. Clin. Oncol. 2007, 25, 4562–4568. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.K.; Yun, N.H.; Jeong, Y.L.; Park, J.; Doh, J.; Lee, W.Y.; Cho, Y.B. Establishment of patient-derived organotypic tumor spheroid models for tumor microenvironment modeling. Cancer Med. 2021, 10, 5589–5598. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Sa, J.K.; Lee, Y.; Kim, D.; Chang, N.; Cho, H.J.; Son, M.; Oh, M.Y.T.; Shin, K.; Lee, J.K.; et al. PIP4K2A as a negative regulator of PI3K in PTEN-deficient glioblastoma. J. Exp. Med. 2019, 216, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liu, Z.; Sa, J.K.; Shin, S.; Wang, J.; Bordyuh, M.; Cho, H.J.; Elliott, O.; Chu, T.; Choi, S.W.; et al. Pharmacogenomic landscape of patient-derived tumor cells informs precision oncology therapy. Nat. Genet. 2018, 50, 1399–1411. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.J.; Jo, E.H.; Oh, Y.; Kim, D.S.; Hyun, S.; Yu, A.; Hong, H.K.; Cho, Y.B. Improved Drug-Response Prediction Model of APC Mutant Colon Cancer Patient-Derived Organoids for Precision Medicine. Cancers 2023, 15, 5531. https://doi.org/10.3390/cancers15235531
Shin YJ, Jo EH, Oh Y, Kim DS, Hyun S, Yu A, Hong HK, Cho YB. Improved Drug-Response Prediction Model of APC Mutant Colon Cancer Patient-Derived Organoids for Precision Medicine. Cancers. 2023; 15(23):5531. https://doi.org/10.3390/cancers15235531
Chicago/Turabian StyleShin, Yong Jae, Eun Hae Jo, Yunjeong Oh, Da Som Kim, Seungyoon Hyun, Ahran Yu, Hye Kyung Hong, and Yong Beom Cho. 2023. "Improved Drug-Response Prediction Model of APC Mutant Colon Cancer Patient-Derived Organoids for Precision Medicine" Cancers 15, no. 23: 5531. https://doi.org/10.3390/cancers15235531
APA StyleShin, Y. J., Jo, E. H., Oh, Y., Kim, D. S., Hyun, S., Yu, A., Hong, H. K., & Cho, Y. B. (2023). Improved Drug-Response Prediction Model of APC Mutant Colon Cancer Patient-Derived Organoids for Precision Medicine. Cancers, 15(23), 5531. https://doi.org/10.3390/cancers15235531





