Semaphorin 6 Family—An Important Yet Overlooked Group of Signaling Proteins Involved in Cancerogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
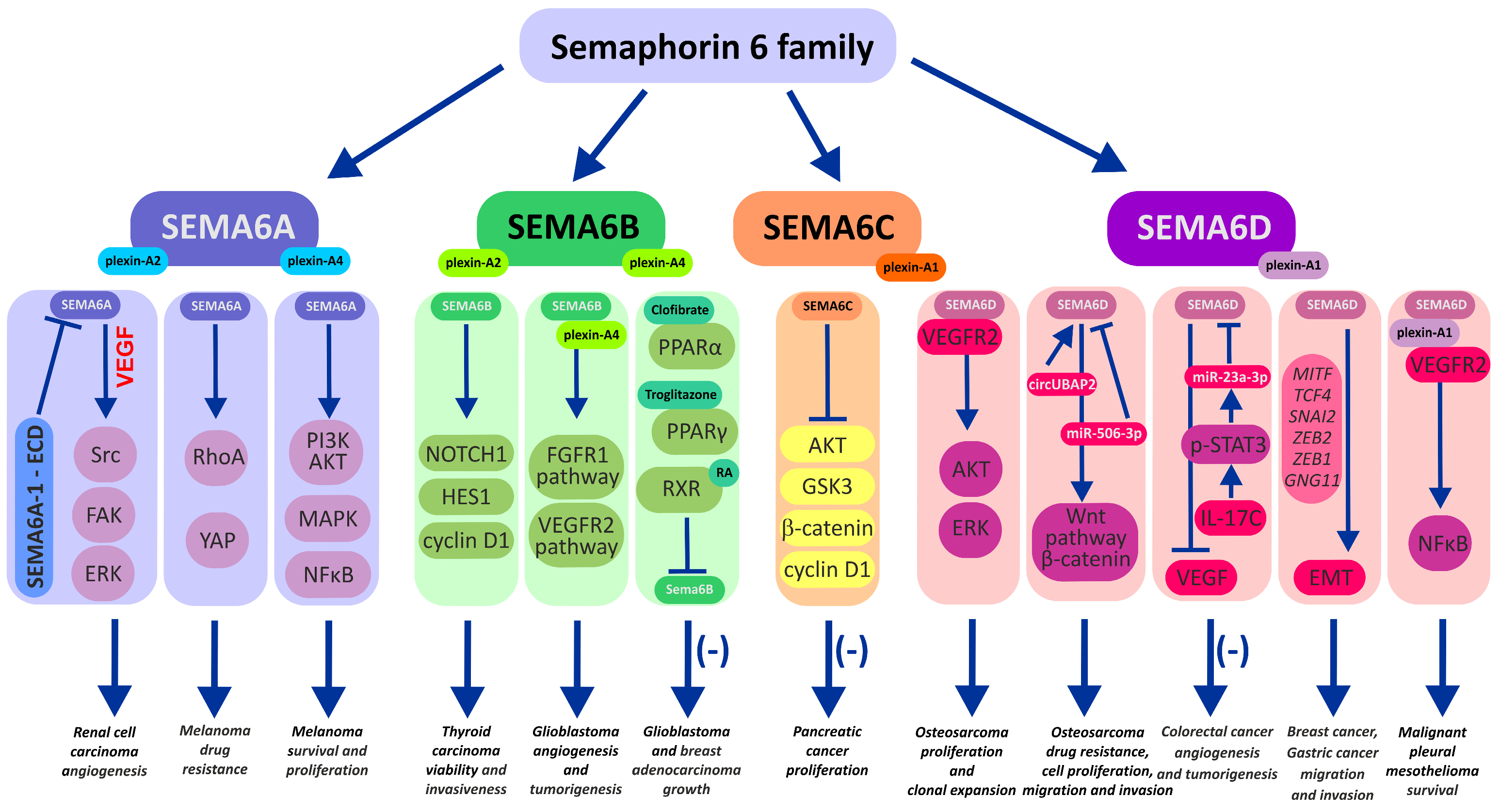
2. Semaphorin 6 Family
2.1. Semaphorin 6A
2.2. Semaphorin 6B
2.3. Semaphorin 6C
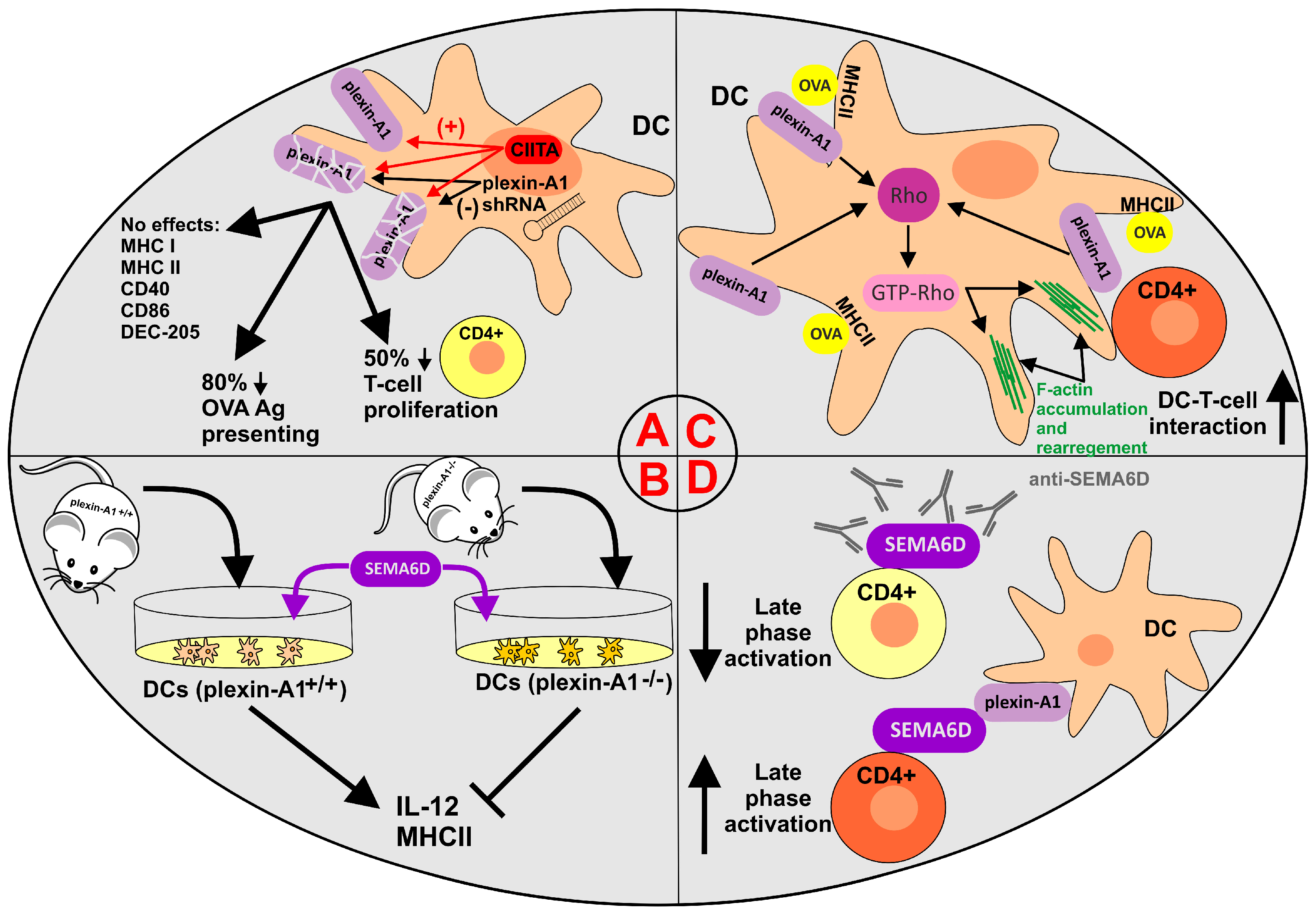
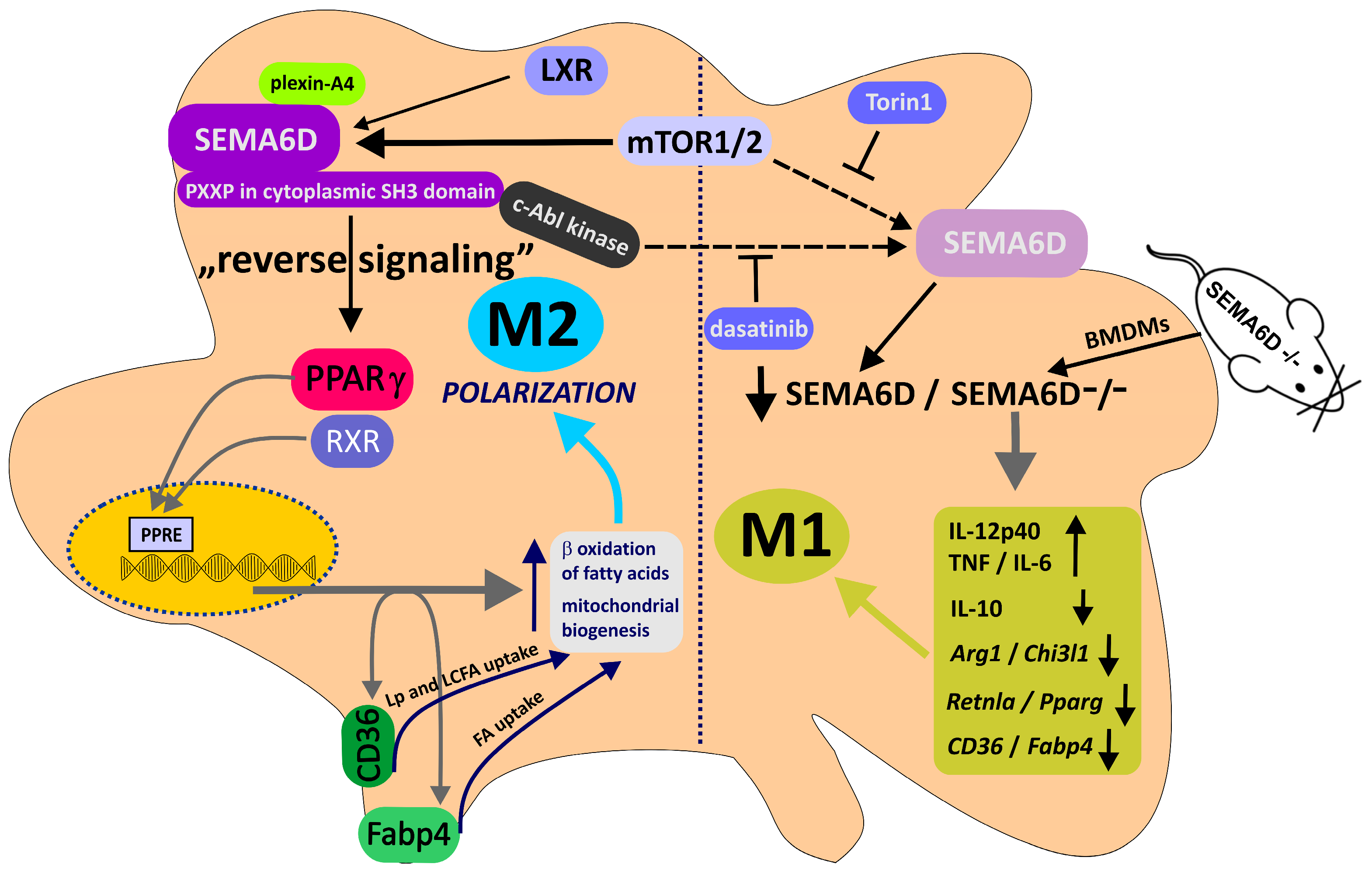
2.4. Semaphorin 6D in the Immunological Landscape of Tumors
2.5. Semaphorin 6D in Cancer Tissues
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Alto, L.T.; Terman, J.R. Semaphorins and their Signaling Mechanisms. Methods Mol. Biol. 2017, 1493, 1–25. [Google Scholar] [CrossRef]
- Fard, D.; Tamagnone, L. Semaphorins in health and disease. Cytokine Growth Factor Rev. 2021, 57, 55–63. [Google Scholar] [CrossRef]
- Koncina, E.; Roth, L.; Gonthier, B.; Bagnard, D. Role of semaphorins during axon growth and guidance. Adv. Exp. Med. Biol. 2007, 621, 50–64. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Ruitenberg, M.J.; Verhaagen, J. Semaphorins and their receptors in olfactory axon guidance. Cell. Mol. Biol. 1999, 45, 763–779. [Google Scholar]
- Feiner, L.; Webber, A.L.; Brown, C.B.; Lu, M.M.; Jia, L.; Feinstein, P.; Mombaerts, P.; Epstein, J.A.; Raper, J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001, 128, 3061–3070. [Google Scholar] [CrossRef]
- Toyofuku, T.; Yoshida, J.; Sugimoto, T.; Yamamoto, M.; Makino, N.; Takamatsu, H.; Takegahara, N.; Suto, F.; Hori, M.; Fujisawa, H.; et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 2008, 321, 251–262. [Google Scholar] [CrossRef]
- Brown, C.B.; Feiner, L.; Lu, M.M.; Li, J.; Ma, X.; Webber, A.L.; Jia, L.; Raper, J.A.; Epstein, J.A. PlexinA2 and semaphorin signaling during cardiac neural crest development. Dev. Camb. Engl. 2001, 128, 3071–3080. [Google Scholar] [CrossRef]
- Hughes, A.; Kleine-Albers, J.; Helfrich, M.H.; Ralston, S.H.; Rogers, M.J. A class III semaphorin (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcif. Tissue Int. 2012, 90, 151–162. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Rafael-Vidal, C.; Malvar-Fernandez, B.; Lopes, A.P.; Pego-Reigosa, J.M.; Radstake, T.R.D.J.; Garcia, S. Semaphorin4A-Plexin D1 Axis Induces Th2 and Th17 While Represses Th1 Skewing in an Autocrine Manner. Int. J. Mol. Sci. 2020, 21, 6965. [Google Scholar] [CrossRef]
- Gurrapu, S.; Tamagnone, L. Semaphorins as Regulators of Phenotypic Plasticity and Functional Reprogramming of Cancer Cells. Trends Mol. Med. 2019, 25, 303–314. [Google Scholar] [CrossRef]
- Oinuma, I.; Ishikawa, Y.; Katoh, H.; Negishi, M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 2004, 305, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Juhaszova, K.; Kolodkin, A.L. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron 2012, 76, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Yabuki, M.; Harada, K.; Hori, M.; Kikutani, H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 2004, 6, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Sweeney, L.B.; Schuldiner, O.; Garcia, K.C.; Luo, L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 2007, 128, 399–410. [Google Scholar] [CrossRef]
- Mauti, O.; Domanitskaya, E.; Andermatt, I.; Sadhu, R.; Stoeckli, E.T. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2007, 2, 28. [Google Scholar] [CrossRef]
- Suto, F.; Tsuboi, M.; Kamiya, H.; Mizuno, H.; Kiyama, Y.; Komai, S.; Shimizu, M.; Sanbo, M.; Yagi, T.; Hiromi, Y.; et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 2007, 53, 535–547. [Google Scholar] [CrossRef]
- Andermatt, I.; Wilson, N.H.; Bergmann, T.; Mauti, O.; Gesemann, M.; Sockanathan, S.; Stoeckli, E.T. Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance. Development 2014, 141, 3709–3720. [Google Scholar] [CrossRef]
- Haklai-Topper, L.; Mlechkovich, G.; Savariego, D.; Gokhman, I.; Yaron, A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J. 2010, 29, 2635–2645. [Google Scholar] [CrossRef]
- Fujisawa, H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J. Neurobiol. 2004, 59, 24–33. [Google Scholar] [CrossRef]
- Perälä, N.; Sariola, H.; Immonen, T. More than nervous: The emerging roles of plexins. Differentiation 2012, 83, 77–91. [Google Scholar] [CrossRef]
- Pascoe, H.G.; Wang, Y.; Zhang, X. Structural mechanisms of plexin signaling. Prog. Biophys. Mol. Biol. 2015, 118, 161–168. [Google Scholar] [CrossRef]
- Fard, D.; Testa, E.; Panzeri, V.; Rizzolio, S.; Bianchetti, G.; Napolitano, V.; Masciarelli, S.; Fazi, F.; Maulucci, G.; Scicchitano, B.M.; et al. SEMA6C: A novel adhesion-independent FAK and YAP activator, required for cancer cell viability and growth. Cell. Mol. Life Sci. 2023, 80, 111. [Google Scholar] [CrossRef]
- Cagnoni, G.; Tamagnone, L. Semaphorin receptors meet receptor tyrosine kinases on the way of tumor progression. Oncogene 2014, 33, 4795–4802. [Google Scholar] [CrossRef] [PubMed]
- Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 1999, 97, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Chédotal, A.; Hanafusa, H.; Ujimasa, Y.; de Castro, F.; Goodman, C.S.; Kimura, T. Cloning and characterization of a novel class VI semaphorin, semaphorin Y. Mol. Cell. Neurosci. 1999, 13, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Matthes, D.J.; Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993, 75, 1389–1399. [Google Scholar] [CrossRef]
- Hota, P.K.; Buck, M. Plexin structures are coming: Opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell. Mol. Life Sci. 2012, 69, 3765–3805. [Google Scholar] [CrossRef]
- Love, C.A.; Harlos, K.; Mavaddat, N.; Davis, S.J.; Stuart, D.I.; Jones, E.Y.; Esnouf, R.M. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat. Struct. Biol. 2003, 10, 843–848. [Google Scholar] [CrossRef]
- Janssen, B.J.C.; Robinson, R.A.; Pérez-Brangulí, F.; Bell, C.H.; Mitchell, K.J.; Siebold, C.; Jones, E.Y. Structural basis of semaphorin-plexin signalling. Nature 2010, 467, 1118–1122. [Google Scholar] [CrossRef]
- Tanaka, T.; Ekimoto, T.; Nagatomo, M.; Neyazaki, M.; Shimoji, E.; Yamane, T.; Kanagawa, S.; Oi, R.; Mihara, E.; Takagi, J.; et al. Hybrid in vitro/in silico analysis of low-affinity protein-protein interactions that regulate signal transduction by Sema6D. Protein Sci. 2022, 31, e4452. [Google Scholar] [CrossRef]
- Tran, T.S.; Kolodkin, A.L.; Bharadwaj, R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 2007, 23, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Nogi, T.; Yasui, N.; Mihara, E.; Matsunaga, Y.; Noda, M.; Yamashita, N.; Toyofuku, T.; Uchiyama, S.; Goshima, Y.; Kumanogoh, A.; et al. Structural basis for semaphorin signalling through the plexin receptor. Nature 2010, 467, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Suto, F.; Kamei, J.; Aoki, K.; Yabuki, M.; Hori, M.; Fujisawa, H.; et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004, 18, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Rozbesky, D.; Verhagen, M.G.; Karia, D.; Nagy, G.N.; Alvarez, L.; Robinson, R.A.; Harlos, K.; Padilla-Parra, S.; Pasterkamp, R.J.; Jones, E.Y. Structural basis of semaphorin-plexin cis interaction. EMBO J. 2020, 39, e102926. [Google Scholar] [CrossRef]
- Seiradake, E.; Jones, E.Y.; Klein, R. Structural Perspectives on Axon Guidance. Annu. Rev. Cell Dev. Biol. 2016, 32, 577–608. [Google Scholar] [CrossRef]
- Renaud, J.; Kerjan, G.; Sumita, I.; Zagar, Y.; Georget, V.; Kim, D.; Fouquet, C.; Suda, K.; Sanbo, M.; Suto, F.; et al. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat. Neurosci. 2008, 11, 440–449. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Kawasaki, T.; Abe, T.; Shioi, G.; Kohno, T.; Hattori, M.; Sakakibara, A.; Kawaguchi, Y.; Hirata, T. Semaphorin 6A-Plexin A2/A4 Interactions with Radial Glia Regulate Migration Termination of Superficial Layer Cortical Neurons. iScience 2019, 21, 359–374. [Google Scholar] [CrossRef]
- Castellotti, B.; Canafoglia, L.; Freri, E.; Tappatà, M.; Messina, G.; Magri, S.; DiFrancesco, J.C.; Fanella, M.; Di Bonaventura, C.; Morano, A.; et al. Progressive myoclonus epilepsies due to SEMA6B mutations. New variants and appraisal of published phenotypes. Epilepsia Open 2023, 8, 645–650. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Huang, D.-P.; Li, T.; Huang, J.; Jiang, P.; Ling, W.-H.; Chen, X.-Q. A De Novo SEMA6B Variant in a Chinese Patient with Progressive Myoclonic Epilepsy-11 and Review of the Literature. J. Mol. Neurosci. 2021, 71, 1944–1950. [Google Scholar] [CrossRef]
- Yoshida, Y.; Han, B.; Mendelsohn, M.; Jessell, T.M. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 2006, 52, 775–788. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, W.; Shen, W.; Cheng, J.; Xi, Y.; Yuan, S.; Fu, F.; Ding, T.; Luo, A.; Wang, S. Low expression of SEMA6C accelerates the primordial follicle activation in the neonatal mouse ovary. J. Cell. Mol. Med. 2018, 22, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Libelius, R.; Tågerud, S. Semaphorin 6C expression in innervated and denervated skeletal muscle. J. Mol. Histol. 2008, 39, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Takegahara, N.; Takamatsu, H.; Toyofuku, T.; Tsujimura, T.; Okuno, T.; Yukawa, K.; Mizui, M.; Yamamoto, M.; Prasad, D.V.R.; Suzuki, K.; et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 2006, 8, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Hoek, R.M.; Cerwenka, A.; Blom, B.; Lucian, L.; McNeil, T.; Murray, R.; Phillips, L.H.; Sedgwick, J.D.; Lanier, L.L. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 2000, 13, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wolter, M.; Werner, T.; Malzkorn, B.; Reifenberger, G. Role of microRNAs Located on Chromosome Arm 10q in Malignant Gliomas. Brain Pathol. 2016, 26, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tang, H.; Zhao, H.; Che, W.; Zhang, L.; Liang, P. SEMA6A is a prognostic biomarker in glioblastoma. Tumour Biol. 2015, 36, 8333–8340. [Google Scholar] [CrossRef]
- Kigel, B.; Rabinowicz, N.; Varshavsky, A.; Kessler, O.; Neufeld, G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood 2011, 118, 4285–4296. [Google Scholar] [CrossRef]
- Tian, R.; Hu, J.; Ma, X.; Liang, L.; Guo, S. Immune-related gene signature predicts overall survival of gastric cancer patients with varying microsatellite instability status. Aging 2020, 13, 2418–2435. [Google Scholar] [CrossRef]
- Ge, C.; Li, Q.; Wang, L.; Xu, X. The role of axon guidance factor semaphorin 6B in the invasion and metastasis of gastric cancer. J. Int. Med. Res. 2013, 41, 284–292. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Chen, L.; Xu, Q.; Li, Y.-H. Expression of semaphorin 6D in gastric carcinoma and its significance. World J. Gastroenterol. 2006, 12, 7388–7390. [Google Scholar] [CrossRef]
- Qu, S.; Yang, Z.; Tao, H.; Ji, F.; Chen, P.; Liang, J.; Lu, Y. Semaphorin 6D and Snail are highly expressed in gastric cancer and positively correlated with malignant clinicopathological indexes. Chin. J. Cell. Mol. Immunol. 2019, 35, 932–937. [Google Scholar]
- Lim, H.-S.; Kim, C.S.; Kim, J.-S.; Yu, S.-K.; Go, D.-S.; Lee, S.A.; Moon, S.M.; Chun, H.S.; Kim, S.G.; Kim, D.K. Suppression of Oral Carcinoma Oncogenic Activity by microRNA-203 via Down-regulation of SEMA6A. Anticancer Res. 2017, 37, 5425–5433. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, X.; Jin, C.; Li, L.; Cui, Q.; Guo, Y.; Dong, Y.; Yang, X.; Guo, L.; Zhang, M. Identification and verification of differentially expressed microRNAs and their target genes for the diagnosis of esophageal cancer. Oncol. Lett. 2018, 16, 3642–3650. [Google Scholar] [CrossRef]
- Yu, S.; Li, N.; Wang, J.; Fu, Y.; Huang, Y.; Yi, P.; Chen, R.; Tang, D.; Hu, X.; Fan, X. Correlation of Long Noncoding RNA SEMA6A-AS1 Expression with Clinical Outcome in HBV-Related Hepatocellular Carcinoma. Clin. Ther. 2020, 42, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-B.; Yu, Y.; Zhang, G.-P.; Li, S.-Q. Genomic Instability of Mutation-Derived Gene Prognostic Signatures for Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2021, 9, 728574. [Google Scholar] [CrossRef]
- Cui, K.; Bian, X. The microRNA cluster miR-30b/-30d prevents tumor cell switch from an epithelial to a mesenchymal-like phenotype in GBC. Mol. Ther. Methods Clin. Dev. 2021, 20, 716–725. [Google Scholar] [CrossRef]
- Zhou, S.; Szöllősi, A.G.; Huang, X.; Chang-Chien, Y.-C.; Hajdu, A. A Novel Immune-Related Gene Prognostic Index (IRGPI) in Pancreatic Adenocarcinoma (PAAD) and Its Implications in the Tumor Microenvironment. Cancers 2022, 14, 5652. [Google Scholar] [CrossRef]
- Liu, H.; Brannon, A.R.; Reddy, A.R.; Alexe, G.; Seiler, M.W.; Arreola, A.; Oza, J.H.; Yao, M.; Juan, D.; Liou, L.S.; et al. Identifying mRNA targets of microRNA dysregulated in cancer: With application to clear cell Renal Cell Carcinoma. BMC Syst. Biol. 2010, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jin, M.; Qiao, B. Semaphorin 6D as an independent predictor for better prognosis in clear cell renal cell carcinoma. Transl. Oncol. 2022, 22, 101453. [Google Scholar] [CrossRef]
- Chen, L.-H.; Liao, C.-Y.; Lai, L.-C.; Tsai, M.-H.; Chuang, E.Y. Semaphorin 6A Attenuates the Migration Capability of Lung Cancer Cells via the NRF2/HMOX1 Axis. Sci. Rep. 2019, 9, 13302. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Chen, Y.; Li, M.; Ha, M.; Li, S. Screening and identification of biomarkers associated with the diagnosis and prognosis of lung adenocarcinoma. J. Clin. Lab. Anal. 2020, 34, e23450. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.B.; Kekeeva, T.V.; Larin, S.S.; Zemliakova, V.V.; Babenko, O.V.; Nemtsova, M.V.; Zaletaev, D.V.; Strel’nikov, V.V. Novel methylation and expression markers associated with breast cancer. Mol. Biol. 2007, 41, 624–633. [Google Scholar] [CrossRef]
- Baxter, D.E.; Allinson, L.M.; Al Amri, W.S.; Poulter, J.A.; Pramanik, A.; Thorne, J.L.; Verghese, E.T.; Hughes, T.A. MiR-195 and Its Target SEMA6D Regulate Chemoresponse in Breast Cancer. Cancers 2021, 13, 5979. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.; Bon, G.; Perotti, V.; Gallo, E.; Bersani, I.; Baldassari, P.; Porru, M.; Leonetti, C.; Di Carlo, S.; Visca, P.; et al. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget 2015, 6, 2779–2793. [Google Scholar] [CrossRef]
- Lv, W.; Zhan, Y.; Tan, Y.; Wu, Y.; Chen, H. A combined aging and immune prognostic signature predict prognosis and responsiveness to immunotherapy in melanoma. Front. Pharmacol. 2022, 13, 943944. [Google Scholar] [CrossRef]
- Loria, R.; Laquintana, V.; Scalera, S.; Fraioli, R.; Caprara, V.; Falcone, I.; Bazzichetto, C.; Di Martile, M.; Rosanò, L.; Del Bufalo, D.; et al. SEMA6A/RhoA/YAP axis mediates tumor-stroma interactions and prevents response to dual BRAF/MEK inhibition in BRAF-mutant melanoma. J. Exp. Clin. Cancer Res. 2022, 41, 148. [Google Scholar] [CrossRef]
- Lv, X.-J.; Chen, X.; Wang, Y.; Yu, S.; Pang, L.; Huang, C. Aberrant expression of semaphorin 6B affects cell phenotypes in thyroid carcinoma by activating the Notch signalling pathway. Endokrynol. Pol. 2021, 72, 29–36. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Wang, Y.; Zhan, Y.; Li, J.; Nong, X.; Gao, B. Association of a Novel Prognosis Model with Tumor Mutation Burden and Tumor-Infiltrating Immune Cells in Thyroid Carcinoma. Front. Genet. 2021, 12, 744304. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Wang, W.; Zhang, R.; Gan, W.; Lv, S.; Zeng, Z.; Hou, Y.; Yang, M. SEMA6B Overexpression Predicts Poor Prognosis and Correlates With the Tumor Immunosuppressive Microenvironment in Colorectal Cancer. Front. Mol. Biosci. 2021, 8, 687319. [Google Scholar] [CrossRef]
- Moriarity, B.S.; Otto, G.M.; Rahrmann, E.P.; Rathe, S.K.; Wolf, N.K.; Weg, M.T.; Manlove, L.A.; LaRue, R.S.; Temiz, N.A.; Molyneux, S.D.; et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat. Genet. 2015, 47, 615–624. [Google Scholar] [CrossRef]
- Catalano, A.; Lazzarini, R.; Di Nuzzo, S.; Orciari, S.; Procopio, A. The Plexin-A1 Receptor Activates Vascular Endothelial Growth Factor-Receptor 2 and Nuclear Factor-κB to Mediate Survival and Anchorage-Independent Growth of Malignant Mesothelioma Cells. Cancer Res. 2009, 69, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.L.; Catig, G.C.; Horn-Ranney, E.L.; Moore, M.J. Sensory axon guidance with semaphorin 6A and nerve growth factor in a biomimetic choice point model. Biofabrication 2014, 6, 035026. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Fisher, D.A.; Zhou, L.; White, F.A.; Ng, S.; Snider, W.D.; Luo, Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J. Neurosci. 2000, 20, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Rünker, A.E.; Little, G.E.; Suto, F.; Fujisawa, H.; Mitchell, K.J. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Ohnuki, H.; Maric, D.; Salvucci, O.; Hou, X.; Kumar, A.; Li, X.; Tosato, G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood 2012, 120, 4104–4115. [Google Scholar] [CrossRef]
- Dhanabal, M.; Wu, F.; Alvarez, E.; McQueeney, K.D.; Jeffers, M.; MacDougall, J.; Boldog, F.L.; Hackett, C.; Shenoy, S.; Khramtsov, N.; et al. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol. Ther. 2005, 4, 659–668. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Chang, Y.-C.; Chen, L.-H.; Lin, W.-C.; Lee, Y.-H.; Yeh, S.-T.; Chen, H.-K.; Fang, W.; Hsu, C.-P.; Lee, J.-M.; et al. The extracellular SEMA domain attenuates intracellular apoptotic signaling of semaphorin 6A in lung cancer cells. Oncogenesis 2018, 7, 95. [Google Scholar] [CrossRef]
- Hasan, A.N.; Ahmad, M.W.; Madar, I.H.; Grace, B.L.; Hasan, T.N. An in silico analytical study of lung cancer and smokers datasets from gene expression omnibus (GEO) for prediction of differentially expressed genes. Bioinformation 2015, 11, 229–235. [Google Scholar] [CrossRef]
- Lu, T.-P.; Tsai, M.-H.; Lee, J.-M.; Hsu, C.-P.; Chen, P.-C.; Lin, C.-W.; Shih, J.-Y.; Yang, P.-C.; Hsiao, C.K.; Lai, L.-C.; et al. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2590–2597. [Google Scholar] [CrossRef]
- Prislei, S.; Mozzetti, S.; Filippetti, F.; De Donato, M.; Raspaglio, G.; Cicchillitti, L.; Scambia, G.; Ferlini, C. From plasma membrane to cytoskeleton: A novel function for semaphorin 6A. Mol. Cancer Ther. 2008, 7, 233–241. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Cordovado, A.; Schaettin, M.; Jeanne, M.; Panasenkava, V.; Denommé-Pichon, A.-S.; Keren, B.; Mignot, C.; Doco-Fenzy, M.; Rodan, L.; Ramsey, K.; et al. SEMA6B variants cause intellectual disability and alter dendritic spine density and axon guidance. Hum. Mol. Genet. 2022, 31, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- Tawarayama, H.; Yoshida, Y.; Suto, F.; Mitchell, K.J.; Fujisawa, H. Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. J. Neurosci. 2010, 30, 7049–7060. [Google Scholar] [CrossRef] [PubMed]
- Küry, P.; Abankwa, D.; Kruse, F.; Greiner-Petter, R.; Müller, H.W. Gene expression profiling reveals multiple novel intrinsic and extrinsic factors associated with axonal regeneration failure. Eur. J. Neurosci. 2004, 19, 32–42. [Google Scholar] [CrossRef]
- Xiaozhen, S.; Fan, Y.; Fang, Y.; Xiaoping, L.; Jia, J.; Wuhen, X.; Xiaojun, T.; Jun, S.; Yucai, C.; Hong, Z.; et al. Novel Truncating and Missense Variants in SEMA6B in Patients With Early-Onset Epilepsy. Front. Cell Dev. Biol. 2021, 9, 633819. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Hellenbroich, Y.; Brüggemann, N.; Lohmann, K.; Grimmel, M.; Haack, T.B.; von Spiczak, S.; Münchau, A. Zonisamide-responsive myoclonus in SEMA6B-associated progressive myoclonic epilepsy. Ann. Clin. Transl. Neurol. 2021, 8, 1524–1527. [Google Scholar] [CrossRef]
- Duan, J.; Chen, Y.; Hu, Z.; Ye, Y.; Zhang, T.; Li, C.; Zeng, Q.; Zhao, X.; Mai, J.; Sun, Y.; et al. Non-convulsive Status Epilepticus in SEMA6B-Related Progressive Myoclonic Epilepsy: A Case Report With Literature Review. Front. Pediatr. 2022, 10, 859183. [Google Scholar] [CrossRef]
- Hamanaka, K.; Imagawa, E.; Koshimizu, E.; Miyatake, S.; Tohyama, J.; Yamagata, T.; Miyauchi, A.; Ekhilevitch, N.; Nakamura, F.; Kawashima, T.; et al. De Novo Truncating Variants in the Last Exon of SEMA6B Cause Progressive Myoclonic Epilepsy. Am. J. Hum. Genet. 2020, 106, 549–558. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Yan, X.; Shen, L.; Guo, J.; Xu, Q. A novel SEMA6B variant causes adult-onset progressive myoclonic epilepsy-11 in a Chinese family: A case report and literature review. Front. Genet. 2023, 14, 1110310. [Google Scholar] [CrossRef]
- D’Apice, L.; Costa, V.; Valente, C.; Trovato, M.; Pagani, A.; Manera, S.; Regolo, L.; Zambelli, A.; Ciccodicola, A.; De Berardinis, P. Analysis of SEMA6B gene expression in breast cancer: Identification of a new isoform. Biochim. Biophys. Acta 2013, 1830, 4543–4553. [Google Scholar] [CrossRef]
- Collet, P.; Domenjoud, L.; Devignes, M.D.; Murad, H.; Schohn, H.; Dauça, M. The human semaphorin 6B gene is down regulated by PPARs. Genomics 2004, 83, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Collet, P.; Huin-Schohn, C.; Al-Makdissy, N.; Kerjan, G.; Chedotal, A.; Donner, M.; Devignes, M.D.; Becuwe, P.; Schohn, H.; et al. Effects of PPAR and RXR ligands in semaphorin 6B gene expression of human MCF-7 breast cancer cells. Int. J. Oncol. 2006, 28, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Kasies, F.; Azroony, R.; Alya, G.; Madania, A. Effects of fenofibrate on Semaphorin 6B gene expression in rat skeletal muscle. Mol. Med. Rep. 2011, 4, 575–580. [Google Scholar] [CrossRef]
- Burgaya, F.; Fontana, X.; Martínez, A.; Montolio, M.; Mingorance, A.; Simó, S.; del Río, J.A.; Soriano, E. Semaphorin 6C leads to GSK-3-dependent growth cone collapse and redistributes after entorhino-hippocampal axotomy. Mol. Cell. Neurosci. 2006, 33, 321–334. [Google Scholar] [CrossRef]
- Qu, X.; Wei, H.; Zhai, Y.; Que, H.; Chen, Q.; Tang, F.; Wu, Y.; Xing, G.; Zhu, Y.; Liu, S.; et al. Identification, characterization, and functional study of the two novel human members of the semaphorin gene family. J. Biol. Chem. 2002, 277, 35574–35585. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-H.; Hsu, S.-H.; Hou, Y.-C.; Chu, P.-Y.; Su, Y.-Y.; Shan, Y.-S.; Hung, W.-C.; Chen, L.-T. Semaphorin 6C Suppresses Proliferation of Pancreatic Cancer Cells via Inhibition of the AKT/GSK3/β-Catenin/Cyclin D1 Pathway. Int. J. Mol. Sci. 2022, 23, 2608. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Caprari, P.; Moretti, S.; Faronato, M.; Tamagnone, L.; Procopio, A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 2006, 107, 3321–3329. [Google Scholar] [CrossRef]
- Kanth, S.M.; Gairhe, S.; Torabi-Parizi, P. The Role of Semaphorins and Their Receptors in Innate Immune Responses and Clinical Diseases of Acute Inflammation. Front. Immunol. 2021, 12, 672441. [Google Scholar] [CrossRef]
- Naito, Y.; Koyama, S.; Masuhiro, K.; Hirai, T.; Uenami, T.; Inoue, T.; Osa, A.; Machiyama, H.; Watanabe, G.; Sax, N.; et al. Tumor-derived semaphorin 4A improves PD-1-blocking antibody efficacy by enhancing CD8+ T cell cytotoxicity and proliferation. Sci. Adv. 2023, 9, eade0718. [Google Scholar] [CrossRef]
- Wong, A.W.; Brickey, W.J.; Taxman, D.J.; van Deventer, H.W.; Reed, W.; Gao, J.X.; Zheng, P.; Liu, Y.; Li, P.; Blum, J.S.; et al. CIITA-regulated plexin-A1 affects T-cell–dendritic cell interactions. Nat. Immunol. 2003, 4, 891–898. [Google Scholar] [CrossRef]
- Eun, S.-Y.; O’Connor, B.P.; Wong, A.W.; van Deventer, H.W.; Taxman, D.J.; Reed, W.; Li, P.; Blum, J.S.; McKinnon, K.P.; Ting, J.P.-Y. Cutting edge: Rho activation and actin polarization are dependent on plexin-A1 in dendritic cells. J. Immunol. 2006, 177, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, J.L.; Criado-García, O. The Actin Cytoskeleton at the Immunological Synapse of Dendritic Cells. Front. Cell Dev. Biol. 2021, 9, 679500. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Eun, S.-Y.; Ye, Z.; Zozulya, A.L.; Lich, J.D.; Moore, C.B.; Iocca, H.A.; Roney, K.E.; Holl, E.K.; Wu, Q.P.; et al. Semaphorin 6D regulates the late phase of CD4+ T cell primary immune responses. Proc. Natl. Acad. Sci. USA 2008, 105, 13015–13020. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Ting, J.P.-Y. The evolving role of semaphorins and plexins in the immune system: Plexin-A1 regulation of dendritic cell function. Immunol. Res. 2008, 41, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, K.; Okuno, T.; Ogata, T.; Takegahara, N.; Takamatsu, H.; Mizui, M.; Taniguchi, M.; Chédotal, A.; Suto, F.; et al. Plexin-A4 negatively regulates T lymphocyte responses. Int. Immunol. 2008, 20, 413–420. [Google Scholar] [CrossRef]
- Kang, S.; Nakanishi, Y.; Kioi, Y.; Okuzaki, D.; Kimura, T.; Takamatsu, H.; Koyama, S.; Nojima, S.; Nishide, M.; Hayama, Y.; et al. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat. Immunol. 2018, 19, 561–570. [Google Scholar] [CrossRef]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Sun, Q.; Peng, Y.; Zhao, Q.; Yan, S.; Liu, S.; Yang, Q.; Liu, K.; Rokosh, D.G.; Jiao, K. SEMA6D regulates perinatal cardiomyocyte proliferation and maturation in mice. Dev. Biol. 2019, 452, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Song, L.; Li, D.; Kesterson, R.; Wang, J.; Wang, L.; Rokosh, G.; Wu, B.; Wang, Q.; Jiao, K. Sema6D acts downstream of bone morphogenetic protein signalling to promote atrioventricular cushion development in mice. Cardiovasc. Res. 2016, 112, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Papic, N.; Zidovec Lepej, S.; Gorenec, L.; Grgic, I.; Gasparov, S.; Filipec Kanizaj, T.; Vince, A. The association of semaphorins 3C, 5A and 6D with liver fibrosis stage in chronic hepatitis C. PLoS ONE 2018, 13, e0209481. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Qu, F. CircUBAP2 promotes SEMA6D expression to enhance the cisplatin resistance in osteosarcoma through sponging miR-506-3p by activating Wnt/β-catenin signaling pathway. J. Mol. Histol. 2020, 51, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gunyuz, Z.E.; Sahi-Ilhan, E.; Kucukkose, C.; Ipekgil, D.; Tok, G.; Mese, G.; Ozcivici, E.; Yalcin-Ozuysal, O. SEMA6D Differentially Regulates Proliferation, Migration, and Invasion of Breast Cell Lines. ACS Omega 2022, 7, 15769–15778. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Wang, L.; Jiao, K. SEMA6D Expression and Patient Survival in Breast Invasive Carcinoma. Int. J. Breast Cancer 2015, 2015, 539721. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Q.; Chen, L.; Zuo, Y.; Liu, S.; Hu, Y.; Li, X.; Li, Y.; Zhao, X. Expression of semaphorin 6D and its receptor plexin-A1 in gastric cancer and their association with tumor angiogenesis. Oncol. Lett. 2016, 12, 3967–3974. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.J.; Choo, J.; Heo, G.; Yoo, J.-W.; Jung, Y.; Rhee, S.H.; Im, E. miR-23a-3p is a Key Regulator of IL-17C-Induced Tumor Angiogenesis in Colorectal Cancer. Cells 2020, 9, 1363. [Google Scholar] [CrossRef]
| SEMA6A | SEMA6B | SEMA6C | SEMA6D | ||||
|---|---|---|---|---|---|---|---|
| ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ |
| Malignant glioma [45] | Glioblastoma [46] | U87MG glioblastoma cells [47] | — | — | — | — | — |
| Gastric cancer [48] | — | Gastric cancer [49] | — | — | — | Gastric cancer [50,51] | |
| Oral carcinoma [52] | — | — | — | — | — | Esophageal cancer [53] | — |
| Hepatocellular carcinoma [54,55] | — | Gallbladder cancer [56] | — | — | Pancreatic cancer [57] | — | — |
| Clear cell renal carcinoma [58] | — | — | — | — | — | — | Clear cell renal carcinoma [59] |
| — | Lung cancer [60] | — | — | — | — | — | Lung adenocarcinoma [61] |
| — | — | Breast cancer [62] | — | — | — | MCF7 breast cancer cells [63] | |
| Melanoma [64,65,66] | — | — | — | — | — | — | — |
| — | — | Thyroid carcinoma [67,68] | — | — | — | — | — |
| — | — | Colorectal cancer [69] | — | — | — | Osteosarcoma [70] | Pleural mesothelioma [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, W.; Ochman, B.; Wagner, W. Semaphorin 6 Family—An Important Yet Overlooked Group of Signaling Proteins Involved in Cancerogenesis. Cancers 2023, 15, 5536. https://doi.org/10.3390/cancers15235536
Wagner W, Ochman B, Wagner W. Semaphorin 6 Family—An Important Yet Overlooked Group of Signaling Proteins Involved in Cancerogenesis. Cancers. 2023; 15(23):5536. https://doi.org/10.3390/cancers15235536
Chicago/Turabian StyleWagner, Wiktor, Błażej Ochman, and Waldemar Wagner. 2023. "Semaphorin 6 Family—An Important Yet Overlooked Group of Signaling Proteins Involved in Cancerogenesis" Cancers 15, no. 23: 5536. https://doi.org/10.3390/cancers15235536
APA StyleWagner, W., Ochman, B., & Wagner, W. (2023). Semaphorin 6 Family—An Important Yet Overlooked Group of Signaling Proteins Involved in Cancerogenesis. Cancers, 15(23), 5536. https://doi.org/10.3390/cancers15235536







