Emerging Precision Medicine Approaches for Lung Neuroendocrine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Multidisciplinary Evaluation and Clinical Precision Medicine
3. Molecular Characterization of LNETs
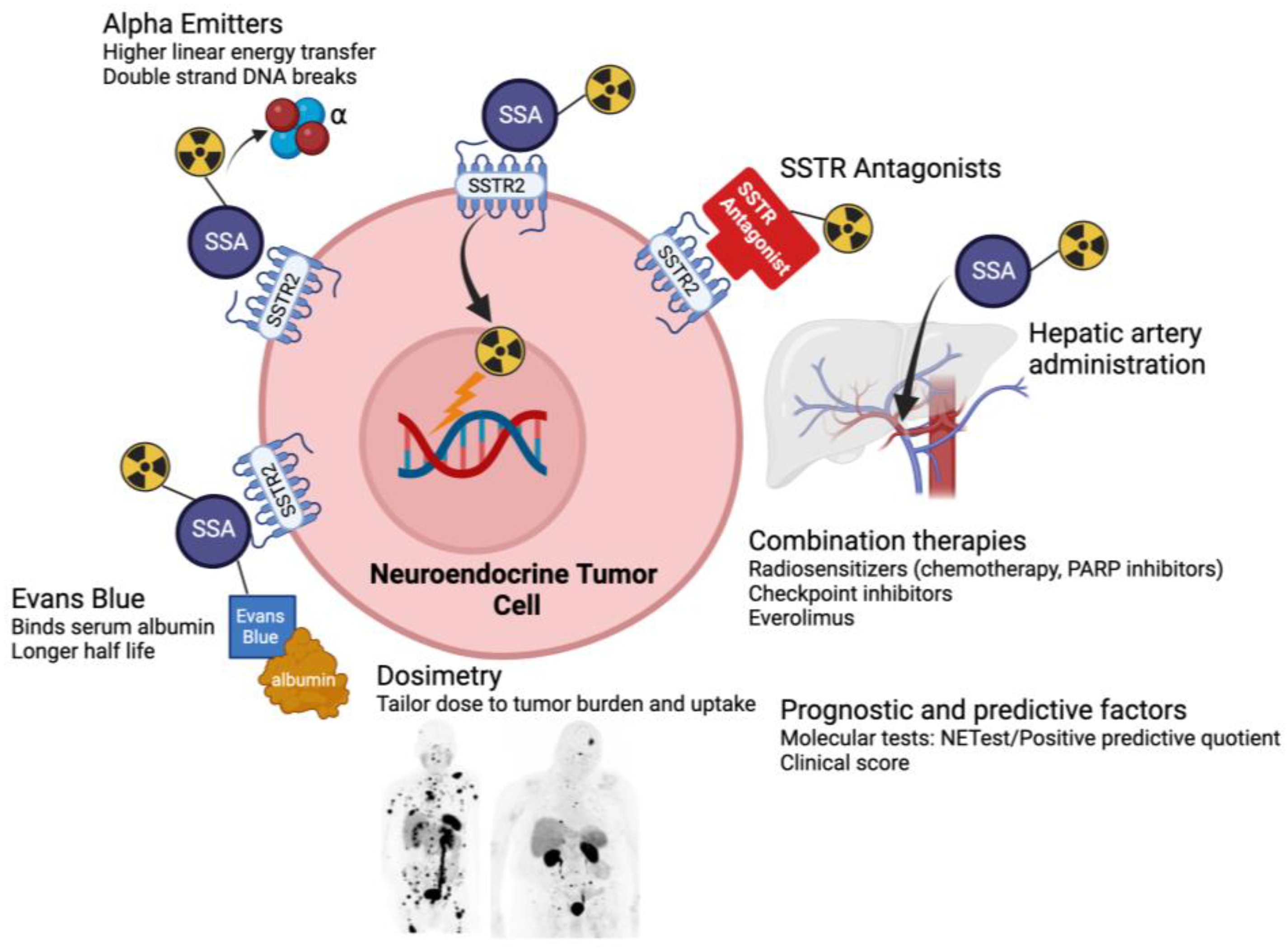
4. Radiotheragnostics
5. Personalized Therapeutic Approaches for LNETs
5.1. Tyrosine Kinase Inhibitors
5.2. Chemotherapy
5.3. Immunotherapy
5.4. Novel PRRT Compounds
6. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol. 2022, 35, 36–50. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X. Incidence trends and risk factors of carcinoid tumors: A nationwide epidemiologic study from Sweden. Cancer 2001, 92, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, C.K.; Paciorek, A.; Moon, F.; Steiding, P.; Shih, B.; Gubens, M.A.; Zhang, L.; Bergsland, E.K.; Cheng, I. Survival outcomes for lung neuroendocrine tumors in California differ by sociodemographic factors. Endocr. Relat. Cancer 2023. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gosain, R.; Groman, A.; Gosain, R.; Dasari, A.; Halfdanarson, T.; Mukherjee, S. Incidence and survival outcomes in patients with lung neuroendocrine neoplasms in the United States. Cancers 2021, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chen, L.-T.; Shan, Y.-S.; Chu, P.-Y.; Tsai, C.-R.; Tsai, H.-J. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Spyroglou, A.; Kykalos, S.; Daskalakis, K.; Kyriakopoulos, G.; Sotiropoulos, G.C.; A Kaltsas, G.; Grossman, A.B. Changing biological behaviour of NETs during the evolution of the disease: Progress on progression. Endocr. Relat. Cancer 2021, 28, R121–R140. [Google Scholar] [CrossRef]
- Kvols, L.K.; Brendtro, K.L. The North American Neuroendocrine Tumor Society (NANETS) guidelines: Mission, goals, and process. Pancreas 2010, 39, 705–706. [Google Scholar] [CrossRef]
- de Herder, W.W.; Capdevila, J. Unmet Needs in the Field of Neuroendocrine Neoplasms of the Gastrointestinal Tract, Pancreas, and Respiratory System: Reports by the ENETS Group. Neuroendocrinology 2019, 108, 5–6. [Google Scholar] [CrossRef]
- Derks, J.L.; Leblay, N.; Lantuejoul, S.; Dingemans, A.-M.C.; Speel, E.-J.M.; Fernandez-Cuesta, L. New Insights into the Molecular Characteristics of Pulmonary Carcinoids and Large Cell Neuroendocrine Carcinomas, and the Impact on Their Clinical Management. J. Thorac. Oncol. 2018, 13, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Mafficini, A.; O Sikora, K.; Fassan, M.; Barbi, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Grillo, F.; Vicentini, C.; et al. Lung neuroendocrine tumours: Deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J. Pathol. 2017, 241, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, L.; Eymin, B.; Brambilla, E.; Gazzeri, S. Expression of p15 and p15.5 products in neuroendocrine lung tumours: Relationship with p15(INK4b) methylation status. Oncogene 2001, 20, 6587–6596. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Fumagalli, C.; Trubia, M.; Sonzogni, A.; Rekhtman, N.; Maisonneuve, P.; Galetta, D.; Spaggiari, L.; Veronesi, G.; Scarpa, A.; et al. Dual role of RASSF1 as a tumor suppressor and an oncogene in neuroendocrine tumors of the lung. Anticancer. Res. 2010, 30, 4269–4281. [Google Scholar] [PubMed]
- Li, F.; Ye, B.; Hong, L.; Xu, H.; Fishbein, M.C. Epigenetic modifications of histone h4 in lung neuroendocrine tumors. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zheng, M.; Jin, Y.; Shen, X.; Shan, L.; Shen, L.; Sun, Y.; Chen, H.; Li, Y. ALK-rearrangement neuroendocrine carcinoma of the lung: A comprehensive study of a rare case series and review of literature. Onco Targets Ther. 2018, 11, 4991–4998. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Li, Y.-F.; Zhang, C.-Y.; Zhang, S.-L.; Lv, Z.-Y.; Dong, S.; Chen, H.-J.; Zhang, X.-C.; Wu, Y.-L.; Yang, J.-J. Response to Icotinib Plus Chemotherapy in Pulmonary Atypical Carcinoid Harboring the EGFR L858R Mutation: A Brief Report. JTO Clin. Res. Rep. 2021, 2, 100258. [Google Scholar] [CrossRef]
- Kander, E.M.; Shah, M.H.; Zhou, Y.; Goyal, A.; Palmer, J.D.; Owen, D.H.; Shilo, K.; Patel, G.; Raval, R.R.; Gonzalez, J.; et al. Response to the Selective RET Inhibitor Selpercatinib (LOXO-292) in a Patient With RET Fusion-positive Atypical Lung Carcinoid. Clin. Lung Cancer 2021, 22, e442–e445. [Google Scholar] [CrossRef]
- Hermans, B.; Derks, J.; Moonen, L.; Habraken, C.; von der Thüsen, J.; Hillen, L.; Speel, E.; Dingemans, A.-M. Pulmonary neuroendocrine neoplasms with well differentiated morphology and high proliferative activity: Illustrated by a case series and review of the literature. Lung Cancer 2020, 150, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Alcala, N.; Leblay, N.; Gabriel, A.A.G.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.S.; Ferrari, A.; Derks, J.; Ghantous, A.; et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat. Commun. 2019, 10, 3407. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; DeBerardinis, R.J.; Xiao, G.; Minna, J.D.; Xie, Y. A Pan-Cancer Assessment of RB1/TP53 Co-Mutations. Cancers 2022, 14, 4199. [Google Scholar] [CrossRef]
- Laddha, S.V.; da Silva, E.M.; Robzyk, K.; Untch, B.R.; Ke, H.; Rekhtman, N.; Poirier, J.T.; Travis, W.D.; Tang, L.H.; Chan, C.S. Integrative Genomic Characterization Identifies Molecular Subtypes of Lung Carcinoids. Cancer Res. 2019, 79, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Barbi, S.; Fassan, M.; Mafficini, A.; Ali, G.; Vicentini, C.; Sperandio, N.; Corbo, V.; Rusev, B.; Mastracci, L.; et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes with Specific Genomic Alterations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.; Grimelius, L.; Spathis, A.; Tringidou, R.; Rassidakis, G.Z.; Öberg, K.; Kaltsas, G.; Tsolakis, A.V. Expression of Somatostatin Receptors 1-5 and Dopamine Receptor 2 in Lung Carcinoids: Implications for a Therapeutic Role. Neuroendocrinology 2015, 101, 211–222. [Google Scholar] [CrossRef]
- Zidan, L.; Iravani, A.; Kong, G.; Akhurst, T.; Michael, M.; Hicks, R.J. Theranostic implications of molecular imaging phenotype of well-differentiated pulmonary carcinoid based on (68)Ga-DOTATATE PET/CT and (18)F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 204–216. [Google Scholar] [CrossRef]
- Ianniello, A.; Sansovini, M.; Severi, S.; Nicolini, S.; Grana, C.M.; Massri, K.; Bongiovanni, A.; Antonuzzo, L.; Di Iorio, V.; Sarnelli, A.; et al. Peptide receptor radionuclide therapy with (177)Lu-DOTATATE in advanced bronchial carcinoids: Prognostic role of thyroid transcription factor 1 and (18)F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1040–1046. [Google Scholar] [CrossRef]
- Parghane, R.V.; Talole, S.; Prabhash, K.; Basu, S. Clinical Response Profile of Metastatic/Advanced Pulmonary Neuroendocrine Tumors to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. Clin. Nucl. Med. 2017, 42, 428–435. [Google Scholar] [CrossRef]
- Rufini, V.; Lorusso, M.; Inzani, F.; Pasciuto, T.; Triumbari, E.K.A.; Grillo, L.R.; Locco, F.; Margaritora, S.; Pescarmona, E.; Rindi, G. Correlation of somatostatin receptor PET/CT imaging features and immunohistochemistry in neuroendocrine tumors of the lung: A retrospective observational study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, B.; Mazal, P.; Kretschmer-Chott, E.; Mayerhoefer, M.; Raderer, M. Pulmonary neuroendocrine tumours and somatostatin receptor status: An assessment of unlicensed use of somatostatin analogues in the clinical practice. ESMO Open. 2022, 7, 100478. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.; Garske, U.; Granberg, D.; Sundin, A.; Lundqvist, H. Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Du, L.; Schad, A.; Jain, S.; Jessop, A.; Shah, C.; Eisner, D.; Cardin, D.; Ciombor, K.; Goff, L.; et al. A clinical score for neuroendocrine tumor patients under consideration for Lu-177-DOTATATE therapy. Endocr. Relat. Cancer 2021, 28, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chauhan, A.; Du, L.; Thomas, K.E.; Jacob, A.; Schad, A.; Jain, S.; Jessop, A.; Shah, C.; Eisner, D.; et al. External Validation of a Clinical Score for Patients With Neuroendocrine Tumors Under Consideration for Peptide Receptor Radionuclide Therapy. JAMA Netw. Open 2022, 5, e2144170. [Google Scholar] [CrossRef]
- Bodei, L.; Schöder, H.; Baum, R.P.; Herrmann, K.; Strosberg, J.; Caplin, M.; Öberg, K.; Modlin, I.M. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020, 21, e431–e443. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Modlin, I.M.; Severi, S.; Drozdov, I.; Nicolini, S.; Kwekkeboom, D.J.; Krenning, E.P.; Baum, R.P.; Paganelli, G. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 839–851. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Capdevila, J.; Salazar, R.; Halperín, I.; Abad, A.; Yao, J.C. Innovations therapy: Mammalian target of rapamycin (mTOR) inhibitors for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011, 30 (Suppl. S1), 27–34. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Terris, B.; Scoazec, J.Y.; Rubbia, L.; Bregeaud, L.; Pepper, M.S.; Ruszniewski, P.; Belghiti, J.; Fléjou, J.; Degott, C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology 1998, 32, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Sennino, B.; Ishiguro-Oonuma, T.; Wei, Y.; Naylor, R.M.; Williamson, C.W.; Bhagwandin, V.; Tabruyn, S.P.; You, W.-K.; Chapman, H.A.; Christensen, J.G.; et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Faris, J.E.; Murphy, J.E.; Blaszkowsky, L.S.; Kwak, E.L.; McCleary, N.J.; Fuchs, C.S.; Meyerhardt, J.A.; Ng, K.; Zhu, A.X.; et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J. Clin. Oncol. 2017, 35 (Suppl. S4), 228. [Google Scholar] [CrossRef]
- Suyama, K.; Iwase, H. Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control 2018, 25, 1073274818789361. [Google Scholar] [CrossRef]
- Syed, Y.Y. Surufatinib: First Approval. Drugs. 2021, 81, 727–732. [Google Scholar] [CrossRef]
- Granberg, D.; Eriksson, B.; Wilander, E.; Grimfjärd, P.; Fjällskog, M.-L.; Öberg, K.; Skogseid, B. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann. Oncol. 2001, 12, 1383–1391. [Google Scholar] [CrossRef]
- Forde, P.M.; Hooker, C.M.; Boikos, S.A.; Petrini, I.; Giaccone, G.; Rudin, C.; Yang, S.C.; Illei, P.B.; Hann, C.L.; Ettinger, D.S.; et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: A brief report. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 414–418. [Google Scholar] [CrossRef]
- Chong, C.R.; Wirth, L.J.; Nishino, M.; Chen, A.B.; Sholl, L.M.; Kulke, M.H.; McNamee, C.J.; Jänne, P.A.; Johnson, B.E. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014, 86, 241–246. [Google Scholar] [CrossRef]
- Crona, J.; Fanola, I.; Lindholm, D.P.; Antonodimitrakis, P.; Öberg, K.; Eriksson, B.; Granberg, D. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013, 98, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ekeblad, S.; Sundin, A.; Janson, E.T.; Welin, S.; Granberg, D.; Kindmark, H.; Dunder, K.; Kozlovacki, G.; Orlefors, H.; Sigurd, M.; et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin. Cancer Res. 2007, 13, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Voros, B.A.; Meadows-Taylor, M.; Smeltzer, M.P.; Griffin, R.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers 2020, 12, 206. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Morse, B.; Strosberg, J. Capecitabine and Temozolomide in Advanced Lung Neuroendocrine Neoplasms. Oncologist 2020, 25, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Defining Driver DNA Methylation Changes in Human Cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef]
- Rivera, A.L.; Pelloski, C.E.; Gilbert, M.R.; Colman, H.; De La Cruz, C.; Sulman, E.P.; Bekele, B.N.; Aldape, K.D. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010, 12, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Oberg, K.; Funa, K.; Alm, G. Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N. Engl. J. Med. 1983, 309, 129–133. [Google Scholar] [CrossRef]
- Kulke, M.H.; Shah, M.H.; Benson, A.B., 3rd; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T.; et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Canc Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.R.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.L.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Mandriani, B.; Pellè, E.; Mannavola, F.; Palazzo, A.; Marsano, R.M.; Ingravallo, G.; Cazzato, G.; Ramello, M.C.; Porta, C.; Strosberg, J.; et al. Development of anti-somatostatin receptors CAR T cells for treatment of neuroendocrine tumors. J. Immunother. Cancer 2022, 10, e004854. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with (225)Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Rubira, L.; Deshayes, E.; Santoro, L.; Kotzki, P.O.; Fersing, C. (225)Ac-Labeled Somatostatin Analogs in the Management of Neuroendocrine Tumors: From Radiochemistry to Clinic. Pharmaceutics 2023, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with (225)Actinium and (213)Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Weber, W.A. Somatostatin Receptor Imaging of Neuroendocrine Tumors: From Agonists to Antagonists. J. Nucl. Med. 2018, 59, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, Y.; Zang, J.; Sui, H.; Wang, H.; Jacobson, O.; Zhu, Z.; Chen, X. Dose escalation of an Evans blue-modified radiolabeled somatostatin analog (177)Lu-DOTA-EB-TATE in the treatment of metastatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 947–957. [Google Scholar] [CrossRef]
| Trial Name | Sponsor City, Country/Region | Phase | Treatment Regimen |
|---|---|---|---|
| TKI Studies and Combination Studies | |||
| A Trial Evaluating the Activity and Safety of Combination Between Cabozantinib and Temozolomide in Lung and GEP-NENS Progressive After Everolimus, Sunitinib or PRRT (CABOTEM) | National Cancer Institute, Naples Naples, Italy | Phase II | Cabozantinib + temozolomide |
| Testing Lutetium Lu 177 Dotatate in Patients With Somatostatin Receptor Positive Advanced Bronchial Neuroendocrine Tumors | National Cancer Institute Bethesda, USA | Phase II | 177Lu-DOTATATE vs. everolimus |
| Testing Cabozantinib in Patients With Advanced Pancreatic Neuroendocrine and Carcinoid Tumors | National Cancer Institute Bethesda, USA | Phase III | Cabozantinib vs. placebo |
| Cabozantinib and Nivolumab for Carcinoid Tumors | Dana-Farber Cancer Institute Boston, USA | Phase II | Cabozantinib + nivolumab |
| Lenvatinib and Everolimus in Treating Patients With Advanced, Unresectable Carcinoid Tumors | MD Anderson Cancer Center Houston, USA | Phase II | Lenvatinib + everolimus |
| Phase II Study of Pembrolizumab and Lenvatinib in Advanced Well-differentiated Neuroendocrine Tumors | H. Lee Moffitt Cancer Center and Research Institute Tampa, USA | Phase II | Lenvatinib + pembrolizumab |
| An Open-Label Phase 2 Study of Surufatinib in Patients With Neuroendocrine Tumours in Europe | Hutchmed Hong Kong, China | Phase II | Surufatinib |
| Immunotherapy Studies | |||
| Survivin Long Peptide Vaccine in Treating Patients With Metastatic Neuroendocrine Tumors | Rosewell Park Buffalo, USA | Phase I | Survivin peptide vaccine + GM-CSF + sandostatin |
| PRRT Studies | |||
| Lutathera in People With Gastroenteropancreatic, Bronchial or Unknown Primary Neuroendocrine Tumors That Have Spread to the Liver | Memorial Sloan Kettering Cancer Center New York, USA | Phase I | Intra-arterial 177Lu-DOTATATE |
| Personalized PRRT of Neuroendocrine Tumors (P-PRRT) | CHU de Quebec-Universite Laval Quebec City, Canada | Phase II | 177Lu-DOTATATE dosimetry |
| A Clinical Study to Assess the Combination of Two Drugs (177Lu-DOTATATE and Nivolumab) in Neuroendocrine Tumors | Fundación de Investigación HM Madrid, Spain | Phase II | 177Lu-DOTATATE + nivolumab |
| Targeted Alpha-emitter Therapy of PRRT Naive Neuroendocrine Tumor Patients (ALPHAMEDIX02) | Radiomedix Houston, USA | Phase II | 212Pb-DOTAMTATE |
| Treatment Using 177Lu-DOTA-EB-TATE in Patients With Advanced Neuroendocrine Tumors | Peking Union Medical College Hospital Beijing, China | Phase I | 177Lu-DOTA-EB-TATE |
| Chemotherapy Studies | |||
| CapTemY90 for Grade 2 NET Liver Metastases (CapTemY90) | Abramson Cancer Center at Penn Medicine Philadelphia, USA | Phase II | Capecitabine/temozolomide and Y90 radioembolization |
| Miscellaneous Agents | |||
| Study to Evaluate the Safety, PK, and Dose Response of Paltusotine in Subjects With Carcinoid Syndrome | Crinetics Pharmaceuticals San Diego, USA | Phase II | Paltusotine |
| Study of CVM-1118 for Patients With Advanced Neuroendocrine Tumors | TaiRx, Inc. Taipei City, Taiwan | Phase II | CVM-1118 (TRX-818) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulvey, C.K. Emerging Precision Medicine Approaches for Lung Neuroendocrine Tumors. Cancers 2023, 15, 5575. https://doi.org/10.3390/cancers15235575
Mulvey CK. Emerging Precision Medicine Approaches for Lung Neuroendocrine Tumors. Cancers. 2023; 15(23):5575. https://doi.org/10.3390/cancers15235575
Chicago/Turabian StyleMulvey, Claire K. 2023. "Emerging Precision Medicine Approaches for Lung Neuroendocrine Tumors" Cancers 15, no. 23: 5575. https://doi.org/10.3390/cancers15235575





