Gaps and Opportunities in the Diagnosis and Treatment of Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Gaps in the Diagnosis of PDAC
2.1. Unspecific Symptoms
2.2. Inadequacy of Radiological and Endoscopic Diagnosis
2.3. Unsatisfactory Screening
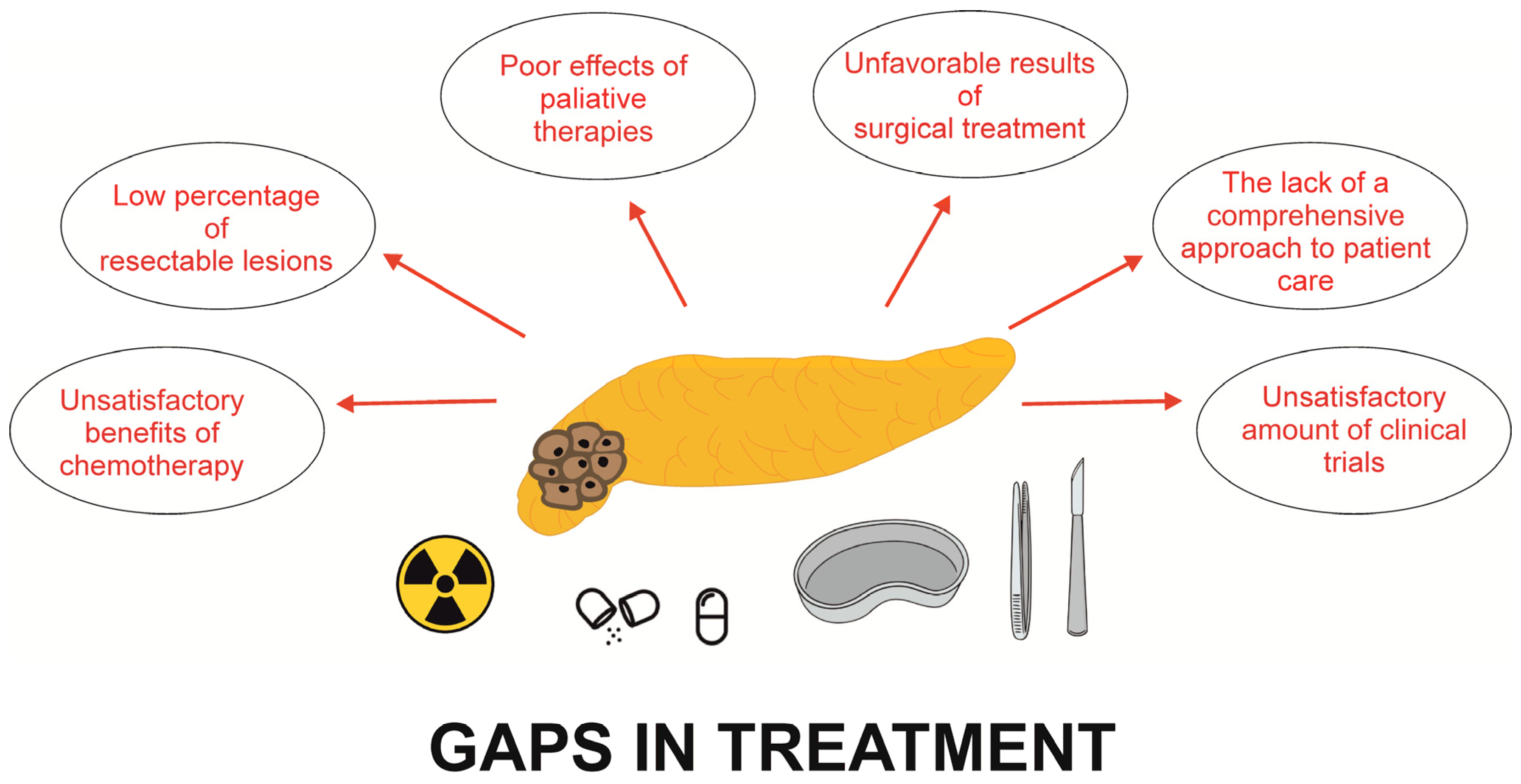
3. Gaps in the Treatment of PDAC
3.1. Gaps in the Surgical Treatment of PDAC
3.2. Gaps in the Chemotherapy of PDAC
3.3. Pancreatic Exocrine Insufficiency
4. Opportunities in the Diagnosis of PC
4.1. Acute Pancreatitis
4.2. Diabetes Mellitus
4.3. New Techniques of Imaging
4.4. Artificial Intelligence
4.5. Circulome in PDAC
5. Opportunities in the Treatment of PDAC
6. Summary
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Okusaka, T.; Furuse, J. Recent advances in chemotherapy for pancreatic cancer: Evidence from Japan and recommendations in guidelines. J. Gastroenterol. 2020, 55, 369–382. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Moutinho-Ribeiro, P.; Iglesias-Garcia, J.; Gaspar, R.; Macedo, G. Early pancreatic cancer—The role of endoscopic ultrasound with or without tissue acquisition in diagnosis and staging. Dig. Liver Dis. 2019, 51, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.M.; Fahlbusch, T.; Munding, J.; Uhl, W.; Braumann, C. The Unusual Suspects of the Pancreas—Understanding Pancreatic Acinar Cell Carcinomas and Adenomas. J. Gastrointest. Cancer 2020, 51, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Małecka-Wojciesko, E. Pancreatic Incidentaloma. J. Clin. Med. 2022, 11, 4648. [Google Scholar] [CrossRef]
- Roth, M.T.; Berlin, J.D. Current Concepts in the Treatment of Resectable Pancreatic Cancer. Curr. Oncol. Rep. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Furuse, J.; Shibahara, J.; Sugiyama, M. Development of chemotherapy and significance of conversion surgery after chemotherapy in unresectable pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2018, 25, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, Y.; Changyong, E.; Sheng, J.; Zhang, X. Screening and Validation of Independent Predictors of Poor Survival in Pancreatic Cancer. Pathol. Oncol. Res. 2021, 27, 1609868. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Futur. Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.M.; Mills, K.; Mendonça, S.C.; Abel, G.A.; Basu, B.; Carroll, N.; Ballard, S.; Lancaster, J.; Hamilton, W.; Rubin, G.P.; et al. Symptoms and patient factors associated with diagnostic intervals for pancreatic cancer (SYMPTOM pancreatic study): A prospective cohort study. Lancet Gastroenterol. Hepatol. 2016, 1, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.N.; Islam, M.M.; Walther, B.A.; Lin, M.C.; Li, Y.C. Proton Pump Inhibitors Use and the Risk of Pancreatic Cancer: Evidence from Eleven Epidemiological Studies, Comprising 1.5 Million Individuals. Cancers 2022, 14, 5357. [Google Scholar] [CrossRef] [PubMed]
- Maguchi, H.; Takashi, K.; Osanai, M.; Katanuma, A. Small pancreatic lesions: Is there need for EUS-FNA preoperatively? What to do with the incidental lesions? Endoscopy 2006, 38, 53–56. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Grassia, R.; Imperatore, N.; Capone, P.; Cereatti, F.; Forti, E.; Antonini, F.; Tanzi, G.P.; Martinotti, M.; Buffoli, F.; Mutignani, M.; et al. EUS-guided tissue acquisition in chronic pancreatitis: Differential diagnosis between pancreatic cancer and pseudotumoral masses using EUS-FNA or core biopsy. Endosc. Ultrasound 2020, 9, 122–129. [Google Scholar] [CrossRef]
- Facciorusso, A.; Singh Bajwa, H.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound 2020, 9, 167–174. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Bunganič, B.; Laclav, M.; Dvořáková, T.; Bradáč, O.; Traboulsi, E.; Suchánek, Š.; Frič, P.; Zavoral, M. Accuracy of EUS and CEH EUS for the diagnosis of pancreatic tumours. Scand. J. Gastroenterol. 2018, 53, 1411–1417. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. 2019, 31, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, N.; Ou, G.; Lam, E.; Enns, R.; Telford, J. When trainees reach competency in performing endoscopic ultrasound: A systematic review. Endosc. Int. Open 2017, 5, E239–E243. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Kwek, A.B.E.; Wang, L.M. Diagnostic endoscopic ultrasound: Technique, current status and future directions. Gut Liver 2018, 12, 483–496. [Google Scholar] [CrossRef]
- Harmsen, F.J.; Domagk, D.; Dietrich, C.F.; Hocke, M. Discriminating chronic pancreatitis from pancreatic cancer: Contrast-enhanced EUS and multidetector computed tomography in direct comparison. Endosc. Ultrasound 2018, 7, 395–403. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Vasen, H.; Ibrahim, I.; Robbers, K.; Van Mil, A.M.; Potjer, T.; Bonsing, B.A.; Bergman, W.; Wasser, M.; Morreau, H.; De Vos Tot Nederveen Cappel, W.H.; et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: Outcome of long-term prospective follow-up studies from three European expert centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Jacob, R.; Manne, U.; Paluri, R. Advances in pancreatic cancer biomarkers. Oncol. Rev. 2019, 13, 69–76. [Google Scholar] [CrossRef]
- Kapszewicz, M.; Małecka-Wojciesko, E. Simple serum pancreatic ductal adenocarcinoma (Pdac) protein biomarkers—Is there anything in sight? J. Clin. Med. 2021, 10, 5463. [Google Scholar] [CrossRef] [PubMed]
- Rychlíková, J.; Vecka, M.; Jáchymová, M.; Macášek, J.; Hrabák, P.; Zeman, M.; Vávrová, L.; Řoupal, J.; Krechler, T.; Ák, A. Osteopontin as a discriminating marker for pancreatic cancer and chronic pancreatitis. Cancer Biomark. 2016, 17, 55–65. [Google Scholar] [CrossRef]
- Aronen, A.; Aittoniemi, J.; Huttunen, R.; Nikkola, A.; Rinta-Kiikka, I.; Nikkola, J.; Limnell, O.; Nordback, I.; Sand, J.; Laukkarinen, J. Plasma suPAR may help to distinguish between chronic pancreatitis and pancreatic cancer. Scand. J. Gastroenterol. 2021, 56, 81–85. [Google Scholar] [CrossRef]
- Martínez-Bosch, N.; Cristóbal, H.; Iglesias, M.; Gironella, M.; Barranco, L.; Visa, L.; Calafato, D.; Jiménez-Parrado, S.; Earl, J.; Carrato, A.; et al. Soluble AXL is a novel blood marker for early detection of pancreatic ductal adenocarcinoma and differential diagnosis from chronic pancreatitis. eBioMedicine 2022, 75, 103797. [Google Scholar] [CrossRef]
- Ghassem-Zadeh, S.; Gaida, M.M.; Szanyi, S.; Acha-Orbea, H.; Frossard, J.L.; Hinz, U.; Hackert, T.; Strobel, O.; Felix, K. Distinct pathophysiological cytokine profiles for discrimination between autoimmune pancreatitis, chronic pancreatitis, and pancreatic ductal adenocarcinoma. J. Transl. Med. 2017, 15, 126. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Halloran, C.M.; Ghaneh, P.; Bosonnet, L.; Hartley, M.N.; Sutton, R.; Neoptolemos, J.P. Complicaions of pancreatic cancer resection. Dig. Surg. 2002, 19, 138–146. [Google Scholar] [CrossRef]
- Robatel, S.; Schenk, M. Current Limitations and Novel Perspectives in Pancreatic Cancer Treatment. Cancers 2022, 14, 985. [Google Scholar] [CrossRef]
- Simon, R. Complications After Pancreaticoduodenectomy. Surg. Clin. N. Am. 2021, 101, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.C.; Van Dongen, J.C.; Van Goor, I.W.J.M.; Smits, F.J.; Nagelhout, A.; Besselink, M.G.; Busch, O.R.; Bonsing, B.A.; Bosscha, K.; Van Dam, R.M.; et al. Impact of complications after resection of pancreatic cancer on disease recurrence and survival, and mediation effect of adjuvant chemotherapy: Nationwide, observational cohort study. BJS Open 2023, 7, zrac174. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Murakawa, M.; Katayama, Y.; Yamaoku, K.; Kanazawa, A.; Higuchi, A.; Shiozawa, M.; Morimoto, M.; Yoshikawa, T.; Yamamoto, N.; et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015, 35, 2401–2409. [Google Scholar] [PubMed]
- Zhang, B.; Xu, Z.; Gu, W.; Zhou, J.; Tang, N.; Zhang, S.; Chen, C.; Zhang, Z. Postoperative complications and short-term prognosis of laparoscopic pancreaticoduodenectomy vs. open pancreaticoduodenectomy for treating pancreatic ductal adenocarcinoma: A retrospective cohort study. World J. Surg. Oncol. 2023, 21, 26. [Google Scholar] [CrossRef]
- Handgraaf, H.J.M.; Boonstra, M.C.; Van Erkel, A.R.; Bonsing, B.A.; Putter, H.; Van De Velde, C.J.H.; Vahrmeijer, A.L.; Mieog, J.S.D. Current and Future Intraoperative Imaging Strategies to Increase Radical Resection Rates in Pancreatic Cancer Surgery. Biomed Res. Int. 2014, 2014, 890230. [Google Scholar] [CrossRef]
- Springfeld, C.; Jäger, D.; Büchler, M.W.; Strobel, O.; Hackert, T.; Palmer, D.H.; Neoptolemos, J.P. Chemotherapy for pancreatic cancer. Press. Medicale 2019, 48, e159–e174. [Google Scholar] [CrossRef]
- Barnes, J.A.; Ellis, M.L.; Hwang, S.; Emarine, J.; Merwin, P.; Salinas, G.D.; Musher, B.L. Identification of Educational Gaps among Oncologists Who Manage Patients with Pancreatic Cancer. J. Gastrointest. Cancer 2019, 50, 84–90. [Google Scholar] [CrossRef]
- Hamad, A.; DePuccio, M.; Reames, B.N.; Dave, A.; Kurien, N.; Cloyd, J.M.; Shen, C.; Pawlik, T.M.; Tsung, A.; McAlearney, A.S.; et al. Disparities in Stage-Specific Guideline-Concordant Cancer-Directed Treatment for Patients with Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2021, 25, 2889–2901. [Google Scholar] [CrossRef]
- Lima, H.A.; Alaimo, L.; Moazzam, Z.; Endo, Y.; Woldesenbet, S.; Katayama, E.; Munir, M.M.; Shaikh, C.; Ruff, S.M.; Dillhoff, M.; et al. Disparities in NCCN Guideline-Compliant Care for Patients with Early-Stage Pancreatic Adenocarcinoma at Minority-Serving versus Non-Minority-Serving Hospitals. Ann. Surg. Oncol. 2023, 30, 4363–4372. [Google Scholar] [CrossRef]
- Lan, X.; Robin, G.; Kasnik, J.; Wong, G.; Abdel-Rahman, O. Challenges in Diagnosis and Treatment of Pancreatic Exocrine Insufficiency among Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 1331. [Google Scholar] [CrossRef]
- de la Iglesia, D.; Avci, B.; Kiriukova, M.; Panic, N.; Bozhychko, M.; Sandru, V.; de-Madaria, E.; Capurso, G. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 1115–1125. [Google Scholar] [CrossRef]
- Roeyen, G.; Jansen, M.; Hartman, V.; Chapelle, T.; Bracke, B.; Ysebaert, D.; De Block, C. The impact of pancreaticoduodenectomy on endocrine and exocrine pancreatic function: A prospective cohort study based on pre- and postoperative function tests. Pancreatology 2017, 17, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.S.J.; Molenaar, I.Q.; Besselink, M.G.; Van Eijck, C.H.; Rinkes, I.H.B.; Van Santvoort, H.C. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer a systematic review. Pancreas 2016, 45, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Budipramana, V.S.; Witarto, A.P.; Witarto, B.S.; Pramudito, S.L.; Ratri, L.C.; Wairooy, N.A.P.; Putra, A.J.E. Risk factors for exocrine pancreatic insufficiency after pancreatic surgery: A systematic review and meta-analysis. Can. J. Surg. 2022, 65, E770–E781. [Google Scholar] [CrossRef]
- Pezzilli, R.; Caccialanza, R.; Capurso, G.; Brunetti, O.; Milella, M.; Falconi, M. Pancreatic enzyme replacement therapy in pancreatic cancer. Cancers 2020, 12, 275. [Google Scholar] [CrossRef]
- Landers, A.; Muircroft, W.; Brown, H. Pancreatic enzyme replacement therapy (PERT) for malabsorption in patients with metastatic pancreatic cancer. BMJ Support. Palliat. Care 2016, 6, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Joo, J.; Kim, S.Y.; Park, S.J.; Han, S.S.; Kim, T.H.; Koh, Y.H.; Chung, S.H.; Kim, Y.H.; Moon, H.; et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 2016, 16, 1099–1105. [Google Scholar] [CrossRef]
- Zdenkowski, N.; Radvan, G.; Pugliese, L.; Charlton, J.; Oldmeadow, C.; Fraser, A.; Bonaventura, A. Treatment of pancreatic insufficiency using pancreatic extract in patients with advanced pancreatic cancer: A pilot study (PICNIC). Support. Care Cancer 2017, 25, 1963–1971. [Google Scholar] [CrossRef]
- Saito, T.; Nakai, Y.; Isayama, H.; Hirano, K.; Ishigaki, K.; Hakuta, R.; Takeda, T.; Saito, K.; Umefune, G.; Akiyama, D.; et al. A Multicenter Open-Label Randomized Controlled Trial of Pancreatic Enzyme Replacement Therapy in Unresectable Pancreatic Cancer. Pancreas 2018, 47, 800–806. [Google Scholar] [CrossRef]
- Wos-Wroniewicz, E.; Caban, M.; Malecka-Panas, E. Role of adipokines in the assessment of severity and predicting the clinical course of acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 605–614. [Google Scholar] [CrossRef]
- Munigala, S.; Almaskeen, S.; Subramaniam, D.S.; Bandi, S.; Bowe, B.; Xian, H.; Sheth, S.G.; Burroughs, T.E.; Agarwal, B. Acute Pancreatitis Recurrences Augment Long-Term Pancreatic Cancer Risk. Am. J. Gastroenterol. 2023, 118, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Cronin-Fenton, D.; Heide-Jørgensen, U.; Mortensen, F.V. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology 2018, 154, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; An, R.; Tian, H.; Zhao, J. Increased risk of pancreatic cancer after acute pancreatitis: A meta-analysis of prospective cohort studies. Clin. Res. Hepatol. Gastroenterol. 2019, 43, e39–e41. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Yu, Y. Meta-analysis reveals an association between acute pancreatitis and the risk of pancreatic cancer. World J. Clin. Cases 2020, 8, 4416–4430. [Google Scholar] [CrossRef] [PubMed]
- Alhobayb, T.; Peravali, R.; Ashkar, M. The Relationship between Acute and Chronic Pancreatitis with Pancreatic Adenocarcinoma: Review. Diseases 2021, 9, 93. [Google Scholar] [CrossRef]
- Kirkegård, J.; Gaber, C.; Lund, J.L.; Hinton, S.P.; Ladekarl, M.; Heide-Jørgensen, U.; Cronin-Fenton, D.; Mortensen, F.V. Acute pancreatitis as an early marker of pancreatic cancer and cancer stage, treatment, and prognosis. Cancer Epidemiol. 2020, 64, 101647. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhao, Y.; Han, X.; Chen, G.; Windsor, J.; Wu, D.; Qian, J. Clinical characteristics and outcome of tumor-associated acute pancreatitis: A single-center cohort study. Ann. Transl. Med. 2021, 9, 639. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Heide-Jørgensen, U.; Cronin-Fenton, D. Predictors of underlying pancreatic cancer in patients with acute pancreatitis: A Danish nationwide cohort study. Hpb 2020, 22, 553–562. [Google Scholar] [CrossRef]
- Dzeletovic, I.; Harrison, M.E.; Crowell, M.D.; Pannala, R.; Nguyen, C.C.; Wu, Q.; Faigel, D.O. Pancreatitis before pancreatic cancer clinical features and influence on outcome. J. Clin. Gastroenterol. 2014, 48, 801–805. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Qian, Y.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Liu, C. Prior history of acute pancreatitis predicts poor survival in patients with resectable pancreatic ductal adenocarcinoma. Pancreatology 2020, 20, 716–721. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Faron, M.; Bachellier, P.; Sauvanet, A.; Beauchet, A.; Le Treut, Y.P.; Adham, M.; Mabrut, J.Y.; Delpero, J.R.; Paye, F. Acute Pancreatitis as the Initial Presentation of Pancreatic Adenocarcinoma does not Impact Short- and Long-term Outcomes of Curative Intent Surgery: A Study of the French Surgical Association. World J. Surg. 2021, 45, 3146–3156. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Klein, A.P.; Sharma, N.R.; O’Reilly, E.M. Does acute pancreatitis herald pancreatic ductal adenocarcinoma? A multicenter electronic health research network study. Cancer Med. 2023, 12, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Wu, K.; Sun, Y.; Zhang, M.; Wang, D.; Wu, J.; Yin, T.; Gong, W.; Ding, Y.; Xiao, W.; et al. Significant increased CA199 levels in acute pancreatitis patients predicts the presence of pancreatic cancer. Oncotarget 2018, 9, 12745–12753. [Google Scholar] [CrossRef]
- Feng, Q.; Li, C.; Zhang, S.; Tan, C.L.; Mai, G.; Liu, X.B.; Chen, Y.H. Recurrence and survival after surgery for pancreatic cancer with or without acute pancreatitis. World J. Gastroenterol. 2019, 25, 6006–6015. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xie, S.M.; Zhang, H.; Tan, C.L.; Ke, N.W.; Mai, G.; Liu, X.B. Clinical impact of preoperative acute pancreatitis in patients who undergo pancreaticoduodenectomy for periampullary tumors. World J. Gastroenterol. 2015, 21, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Tian, W.; Hao, X.; Koirala, R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018, 52, 342–346. [Google Scholar] [CrossRef]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939–4962. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef]
- Batabyal, P.; Vander Hoorn, S.; Christophi, C.; Nikfarjam, M. Association of diabetes mellitus and pancreatic adenocarcinoma: A meta-analysis of 88 studies. Ann. Surg. Oncol. 2014, 21, 2453–2462. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Dankner, R.; Boffetta, P.; Balicer, R.D.; Boker, L.K.; Sadeh, M.; Berlin, A.; Olmer, L.; Goldfracht, M.; Freedman, L.S. Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2.3 million adults. Am. J. Epidemiol. 2016, 183, 1098–1106. [Google Scholar] [CrossRef]
- Aggarwal, G.; Kamada, P.; Chari, S.T. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas 2013, 42, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rodríguez, L.A.G.; Malgerud, L.; González-Pérez, A.; Martín-Pérez, M.; Lagergren, J.; Bexelius, T.S. New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br. J. Cancer 2015, 113, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and Clinical Profile of Pancreatic Cancer-Associated Diabetes Mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Pizzato, M.; Turati, F.; Rosato, V.; La Vecchia, C. Exploring the link between diabetes and pancreatic cancer. Expert Rev. Anticancer Ther. 2019, 19, 681–687. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients with New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer among Patients with New-Onset Diabetes. Gastroenterology 2017, 152, 840–850.e3. [Google Scholar] [CrossRef]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer among Patients with Prediabetes. Eur. J. Gastroenterol. Hepatol. 2022, 34, 33–38. [Google Scholar] [CrossRef]
- Konno, Y.; Sugai, Y.; Kanoto, M.; Suzuki, K.; Hiraka, T.; Toyoguchi, Y.; Niino, K. A retrospective preliminary study of intrapancreatic late enhancement as a noteworthy imaging finding in the early stages of pancreatic adenocarcinoma. Eur. Radiol. 2023, 33, 5131–5141. [Google Scholar] [CrossRef]
- Rogowska, J.O.; Durko, Ł.; Malecka-Wojciesko, E. The Latest Advancements in Diagnostic Role of Endosonography of Pancreatic Lesions. J. Clin. Med. 2023, 12, 4630. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Martina, M.; Buccino, R.V.; Nacchiero, M.C.; Muscatiello, N. Diagnostic accuracy of fine-needle aspiration of solid pancreatic lesions guided by endoscopic ultrasound elastography. Ann. Gastroenterol. 2018, 31, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bournet, B.; Selves, J.; Grand, D.; Danjoux, M.; Hanoun, N.; Cordelier, P.; Buscail, L. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with a KRAS mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. J. Clin. Gastroenterol. 2014, 49, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, P.; Shang, D.; Liu, Y. Spatial-domain low-coherence quantitative phase microscopy to improve the cytological diagnosis of pancreatic cancer. J. Investig. Med. 2020, 68, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.S.; Al-Haddad, M.; Chandan, S.; Gangwani, M.K.; Aziz, M.; Mohan, B.P.; Ramai, D.; Canakis, A.; Bapaye, J.; Sharma, N. Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Cancer: Where Are We Now and What Does the Future Entail? J. Clin. Med. 2022, 11, 7476. [Google Scholar] [CrossRef] [PubMed]
- Katta, M.R.; Kalluru, P.K.R.; Bavishi, D.A.; Hameed, M.; Valisekka, S.S. Artificial intelligence in pancreatic cancer: Diagnosis, limitations, and the future prospects—A narrative review. J. Cancer Res. Clin. Oncol. 2023, 149, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Park, S.; Kawamoto, S.; Fouladi, D.F.; Shayesteh, S.; Zinreich, E.S.; Graves, J.S.; Horton, K.M.; Hruban, R.H.; Yuille, A.L.; et al. Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. Am. J. Roentgenol. 2019, 213, 349–357. [Google Scholar] [CrossRef]
- Fu, M.; Wu, W.; Hong, X.; Liu, Q.; Jiang, J.; Ou, Y.; Zhao, Y.; Gong, X. Hierarchical combinatorial deep learning architecture for pancreas segmentation of medical computed tomography cancer images. BMC Syst. Biol. 2018, 12, 56–127. [Google Scholar] [CrossRef]
- Liu, S.L.; Li, S.; Guo, Y.T.; Zhou, Y.P.; Zhang, Z.D.; Li, S.; Lu, Y. Establishment and application of an artificial intelligence diagnosis system for pancreatic cancer with a faster region-based convolutional neural network. Chin. Med. J. 2019, 132, 2795–2803. [Google Scholar] [CrossRef]
- Young, M.R.; Abrams, N.; Ghosh, S.; Rinaudo, J.A.S.; Marquez, G.; Srivastava, S. Prediagnostic Image Data, Artificial Intelligence, and Pancreatic Cancer: A Tell-Tale Sign to Early Detection. Pancreas 2020, 49, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Santus, E.; Schuster, T.; Tahmasebi, A.M.; Li, C.; Yala, A.; Lanahan, C.R.; Prinsen, P.; Thompson, S.F.; Coons, S.; Mynderse, L.; et al. Exploiting Rules to Enhance Machine Learning in Extracting Information From Multi-Institutional Prostate Pathology Reports. JCO Clin. Cancer Inform. 2020, 4, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Kurita, Y.; Koda, H.; Toriyama, K.; Onishi, S.; et al. Usefulness of deep learning analysis for the diagnosis of malignancy in intraductal papillary mucinous neoplasms of the pancreas. Clin. Transl. Gastroenterol. 2019, 10, e00045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Yang, H.; Jin, Z.D.; Yu, J.G.; Cai, Z.Y.; Li, Z.S. Differential diagnosis of pancreatic cancer from normal tissue with digital imaging processing and pattern recognition based on a support vector machine of EUS images. Gastrointest. Endosc. 2010, 72, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Sherazi, S.A.A.; Gupta, S.; Perisetti, A.; Achebe, I.; Ali, A.; Tharian, B.; Thosani, N.; Sharma, N.R. Application of artificial intelligence in diagnosis of pancreatic malignancies by endoscopic ultrasound: A systemic review. Therap. Adv. Gastroenterol. 2022, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nguyen, C.C.; Li, F.; Li, B. Digital image analysis of EUS images accurately differentiates pancreatic cancer from chronic pancreatitis and normal tissue. Gastrointest. Endosc. 2008, 67, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Sǎftoiu, A.; Vilmann, P.; Gorunescu, F.; Gheonea, D.I.; Gorunescu, M.; Ciurea, T.; Popescu, G.L.; Iordache, A.; Hassan, H.; Iordache, S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest. Endosc. 2008, 68, 1086–1094. [Google Scholar] [CrossRef]
- Marya, N.B.; Powers, P.D.; Chari, S.T.; Gleeson, F.C.; Leggett, C.L.; Abu Dayyeh, B.K.; Chandrasekhara, V.; Iyer, P.G.; Majumder, S.; Pearson, R.K.; et al. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut 2021, 70, 1335–1344. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.T.; Xie, K.P. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol. Cancer 2021, 20, 34. [Google Scholar] [CrossRef]
- Uesato, Y.; Sasahira, N.; Ozaka, M.; Sasaki, T.; Takatsuki, M.; Zembutsu, H. Evaluation of circulating tumor DNA as a biomarker in pancreatic cancer with liver metastasis. PLoS ONE 2020, 15, e0235623. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Woo, S.M.; Park, B.; Yoon, K.A.; Kim, Y.H.; Joo, J.; Lee, W.J.; Han, S.S.; Park, S.J.; Kong, S.Y. Prognostic implications of multiplex detection of KRAS mutations in cell-Free DNA from patients with pancreatic ductal adenocarcinoma. Clin. Chem. 2018, 64, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Sausen, M.; Phallen, J.; Adleff, V.; Jones, S.; Leary, R.J.; Barrett, M.T.; Anagnostou, V.; Parpart-Li, S.; Murphy, D.; Li, Q.K.; et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015, 6, 7686. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Zhang, C.Y.; Song, B.B.; Wang, B.L.; Pan, B.S.; Lou, W.H.; Guo, W. Co-amplification at lower denaturation-temperature PCR combined with unlabled-probe high-resolution melting to detect KRAS codon 12 and 13 mutations in plasma-circulating DNA of pancreatic adenocarcinoma cases. Asian Pacific J. Cancer Prev. 2014, 15, 10647–10652. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef]
- Kinugasa, H.; Nouso, K.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Tsutsumi, K.; Kato, H.; Matsubara, T.; Okada, H.; Yamamoto, K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015, 121, 2271–2280. [Google Scholar] [CrossRef]
- Van Laethem, J.L.; Riess, H.; Jassem, J.; Haas, M.; Martens, U.M.; Weekes, C.; Peeters, M.; Ross, P.; Bridgewater, J.; Melichar, B.; et al. Phase I/II Study of Refametinib (BAY 86-9766) in Combination with Gemcitabine in Advanced Pancreatic cancer. Target. Oncol. 2017, 12, 97–109. [Google Scholar] [CrossRef]
- Shen, S.Y.; Singhania, R.; Fehringer, G.; Chakravarthy, A.; Roehrl, M.H.A.; Chadwick, D.; Zuzarte, P.C.; Borgida, A.; Wang, T.T.; Li, T.; et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018, 563, 579–583. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Yildirim, H.C.; Aktepe, O.H.; Dizdar, O.; Yalcin, S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit. Rev. Oncol. Hematol. 2021, 168, 103528. [Google Scholar] [CrossRef]
- Sivapalan, L.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. Molecular profiling of ctDNA in pancreatic cancer: Opportunities and challenges for clinical application. Pancreatology 2021, 21, 363–378. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. MicroRNA targeted therapeutic approach for pancreatic cancer. Int. J. Biol. Sci. 2016, 12, 326–337. [Google Scholar] [CrossRef]
- Eun, J.L.; Gusev, Y.; Jiang, J.; Nuovo, G.J.; Lerner, M.R.; Frankel, W.L.; Morgan, D.L.; Postier, R.G.; Brackett, D.J.; Schmittgen, T.D. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer 2007, 120, 1046–1054. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, H.; Fisher, W.E.; Lin, P.H.; Yao, Q.; Chen, C. Profiling of 95 MicroRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J. Surg. 2009, 33, 698–709. [Google Scholar] [CrossRef]
- Hong, T.H.; Park, I.Y. MicroRNA expression profiling of diagnostic needle aspirates from surgical pancreatic cancer specimens. Ann. Surg. Treat. Res. 2014, 87, 290–297. [Google Scholar] [CrossRef]
- Habbe, N.; Koorstra, J.B.M.; Mendell, J.T.; Offerhaus, G.J.; Ji, K.R.; Feldmann, G.; Mullendore, M.E.; Goggins, M.G.; Hong, S.M.; Maitra, A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol. Ther. 2009, 8, 340–346. [Google Scholar] [CrossRef]
- Yu, J.; Ohuchida, K.; Mizumoto, K.; Sato, N.; Kayashima, T.; Fujita, H.; Nakata, K.; Tanaka, M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol. Cancer 2010, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vandenboom, T.G.; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009, 69, 6704–6712. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massagué, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, X.; Qin, F.; Yang, J.; Ai, L.; Wang, Q.; Zhai, Y. The clinical significance of circulating tumor cells and T lymphocyte subtypes in pancreatic cancer patients. Bioengineered 2022, 13, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first & second-line Abiraterone & Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- DiPardo, B.J.; Winograd, P.; Court, C.M.; Tomlinson, J.S. Pancreatic cancer circulating tumor cells: Applications for personalized oncology. Expert Rev. Mol. Diagn. 2018, 18, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Dubash, T.D.; Edd, J.F.; Jewett, M.K.; Garre, S.G.; Karabacak, N.M.; Rabe, D.C.; Mutlu, B.R.; Walsh, J.R.; Kapur, R.; et al. Ultrahigh-throughput magnetic sorting of large blood volumes for epitope-agnostic isolation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2020, 117, 16839–16847. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, M.L.; Sclabas, G.M. Major venous resection during total laparoscopic pancreaticoduodenectomy. Hpb 2011, 13, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; Reid-Lombardo, K.M.; Truty, M.J.; Nagorney, D.M.; Kendrick, M.L. Pancreaticoduodenectomy with Major Vascular Resection: A Comparison of Laparoscopic Versus Open Approaches. J. Gastrointest. Surg. 2015, 19, 189–194. [Google Scholar] [CrossRef]
- Wang, M.; Cai, H.; Meng, L.; Cai, Y.; Wang, X.; Li, Y.; Peng, B. Minimally invasive pancreaticoduodenectomy: A comprehensive review. Int. J. Surg. 2016, 35, 139–146. [Google Scholar] [CrossRef]
- Poves, I.; Burdío, F.; Morató, O.; Iglesias, M.; Radosevic, A.; Ilzarbe, L.; Visa, L.; Grande, L. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: The Padulap randomized controlled trial. Ann. Surg. 2018, 268, 731–739. [Google Scholar] [CrossRef]
- Song, K.B.; Kim, S.C.; Hwang, D.W.; Lee, J.H.; Lee, D.J.; Lee, J.W.; Park, K.M.; Lee, Y.J. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann. Surg. 2015, 262, 146–155. [Google Scholar] [CrossRef]
- Stauffer, J.A.; Coppola, A.; Villacreses, D.; Mody, K.; Johnson, E.; Li, Z.; Asbun, H.J. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: Long-term results at a single institution. Surg. Endosc. 2017, 31, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zhang, R.C.; Zhou, Y.C. Comparison of overall survival and perioperative outcomes of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. BMC Cancer 2019, 19, 781. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jian, Z.; Hou, B.; Jin, H. Surgical and Oncological Outcomes of Laparoscopic Versus Open Pancreaticoduodenectomy in Patients with Pancreatic Duct Adenocarcinoma. Pancreas 2019, 48, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Zureikat, A.H.; Postlewait, L.M.; Liu, Y.; Gillespie, T.W.; Weber, S.M.; Abbott, D.E.; Ahmad, S.A.; Maithel, S.K.; Hogg, M.E.; Zenati, M.; et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann. Surg. 2016, 264, 640–649. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Liu, X.L.; Jiang, G.; Wu, D.Y.; Maher, H.; Cai, X.J. Minimally invasive pancreaticoduodenectomy for periampullary disease: A comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Troncone, E.; Fugazza, A.; Cappello, A.; Del Vecchio Blanco, G.; Monteleone, G.; Repici, A. Lumen-apposing metal stents for malignant biliary obstruction: Is this the ultimate horizon of our experience? World J. Gastroenterol. 2019, 25, 3857–3869. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Fabbri, C.; Di Mitri, R.; Petrone, M.C.; Colombo, M.; Cugia, L.; Amato, A.; Forti, E.; Binda, C.; Maida, M.; et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after failed ERCP: A retrospective nationwide analysis. Gastrointest. Endosc. 2022, 95, 896–904. [Google Scholar] [CrossRef]
- Waldthaler, A.; Rutkowski, W.; Valente, R.; Arnelo, U.; Löhr, J.-M. Palliative therapy in pancreatic cancer-interventional treatment with stents. Transl. Gastroenterol. Hepatol. 2019, 4, 7. [Google Scholar] [CrossRef]
- Hofmann, F.O.; Miksch, R.C.; Weniger, M.; Keck, T.; Anthuber, M.; Witzigmann, H.; Nuessler, N.C.; Reissfelder, C.; Köninger, J.; Ghadimi, M.; et al. Outcomes and risks in palliative pancreatic surgery: An analysis of the German StuDoQ|Pancreas registry. BMC Surg. 2022, 22, 389. [Google Scholar] [CrossRef]
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible electroporation: A new ablation modality-Clinical implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Leor, J.; Rubinsky, B. The effect of irreversible electroporation on blood vessels. Technol. Cancer Res. Treat. 2007, 6, 307–312. [Google Scholar] [CrossRef]
- Martin, R.C.G. Irreversible electroporation of stage 3 locally advanced pancreatic cancer: Optimal technique and outcomes. J. Vis. Surg. 2015, 1, 4. [Google Scholar] [CrossRef]
- Martin, R.C.G.; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Carrato, A.; Vieitez, J.M.; Benavides, M.; Rodriguez-Garrote, M.; Castillo, A.; Ogalla, G.D.; Bermejo, L.G.; Ruiz de Mena, I.; Guillén-Ponce, C.; Aranda, E.; et al. Phase I/II trial of sequential treatment of nab-paclitaxel in combination with gemcitabine followed by modified FOLFOX chemotherapy in patients with untreated metastatic exocrine pancreatic cancer: Phase I results. Eur. J. Cancer 2020, 139, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA -Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Garrido-Laguna, I.; Liu, S.V.; Multani, P.S.; Chow-Maneval, E.; Rolfo, C. Entrectinib in TRK and ROS1 Fusion-Positive Metastatic Pancreatic Cancer. JCO Precis. Oncol. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2019 from the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Alqahtani, A.; Noel, M.S. The Next Frontier in Pancreatic Cancer: Targeting the Tumor Immune Milieu and Molecular Pathways. Cancers 2022, 14, 2619. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Casolino, R.; Paiella, S.; Azzolina, D.; Beer, P.A.; Corbo, V.; Lorenzoni, G.; Gregori, D.; Golan, T.; Braconi, C.; Froeling, F.E.; et al. Homologous Recombination Deficiency in Pancreatic Cancer: A Systematic Review and Prevalence Meta-Analysis. J. Clin. Oncol. 2021, 39, 2617–2632. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, B.; Puccini, A.; Catalano, F.; Borea, R.; Iaia, M.L.; Bruno, W.; Fornarini, G.; Sciallero, S.; Rebuzzi, S.E.; Ghiorzo, P. Beyond BRCA: The Emerging Significance of DNA Damage Response and Personalized Treatment in Pancreatic and Prostate Cancer Patients. Int. J. Mol. Sci. 2022, 23, 4709. [Google Scholar] [CrossRef]
- Leidner, R.; Sanjuan Silva, N.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.-P.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Selvanesan, B.C.; Chandra, D.; Quispe-Tintaya, W.; Jahangir, A.; Patel, A.; Meena, K.; Da Silva, R.A.A.; Friedman, M.; Gabor, L.; Khouri, O.; et al. Listeria delivers tetanus toxoid protein to pancreatic tumors and induces cancer cell death in mice. Sci. Transl. Med. 2022, 14, eabc1600. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair—Deficient Cancer: Results from the Phase II KEYNOTE-158 Study abstract. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Dorman, K.; Heinemann, V.; Kobold, S.; Bergwelt-Baildon, M.; Boeck, S. Novel systemic treatment approaches for metastatic pancreatic cancer. Expert Opin. Investig. Drugs 2022, 31, 249–262. [Google Scholar] [CrossRef]
- Van Tienhoven, G.; Versteijne, E.; Suker, M.; Groothuis, K.B.C.; Busch, O.R.; Bonsing, B.A.; de Hingh, I.H.J.T.; Festen, S.; Patijn, G.A.; de Vos-Geelen, J.; et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A ran-domized, controlled, multicenter phase III trial. J. Clin. Oncol. 2018, 36 (Suppl. 18), LBA4002. [Google Scholar] [CrossRef]
- Taylor, R.J.; Todor, D.; Kaplan, B.J.; Stover, W.; Fields, E.C. CivaSheet intraoperative radiation therapy for pancreatic cancer. Brachytherapy 2022, 21, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; De Pastena, M.; Romeo, F.; D’onofrio, M.; Fontana, M.; Pea, A.; De Marchi, G.; Crinò, S.F.; Bassi, C.; Salvia, R. Ablation treatments in unresectable pancreatic cancer. Minerva Chir. 2019, 74, 263–269. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Zhang, Y.; Cai, Z.; Lin, X.; Li, S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med. 2020, 9, 4699–4710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhou, L.; Lu, J.; Jiang, B.; Liu, C.; Guo, J. Photodynamic therapy of pancreatic cancer: Where have we come from and where are we going? Photodiagnosis Photodyn. Ther. 2020, 31, 101876. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Mukhopadhyay, M.; Shivam, K.; Tripathy, S.; Patra, R.; Pramanik, A. Recent developments in photodynamic therapy and its application against multidrug resistant cancers. Biomed. Mater. 2023, 18, 062005. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.K.; Johnston, M.C.; Scott, C.J. Nanomedicine in Pancreatic Cancer: Current Status and Future Opportunities for Overcoming Therapy Resistance. Cancers 2021, 13, 6175. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.N.L.; Herremans, K.M.; Riner, A.N.; Vudatha, V.; Freudenberger, D.C.; Hughes, S.J.; Triplett, E.W.; Trevino, J.G. Translocation of Oral Microbiota into the Pancreatic Ductal Adenocarcinoma Tumor Microenvironment. Microorganisms 2023, 11, 1466. [Google Scholar] [CrossRef]
- Kabwe, M.; Dashper, S.; Tucci, J. The Microbiome in Pancreatic Cancer-Implications for Diagnosis and Precision Bacteriophage Therapy for This Low Survival Disease. Front. Cell. Infect. Microbiol. 2022, 12, 871293. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Dong, S.; Xu, H.; Zhou, W. Association of the Microbiota and Pancreatic Cancer: Opportunities and Limitations. Front. Immunol. 2022, 13, 844401. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.X. Recurrent antibiotic exposure may promote cancer formation-Another step in understanding the role of the human microbiota? Eur. J. Cancer 2015, 51, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Pisati, F.; Ulaszewska, M.; Andolfo, A.; Villani, A.; Federici, F.; Laura, M.; Rizzi, E.; Potenza, A.; Latiano, T.P.; et al. Tuning gut microbiota through a probiotic blend in gemcitabine-treated pancreatic cancer xenografted mice. Clin. Transl. Med. 2021, 11, e580. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Hamidi-Sofiani, V.; Rakhshi, R.; Moradi, N.; Zeynali, P.; Nakhaie, M.; Behboudi, E. Oncolytic viruses and pancreatic cancer. Cancer Treat. Res. Commun. 2022, 31, 100563. [Google Scholar] [CrossRef]

| Genetic Syndrome | Risk of PC throughout Life |
|---|---|
| FAMMM | to 17% |
| Peutz-Jeghers syndrome | to 36% |
| Lynch syndrome | to 5% |
| Cystic fibrosis | 5% |
| Hereditary pancreatitis | 30–40% |
| BRCA1/BRCA2 mutation | various |
| Genetic Syndrome/Gene Mutation | Criteria of PDAC Family History |
|---|---|
| Peutz-Jeghers syndrome (LKB1/STK11) | Regardless of family history |
| FAMMM (CDKN2A p16) | Regardless of family history |
| Lynch syndrome (MLH1/MSH2/MSH6) | If at least one affected first-degree relative |
| BRCA1 | If at least one affected first-degree relative |
| BRCA2 | If at least one affected first-degree relative or at least two affected relatives of any degree |
| PALB2 | If at least one affected first-degree relative |
| ATM | If at least one affected first-degree relative |
| Strategy | Challenges |
|---|---|
| Awareness | Poor awareness of the early signs and symptoms of PDAC amongst mainly older people and clinicians; Limited awareness of the burden of PDAC on the population with risk factor among clinicians; Poor awareness of the risk factor of PDAC among general population; |
| Detection and diagnosis | The lack of screening with proven efficacy; Limited capacities and use of comprehensive assessment to help shape of diagnosis pathways for patients with PDAC; Limited efficacy of available diagnostic methods for diagnosis of PDAC; The biology of PDAC hard to understand; |
| Treatment and care | Limited access to quality care in some regions of the world; Poor national development/integration of guidelines for the assessment and treatment of patients with PDAC; Limited capacities to manage patients with PDAC, especially these with multiple morbidities; Poor therapeutic response; Delays in time to approval of life-saving treatment; Insufficient development of targeted therapy; |
| Health system integration | Limited integration of training in oncology within core medical and nursing training; Global shortage of specialists to support a multidisciplinary treatment approach; Limited financial and other protection measures for patients with PDAC; Limited availability of oncology clinics; Variable integration of services within national health system and cancer planning; Differences in healthcare and financial outlays in various countries; |
| Research | Insufficient amount of clinical trials in the context of new therapeutic options with high efficacy in PDAC; Poor integration of patients with PDAC into clinical trials, which reduces their relevance; Limited translational research into the needs of patients with PDAC; Limited data on the investment-case; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caban, M.; Małecka-Wojciesko, E. Gaps and Opportunities in the Diagnosis and Treatment of Pancreatic Cancer. Cancers 2023, 15, 5577. https://doi.org/10.3390/cancers15235577
Caban M, Małecka-Wojciesko E. Gaps and Opportunities in the Diagnosis and Treatment of Pancreatic Cancer. Cancers. 2023; 15(23):5577. https://doi.org/10.3390/cancers15235577
Chicago/Turabian StyleCaban, Miłosz, and Ewa Małecka-Wojciesko. 2023. "Gaps and Opportunities in the Diagnosis and Treatment of Pancreatic Cancer" Cancers 15, no. 23: 5577. https://doi.org/10.3390/cancers15235577
APA StyleCaban, M., & Małecka-Wojciesko, E. (2023). Gaps and Opportunities in the Diagnosis and Treatment of Pancreatic Cancer. Cancers, 15(23), 5577. https://doi.org/10.3390/cancers15235577






