Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immune-Mediated Toxicity as a Cause of Death
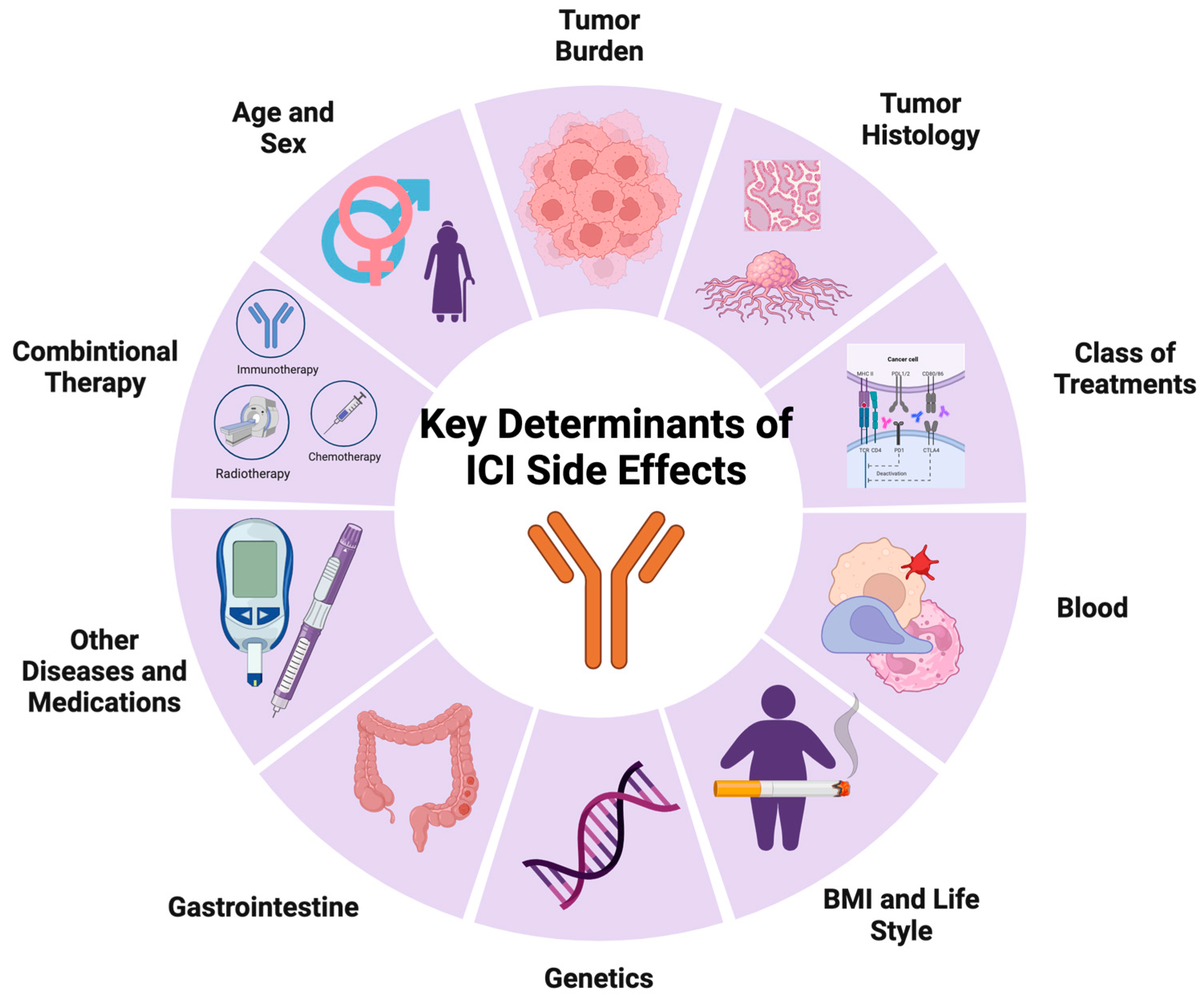
3. Key Determinants of imADRs in ICI Treatment
3.1. Treatment-Related
3.1.1. Class of ICI Treatment
3.1.2. Combined Anti-Cancer Treatments
3.2. Tumor-Related
3.2.1. Tumor Histology
3.2.2. Tumor Burden
3.3. Patient-Related
3.3.1. Demographics
3.3.2. Physiological Parameters and Lifestyle
3.3.3. Genetic Predisposition
3.3.4. Blood Characteristics
3.3.5. Gastrointestinal Characteristics
3.3.6. Other Diseases and Medications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F. Conventional Cancer Therapy: Promise Broken or Promise Delayed? Lancet 1998, 351, SII9–SII16. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Huang, P.-W.; Chang, J.W.-C. Immune Checkpoint Inhibitors Win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency ICH Topic. E2A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. 1995. Available online: https://www.ema.europa.eu/en/ich-e2a-clinical-safety-data-management-definitions-standards-expedited-reporting-scientific (accessed on 24 November 2023).
- Pande, S. Causality or Relatedness Assessment in Adverse Drug Reaction and Its Relevance in Dermatology. Indian J. Dermatol. 2018, 63, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Prasad, V. A Reality Check of the Accelerated Approval of Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.; Naidoo, J. Immune-Related Adverse Events and the Balancing Act of Immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Thienen, H.V.; Blank, C.U. Toxicity Patterns with Immunomodulating Antibodies and Their Combinations. Semin. Oncol. 2015, 42, 423–428. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Gao, W. PD-L1 Is Expressed by Human Renal Tubular Epithelial Cells and Suppresses T Cell Cytokine Synthesis. Clin. Immunol. 2005, 115, 184–191. [Google Scholar] [CrossRef]
- Schoop, R.; Wahl, P.; Le Hir, M.; Heemann, U.; Wang, M.; Wüthrich, R.P. Suppressed T-Cell Activation by IFN-γ-Induced Expression of PD-L1 on Renal Tubular Epithelial Cells. Nephrol. Dial. Transplant. 2004, 19, 2713–2720. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Vanderlugt, C.L.; Miller, S.D. Epitope Spreading in Immune-Mediated Diseases: Implications for Immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- Bang, A.; Wilhite, T.J.; Pike, L.R.G.; Cagney, D.N.; Aizer, A.A.; Taylor, A.; Spektor, A.; Krishnan, M.; Ott, P.A.; Balboni, T.A.; et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounaut, V.; Moro-Sibilot, D.; Michot, J.-M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-Checkpoint Inhibitors Associated with Interstitial Lung Disease in Cancer Patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Mulekar, M.S.; Escobar, D.E.; Alsayed, A.; Sharma, G.; Prodduturvar, P.; Khushman, M.; Howard, J.H.; Gilbert, R.; Alkharabsheh, O. Clinical and Hematological Predictors of High-Grade Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. J. Clin. Med. Res. 2021, 13, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Kawamura, K.; Ichikado, K.; Shingu, N.; Yasuda, Y.; Eguchi, Y.; Anan, K.; Hisanaga, J.; Nitawaki, T.; Iio, M.; et al. The Association between Tumor Burden and Severe Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Responding to Immune-Checkpoint Inhibitor Treatment. Lung Cancer 2019, 130, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Mikami, T.; Niimura, T.; Zamami, Y.; Uesawa, Y.; Chuma, M.; Ishizawa, K. The Risk Factors Associated with Immune Checkpoint Inhibitor-Related Pneumonitis. Oncology 2021, 99, 256–259. [Google Scholar] [CrossRef]
- Betof, A.S.; Nipp, R.D.; Giobbie-Hurder, A.; Johnpulle, R.A.N.; Rubin, K.; Rubinstein, S.M.; Flaherty, K.T.; Lawrence, D.P.; Johnson, D.B.; Sullivan, R.J. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. Oncologist 2017, 22, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Lal, J.C.; Brown, S.-A.; Collier, P.; Cheng, F. A Retrospective Analysis of Cardiovascular Adverse Events Associated with Immune Checkpoint Inhibitors. Cardio-Oncology 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Kim, I.Y.; Sun, J.-M.; Lee, J.; Cha, H.-S.; Koh, E.-M.; Kim, H.; Lee, J. Risk Factors for Immune-Related Adverse Events Associated with Anti-PD-1 Pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Matsuoka, R.; Sakurada, T.; Goda, M.; Chuma, M.; Yagi, K.; Zamami, Y.; Nishioka, Y.; Ishizawa, K. Risk Factors of Immune Checkpoint Inhibitor-Related Interstitial Lung Disease in Patients with Lung Cancer: A Single-Institution Retrospective Study. Sci. Rep. 2020, 10, 13773. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.H.; Berner, F.; Bomze, D.; Fässler, M.; Diem, S.; Cozzio, A.; Jörger, M.; Früh, M.; Driessen, C.; Lenz, T.L.; et al. Human Leukocyte Antigen Variation Is Associated with Adverse Events of Checkpoint Inhibitors. Eur. J. Cancer 2019, 107, 8–14. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Diab, A.; Yu, R.K.; Futreal, A.; Criswell, L.A.; Tayar, J.H.; Dadu, R.; Shannon, V.; Shete, S.S.; Suarez-Almazor, M.E. Genetic Determinants of Immune-Related Adverse Events in Patients with Melanoma Receiving Immune Checkpoint Inhibitors. Cancer Immunol. Immunother. 2021, 70, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of Blood Biomarkers and Autoimmunity with Immune Related Adverse Events in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef]
- Tan, B.; Chen, M.; Guo, Q.; Tang, H.; Li, Y.; Jia, X.; Xu, Y.; Zhu, L.; Wang, M.; Qian, J. Clinical-Radiological Characteristics and Intestinal Microbiota in Patients with Pancreatic Immune-Related Adverse Events. Thorac. Cancer 2021, 12, 1814–1823. [Google Scholar] [CrossRef]
- Cui, P.; Liu, Z.; Wang, G.; Ma, J.; Qian, Y.; Zhang, F.; Han, C.; Long, Y.; Li, Y.; Zheng, X.; et al. Risk Factors for Pneumonitis in Patients Treated with Anti-Programmed Death-1 Therapy: A Case-Control Study. Cancer Med. 2018, 7, 4115–4120. [Google Scholar] [CrossRef]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of Pneumonitis with Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the Toxicity Profile of PD-1 versus PD-L1 Inhibitors in Non–Small Cell Lung Cancer: A Systematic Analysis of the Literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Waterhouse, P.; Penninger, J.M.; Timms, E.; Wakeham, A.; Shahinian, A.; Lee, K.P.; Thompson, C.B.; Griesser, H.; Mak, T.W. Lymphoproliferative Disorders with Early Lethality in Mice Deficient in Ctla-4. Science 1995, 270, 985–988. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-Mediated Thyroid Dysfunction during T-Cell Checkpoint Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Corbière, V.; Chapiro, J.; Stroobant, V.; Ma, W.; Lurquin, C.; Lethé, B.; van Baren, N.; Van den Eynde, B.J.; Boon, T.; Coulie, P.G. Antigen Spreading Contributes to MAGE Vaccination-Induced Regression of Melanoma Metastases. Cancer Res. 2011, 71, 1253–1262. [Google Scholar] [CrossRef]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Antitumor Activity in Solid Malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Horita, N.; Namkoong, H.; Galsky, M.D. The Effect of Adding Immune Checkpoint Inhibitors on the Risk of Pneumonitis for Solid Tumours: A Meta-Analysis of Phase III Randomised Controlled Trials. Eur. J. Cancer 2021, 150, 168–178. [Google Scholar] [CrossRef]
- Vergnenegre, A.; Monnet, I.; Ricordel, C.; Bizieux, A.; Curcio, H.; Bernardi, M.; Corre, R.; Guisier, F.; Hominal, S.; Le Garff, G.; et al. Safety and Efficacy of Second-Line Metronomic Oral Vinorelbine-Atezolizumab Combination in Stage IV Non-Small-Cell Lung Cancer: An Open-Label Phase II Trial (VinMetAtezo). Lung Cancer 2023, 178, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; De Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; Brandi, G.; Garuti, F.; Barbera, M.A.; Tortora, R.; Casadei Gardini, A.; Granito, A.; Tovoli, F.; De Lorenzo, S.; Inghilesi, A.L.; et al. Metronomic Capecitabine as Second-Line Treatment for Hepatocellular Carcinoma after Sorafenib Discontinuation. J. Cancer Res. Clin. Oncol. 2018, 144, 403–414. [Google Scholar] [CrossRef]
- Yang, Y.; Psutka, S.P.; Parikh, A.B.; Li, M.; Collier, K.; Miah, A.; Mori, S.V.; Hinkley, M.; Tykodi, S.S.; Hall, E.; et al. Combining Immune Checkpoint Inhibition plus Tyrosine Kinase Inhibition as First and Subsequent Treatments for Metastatic Renal Cell Carcinoma. Cancer Med. 2022, 11, 3106–3114. [Google Scholar] [CrossRef]
- Tian, T.; Yu, M.; Li, J.; Jiang, M.; Ma, D.; Tang, S.; Lin, Z.; Chen, L.; Gong, Y.; Zhu, J.; et al. Front-Line ICI-Based Combination Therapy Post-TKI Resistance May Improve Survival in NSCLC Patients With EGFR Mutation. Front. Oncol. 2021, 11, 739090. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhan, M.; Li, X.-Y.; Zhang, H.; Dauphars, D.J.; Jiang, J.; Yin, H.; Li, S.-Y.; Luo, S.; Li, Y.; et al. Clinically Approved Combination Immunotherapy: Current Status, Limitations, and Future Perspective. Curr. Res. Immunol. 2022, 3, 118–127. [Google Scholar] [CrossRef]
- Geisberger, R.; Lamers, M.; Achatz, G. The Riddle of the Dual Expression of IgM and IgD. Immunology 2006, 118, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bomze, D.; Hasan Ali, O.; Bate, A.; Flatz, L. Association Between Immune-Related Adverse Events During Anti–PD-1 Therapy and Tumor Mutational Burden. JAMA Oncol. 2019, 5, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Nebhan, C.A.; Johnson, D.B. Impact of Patient Age on Clinical Efficacy and Toxicity of Checkpoint Inhibitor Therapy. Front. Immunol. 2021, 12, 786046. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and Interleukin-6 Are Prognostic Factors for Autoimmune Toxicity Following Treatment with Anti-CTLA4 Blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Novelli, L.; Galdiero, M.R.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; Gerli, R. Immune Checkpoint Inhibitors-Induced Autoimmunity: The Impact of Gender. Autoimmun. Rev. 2020, 19, 102590. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Daly, L.E.; Power, D.G.; O’Reilly, Á.; Donnellan, P.; Cushen, S.J.; O’Sullivan, K.; Twomey, M.; Woodlock, D.P.; Redmond, H.P.; Ryan, A.M. The Impact of Body Composition Parameters on Ipilimumab Toxicity and Survival in Patients with Metastatic Melanoma. Br. J. Cancer 2017, 116, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Mosaad, Y.M. Clinical Role of Human Leukocyte Antigen in Health and Disease. Scand. J. Immunol. 2015, 82, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Ashida, K.; Sakamoto, R.; Sakaguchi, C.; Ogata, M.; Maruyama, K.; Sakamoto, S.; Ikeda, M.; Ohe, K.; Akasu, S.; et al. Human Leucocyte Antigen DR15, a Possible Predictive Marker for Immune Checkpoint Inhibitor–Induced Secondary Adrenal Insufficiency. Eur. J. Cancer 2020, 130, 198–203. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Dorak, M.T.; Bettinotti, M.P.; Bingham, C.O., III; Shah, A.A. Association of HLA-DRB1 Shared Epitope Alleles and Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. Rheumatology 2019, 58, 476–480. [Google Scholar] [CrossRef]
- Inaba, H.; Kaido, Y.; Ito, S.; Hirobata, T.; Inoue, G.; Sugita, T.; Yamamoto, Y.; Jinnin, M.; Kimura, H.; Kobayashi, T.; et al. Human Leukocyte Antigens and Biomarkers in Type 1 Diabetes Mellitus Induced by Immune-Checkpoint Inhibitors. Endocrinol. Metab. 2022, 37, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a Regulates Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef]
- Tyan, K.; Baginska, J.; Brainard, M.; Giobbie-Hurder, A.; Severgnini, M.; Manos, M.; Haq, R.; Buchbinder, E.; Ott, P.; Hodi, F.S.; et al. 648 Cytokine Changes during Immune-Related Adverse Events and Steroid Treatment in Melanoma Patients Receiving Immune Checkpoint Inhibitors. J. Immunother. Cancer 2020, 8, A389. [Google Scholar] [CrossRef]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling Preexisting Antibodies in Patients Treated with Anti–PD-1 Therapy for Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Jiang, N.; Song, X.; Xu, J.; Zhu, X.; Chen, C.; Kong, C.; Wang, X.; Zong, D.; et al. Association of Blood Biochemical Indexes and Antibiotic Exposure with Severe Immune-Related Adverse Events in Patients With Advanced Cancers Receiving PD-1 Inhibitors. J. Immunother. 2022, 45, 210–216. [Google Scholar] [CrossRef]
- Shimozaki, K.; Sukawa, Y.; Sato, Y.; Horie, S.; Chida, A.; Tsugaru, K.; Togasaki, K.; Kawasaki, K.; Hirata, K.; Hayashi, H.; et al. Analysis of Risk Factors for Immune-Related Adverse Events in Various Solid Tumors Using Real-World Data. Future Oncol. 2021, 17, 2593–2603. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Luo, W.; Qiao, W.; Raju, G.S.; Wang, Y. Importance of Endoscopic and Histological Evaluation in the Management of Immune Checkpoint Inhibitor-Induced Colitis. J. Immunother. Cancer 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Gainor, J.F.; Altan, M.; Kravets, S.; Dahlberg, S.E.; Gedmintas, L.; Azimi, R.; Rizvi, H.; Riess, J.W.; Hellmann, M.D.; et al. Safety of Programmed Death–1 Pathway Inhibitors Among Patients with Non–Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J Clin. Oncol. 2018, 36, 1905–1912. [Google Scholar] [CrossRef]
- Johns, A.; Wei, L.; Grogan, M.; Patel, S.; Li, M.; Husain, M.; Kendra, K.L.; Otterson, G.A.; Burkart, J.T.; Owen, D.H.; et al. Association of Medical Comorbidities and Cardiovascular Disease with Toxicity and Survival in Patients Receiving Checkpoint Inhibitor Immunotherapy. JCO 2020, 38, 7039. [Google Scholar] [CrossRef]
- Xie, E.; Yeo, Y.H.; Scheiner, B.; Zhang, Y.; Hiraoka, A.; Tantai, X.; Fessas, P.; De Castro, T.; D’Alessio, A.; Fulgenzi, C.A.M.; et al. Immune Checkpoint Inhibitors for Child-Pugh Class B Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. JAMA Oncol. 2023, 9, 1423. [Google Scholar] [CrossRef]
- Suzman, D.L.; Pelosof, L.; Rosenberg, A.; Avigan, M.I. Hepatotoxicity of Immune Checkpoint Inhibitors: An Evolving Picture of Risk Associated with a Vital Class of Immunotherapy Agents. Liver. Int. 2018, 38, 976–987. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Falcão, D.; Cardoso, C.; Pires, A.L.; Araújo, A.; Castro-Poças, F. Hepatic Immune-Mediatedadverseeffects of Immune Checkpoint Inhibitors: Analysis of Real-Life Experience. Ann. Hepatol. 2021, 26, 100561. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, C.; Zhao, Y.; Li, D.; Guo, M.; Cui, X. Hepatic Failure Associated with Immune Checkpoint Inhibitors: An Analysis of the Food and Drug Administration Adverse Event Reporting System Database. Cancer Med. 2023, 12, 9167–9174. [Google Scholar] [CrossRef]
- Meraz-Muñoz, A.; Amir, E.; Ng, P.; Avila-Casado, C.; Ragobar, C.; Chan, C.; Kim, J.; Wald, R.; Kitchlu, A. Acute Kidney Injury Associated with Immune Checkpoint Inhibitor Therapy: Incidence, Risk Factors and Outcomes. J. Immunother. Cancer 2020, 8, e000467. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chen, X.; Li, K.; Liu, Y.; Zhang, Z.; Chen, Y.; Liu, Y.; Wang, Y.; Lin, S.H.; Diao, L.; et al. Association of Antibiotic Treatment with Immune-Related Adverse Events in Patients with Cancer Receiving Immunotherapy. J. Immunother. Cancer 2022, 10, e003779. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Balmelli, C.; Kaufmann, L.; Stanczak, M.; Syedbasha, M.; Vogt, D.; Hertig, A.; Müller, B.; Gautschi, O.; Stenner, F.; et al. Influenza Vaccination of Cancer Patients during PD-1 Blockade Induces Serological Protection but May Raise the Risk for Immune-Related Adverse Events. J. Immunother. Cancer 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of Acute Interstitial Nephritis with Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Mascaro, F.; Figini, S.; Basile, S.; Gambini, G.; Klersy, C.; Lenti, M.V.; Di Sabatino, A.; Di Benedetto, A.; Calvi, M.; et al. Impact of Proton Pump Inhibitors on the Onset of Gastrointestinal Immune-related Adverse Events during Immunotherapy. Cancer Med. 2023, 12, 19530–19536. [Google Scholar] [CrossRef]
- Husain, M.; Xu, M.; Patel, S.; Johns, A.; Grogan, M.; Li, M.; Lopez, G.; Miah, A.; Hoyd, R.; Liu, Y.; et al. Proton Pump Inhibitor Use (PPI) in Patients Treated with Immune Checkpoint Inhibitors (ICI) for Advanced Cancer: Survival and Prior Therapy. JCO 2021, 39, 2633. [Google Scholar] [CrossRef]
- Dankers, W.; Davelaar, N.; van Hamburg, J.P.; van de Peppel, J.; Colin, E.M.; Lubberts, E. Human Memory Th17 Cell Populations Change Into Anti-Inflammatory Cells With Regulatory Capacity Upon Exposure to Active Vitamin D. Front. Immunol. 2019, 10, 1504. [Google Scholar] [CrossRef]
- Joshi, S.; Pantalena, L.-C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-Dihydroxyvitamin D3 Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
| Key Determinants | Clinical Incident | |
|---|---|---|
| Treatment | Antibody target for ICI | imADR risk: anti-CTLA-4 > anti-PD-1 or anti-PD-L1 Risks of hypophysitis [8], renal imADR [9,10] and hypothyroidism [11]: higher in anti-PD-L1 |
| Combined anti-cancer treatments | Higher imADR incident in combining ipilimumab and nivolumab [12], preceding immunotherapy [13], radiotherapy [11] and chemotherapy [14] | |
| Tumor | Histology | Anti-PD-1 treatment causes dermatological symptoms in melanoma but pneumonitis in NSCLC [11] Anti-PD-1 induce earlier onset of lung diseases in lung cancer [15] |
| Burden | Higher tumor load and metastatic sites associated with severe side effects of anti-CTLA-4 or anti-PD-1 [16,17] | |
| Patient | Demographics | ICI-related pneumonitis: younger > older [18] Arthritis after PD-1/PD-L1 inhibition: older > younger [19] Risk of ipilimumab-mediated toxicity: female > male [20] |
| Physiological Parameter and Lifestyle | Pembrolizumab: obesity > healthy [21] Lung disease in lung cancer: ≥50 pack-year elevated the incidence [22] | |
| Genetic predisposition | Pruritus and colitis: higher risk in certain HLA genotype [23] SNP variants increase risk of ICI-related toxicity [24] | |
| Blood and gastrointestinal | Higher lymphocyte, monocyte, platelet, ratio immune cells associate with ICI-related toxicity [25] Ipilimumab-related colitis: higher risk with higher Firmicutes [26] | |
| Other diseases and medications | Higher risks associated with previous lung disease [27], cardiovascular conditions [28] or autoimmune history [25] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ding, S. Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs. Cancers 2023, 15, 5622. https://doi.org/10.3390/cancers15235622
Zhou Y, Ding S. Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs. Cancers. 2023; 15(23):5622. https://doi.org/10.3390/cancers15235622
Chicago/Turabian StyleZhou, Yihan, and Shan Ding. 2023. "Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs" Cancers 15, no. 23: 5622. https://doi.org/10.3390/cancers15235622
APA StyleZhou, Y., & Ding, S. (2023). Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs. Cancers, 15(23), 5622. https://doi.org/10.3390/cancers15235622






