Computational Modeling of Thermal Ablation Zones in the Liver: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
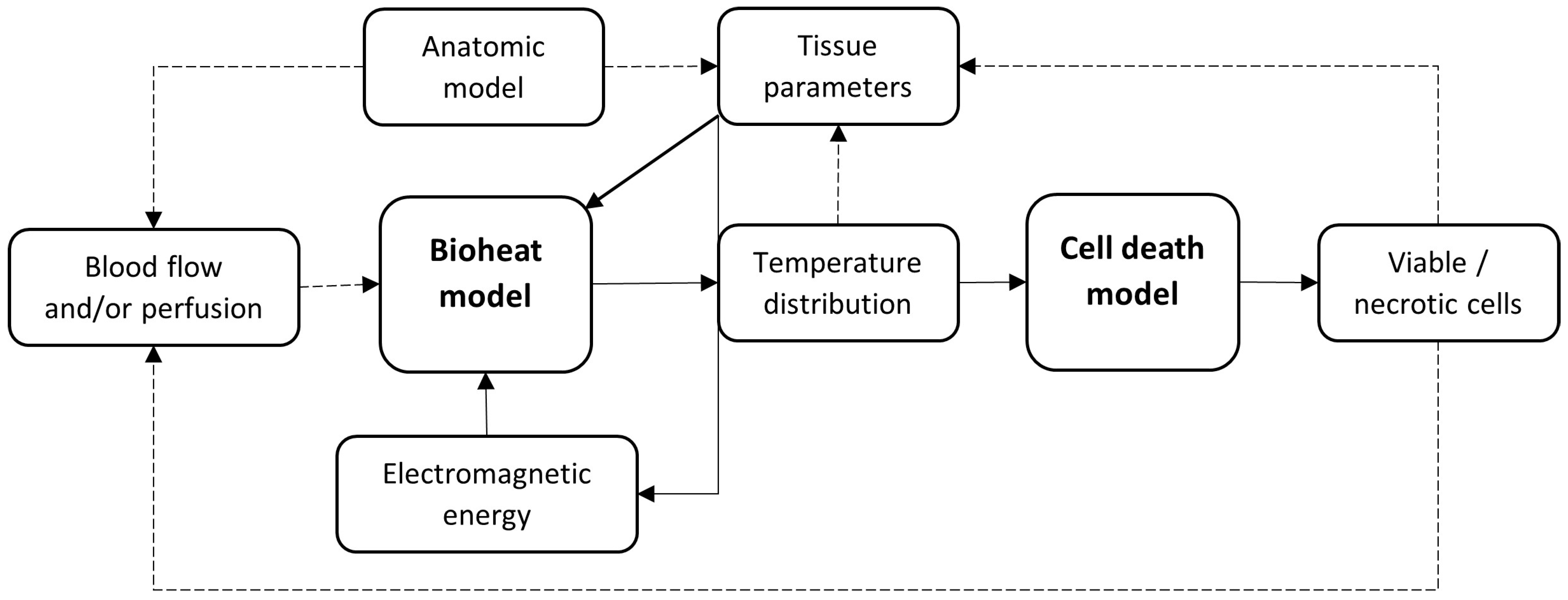
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
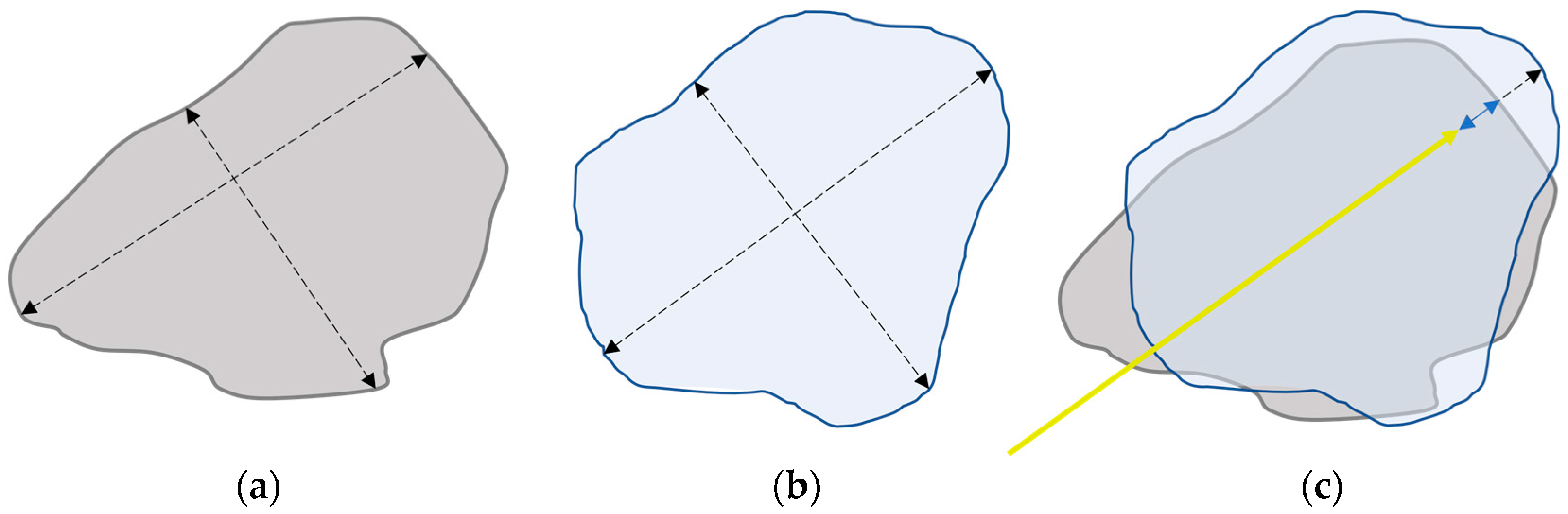
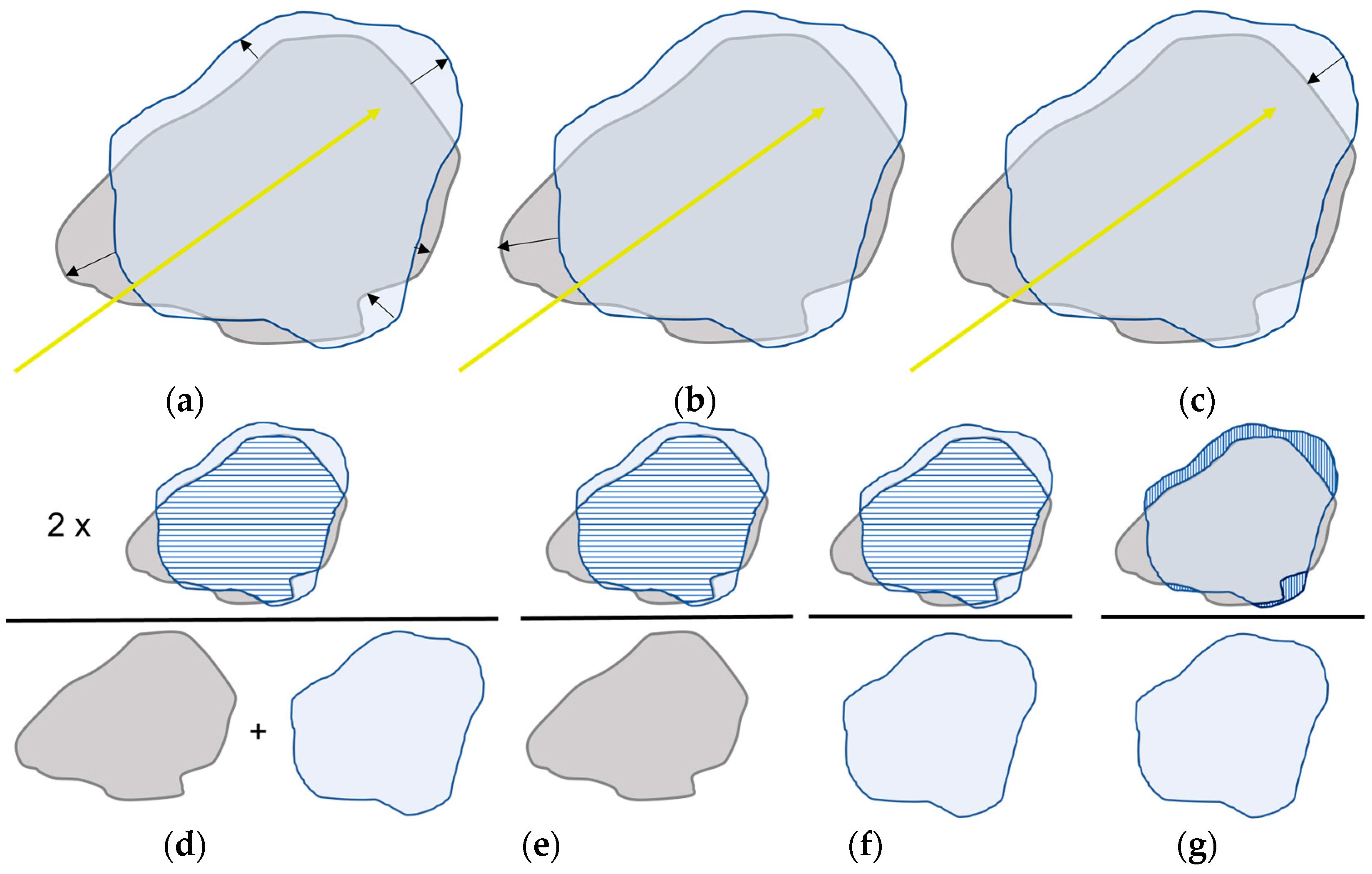
2.3. Data Extraction
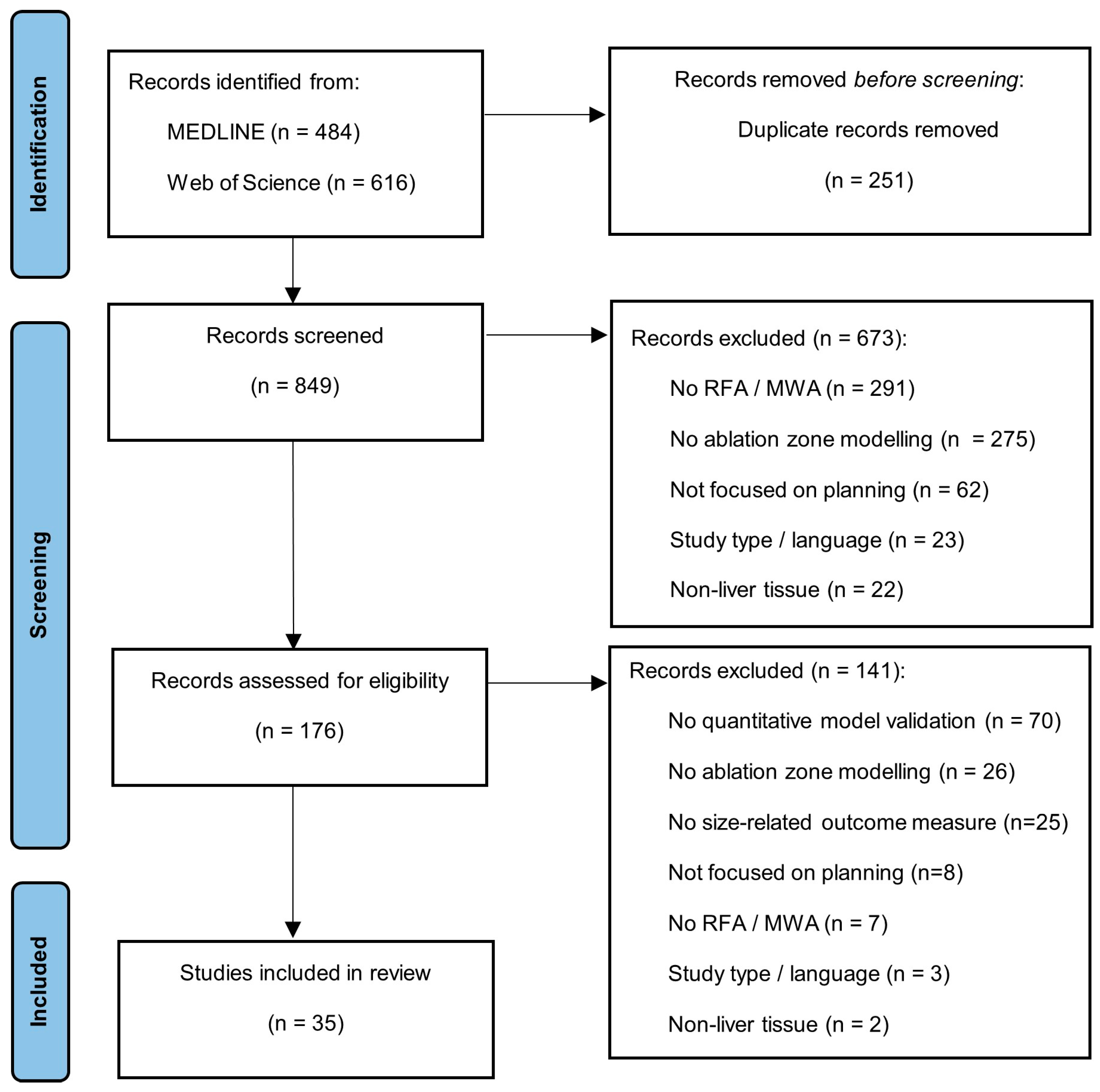
3. Results
3.1. Study Selection
3.2. MWA Data Analysis
3.2.1. MWA Ex Vivo Validation
3.2.2. MWA In Vivo Validation
3.3. RFA Data Analysis
3.3.1. RFA Ex Vivo Validation
3.3.2. RFA In Vivo Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B
| Parameter | Unit | Description | Temperature-Dependent? |
|---|---|---|---|
| Tissue temperature | |||
| Time | |||
| Tissue density | |||
| Specific heat | Yes | ||
| Thermal conductivity | Yes | ||
| Blood perfusion rate | Yes | ||
| Temperature of blood entering the tissue | |||
| Blood volume fraction | |||
| Blood flow velocity | |||
| Interfacial heat transfer coefficient | |||
| Volumetric heat transfer area between tissue and blood | |||
| Coefficient (0 or 1) depending on the thermal damage function |
| Parameter | Unit | Description | Temperature-Dependent? |
|---|---|---|---|
| Electric potential | |||
| Electrical field strength | |||
| Electrical conductivity | Yes | ||
| Angular frequency of the electromagnetic wave | |||
| Permeability | |||
| Complex permittivity | |||
| Relative dielectric constant of biological tissue | Yes | ||
| Relative dielectric constant of vacuum | |||
| Free space wave number |
| Parameter | Unit | Description |
|---|---|---|
| Blood flow velocity | ||
| permeability | ||
| Blood viscosity | ||
| Blood volume fraction | ||
| Blood density | ||
| Pressure | ||
| Heat transfer coefficient | ||
| Local Nusselt number | ||
| W/mK | Blood vessel thermal conductivity | |
| M | Blood vessel diameter |
Appendix C
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Crocetti, L.; de Baére, T.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc. Interv. Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, W.J.; Rhim, H.; Lim, H.K.; Choi, D.; Lee, J.Y. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am. J. Roentgenol. 2010, 195, 758–765. [Google Scholar] [CrossRef]
- Laimer, G.; Schullian, P.; Jaschke, N.; Putzer, D.; Eberle, G.; Alzaga, A.; Odisio, B.; Bale, R. Minimal ablative margin (MAM) assessment with image fusion: An independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur. Radiol. 2020, 30, 2463–2472. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.P.; Petre, E.N.; Gonen, M.; Do, K.G.; Brown, K.T.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc. Interv. Radiol. 2013, 36, 166–175. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yan, G.X.; Xin, H.; Liu, Z.Y. Oncological outcomes and predictors of radiofrequency ablation of colorectal cancer liver metastases. World J. Gastrointest. Oncol. 2020, 12, 1044–1055. [Google Scholar] [CrossRef]
- Faber, R.A.; Burghout, K.S.T.; Bijlstra, O.D.; Hendriks, P.; van Erp, G.C.M.; Broersen, A.; Dijkstra, J.; Vahrmeijer, A.L.; Burgmans, M.C.; Mieog, J.S.D. Three-dimensional quantitative margin assessment in patients with colorectal liver metastases treated with percutaneous thermal ablation using semi-automatic rigid MRI/CECT-CECT co-registration. Eur. J. Radiol. 2022, 156, 110552. [Google Scholar] [CrossRef]
- Hendriks, P.; van Dijk, K.M.; Boekestijn, B.; Broersen, A.; van Duijn-de Vreugd, J.J.; Coenraad, M.J.; Tushuizen, M.E.; van Erkel, A.R.; van der Meer, R.W.; van Rijswijk, C.S.; et al. Intraprocedural assessment of ablation margins using computed tomography co-registration in hepatocellular carcinoma treatment with percutaneous ablation: IAMCOMPLETE study. Diagn. Interv. Imaging 2023. [Google Scholar] [CrossRef]
- Lyons, G.R.; Pua, B.B. Ablation Planning Software for Optimizing Treatment: Challenges, Techniques, and Applications. Tech. Vasc. Interv. Radiol. 2019, 22, 21–25. [Google Scholar] [CrossRef]
- Ruiter, S.J.S.; Heerink, W.J.; de Jong, K.P. Liver microwave ablation: A systematic review of various FDA-approved systems. Eur. Radiol. 2019, 29, 4026–4035. [Google Scholar] [CrossRef]
- Huang, H.W. Influence of blood vessel on the thermal lesion formation during radiofrequency ablation for liver tumors. Med. Phys. 2013, 40, 073303. [Google Scholar] [CrossRef]
- Ahmed, M.; Liu, Z.; Humphries, S.; Goldberg, S.N. Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation. Int. J. Hyperth. 2008, 24, 577–588. [Google Scholar] [CrossRef]
- Liu, Z.; Ahmed, M.; Gervais, D.; Humphries, S.; Goldberg, S.N. Computer modeling of factors that affect the minimum safety distance required for radiofrequency ablation near adjacent nontarget structures. J. Vasc. Interv. Radiol. 2008, 19, 1079–1086. [Google Scholar] [CrossRef]
- Siriwardana, P.N.; Singh, S.; Johnston, E.W.; Watkins, J.; Bandula, S.; Illing, R.O.; Davidson, B.R. Effect of Hepatic Perfusion on Microwave Ablation Zones in an Ex Vivo Porcine Liver Model. J. Vasc. Interv. Radiol. 2017, 28, 732–739. [Google Scholar] [CrossRef]
- Chiang, J.; Wang, P.; Brace, C.L. Computational modelling of microwave tumour ablations. Int. J. Hyperth. 2013, 29, 308–317. [Google Scholar] [CrossRef]
- Singh, S.; Melnik, R. Thermal ablation of biological tissues in disease treatment: A review of computational models and future directions. Electromagn. Biol. Med. 2020, 39, 49–88. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1998, 85, 5–34. [Google Scholar] [CrossRef]
- Trujillo, M.; Bon, J.; Berjano, E. Computational modelling of internally cooled wet (ICW) electrodes for radiofrequency ablation: Impact of rehydration, thermal convection and electrical conductivity. Int. J. Hyperth. 2017, 33, 624–634. [Google Scholar] [CrossRef]
- Haemmerich, D.; Staelin, S.T.; Tungjitkusolmun, S.; Lee, F.T., Jr.; Mahvi, D.M.; Webster, J.G. Hepatic bipolar radio-frequency ablation between separated multiprong electrodes. IEEE Trans. Biomed. Eng. 2001, 48, 1145–1152. [Google Scholar] [CrossRef]
- Antunes, C.L.; Almeida, T.R.O.; Raposeiro, N. Saline-enhanced RF ablation on a cholangiocarcinoma: A numerical simulation. COMPEL J. Comput. Math. Electr. Electron. Eng. 2012, 31, 1055–1066. [Google Scholar] [CrossRef]
- Audigier, C.; Mansi, T.; Delingette, H.; Rapaka, S.; Mihalef, V.; Sharma, P.; Carnegie, D.; Boctor, E.; Choti, M.; Kamen, A.; et al. Lattice Boltzmann method for fast patient-specific simulation of liver tumor ablation from CT images. Med. Image Comput. Comput. Assist. Interv. 2013, 16, 323–330. [Google Scholar] [CrossRef]
- Audigier, C.; Mansi, T.; Delingette, H.; Rapaka, S.; Mihalef, V.; Carnegie, D.; Boctor, E.; Choti, M.; Kamen, A.; Ayache, N.; et al. Efficient Lattice Boltzmann Solver for Patient-Specific Radiofrequency Ablation of Hepatic Tumors. IEEE Trans. Med. Imaging 2015, 34, 1576–1589. [Google Scholar] [CrossRef]
- Audigier, C.; Mansi, T.; Delingette, H.; Rapaka, S.; Passerini, T.; Mihalef, V.; Jolly, M.P.; Pop, R.; Diana, M.; Soler, L.; et al. Comprehensive preclinical evaluation of a multi-physics model of liver tumor radiofrequency ablation. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1543–1559. [Google Scholar] [CrossRef]
- Audigier, C.; Mohaiu, A.T.; Alzaga, A.; Bale, R.; Mansi, T. A comparative study on computational models of multi-electrode radiofrequency ablation of large liver tumors. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1489–1496. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef]
- Chang, I.A.; Nguyen, U.D. Thermal modeling of lesion growth with radiofrequency ablation devices. Biomed. Eng. Online 2004, 3, 27. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Kong, D.; Lou, Q.; Lu, F. Fast calculation of 3D radiofrequency ablation zone based on a closed-form solution of heat conduction equation fitted by ex vivo measurements. Phys. Med. Biol. 2021, 66, 055022. [Google Scholar] [CrossRef]
- Collins, J.A.; Heiselman, J.S.; Clements, L.W.; Weis, J.A.; Brown, D.B.; Miga, M.I. Toward Image Data-Driven Predictive Modeling for Guiding Thermal Ablative Therapy. IEEE Trans. Biomed. Eng. 2020, 67, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Deshazer, G.; Hagmann, M.; Merck, D.; Sebek, J.; Moore, K.B.; Prakash, P. Computational modeling of 915 MHz microwave ablation: Comparative assessment of temperature-dependent tissue dielectric models. Med. Phys. 2017, 44, 4859–4868. [Google Scholar] [CrossRef] [PubMed]
- Deshazer, G.; Prakash, P.; Merck, D.; Haemmerich, D. Experimental measurement of microwave ablation heating pattern and comparison to computer simulations. Int. J. Hyperth. 2017, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wen, R.; Fu, Y.; Chua, K.J.; Chui, C.K. Probabilistic finite element method for large tumor radiofrequency ablation simulation and planning. Med. Eng. Phys. 2016, 38, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Faridi, P.; Keselman, P.; Fallahi, H.; Prakash, P. Experimental assessment of microwave ablation computational modeling with MR thermometry. Med. Phys. 2020, 47, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wei, H.; Zhang, H.; Moser, M.A.J.; Zhang, W.; Qian, Z.; Zhang, B. Radiofrequency ablation for liver tumors abutting complex blood vessel structures: Treatment protocol optimization using response surface method and computer modeling. Int. J. Hyperth. 2022, 39, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.J.; Wu, S.C.; Wang, X.R.; Hu, R.; Zhou, Z.H.; Sun, X.C. Temperature simulation of microwave ablation based on improved specific absorption rate method compared to phantom measurements. Comput. Assist. Surg. 2017, 22, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Wu, S.; Zhou, Z.; Bai, Y.; Wu, W. Conformal coverage of liver tumors by the thermal coagulation zone in 2450-MHz microwave ablation. Int. J. Hyperth. 2019, 36, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.J.; Wang, X.R.; Wu, S.C.; Zhou, Z.H.; Bai, Y.P. 2450-MHz microwave ablation temperature simulation using temperature-dependence feedback of characteristic parameters. Int. J. RF Microw. Comput.-Aided Eng. 2019, 29, e21488. [Google Scholar] [CrossRef]
- Lopresto, V.; Pinto, R.; Farina, L.; Cavagnaro, M. Microwave thermal ablation: Effects of tissue properties variations on predictive models for treatment planning. Med. Eng. Phys. 2017, 46, 63–70. [Google Scholar] [CrossRef]
- Hoffer, E.K.; Borsic, A.; Patel, S.D. Validation of Software for Patient-Specific Real-Time Simulation of Hepatic Radiofrequency Ablation. Acad. Radiol. 2022, 29, e219–e227. [Google Scholar] [CrossRef]
- Mariappan, P.; Weir, P.; Flanagan, R.; Voglreiter, P.; Alhonnoro, T.; Pollari, M.; Moche, M.; Busse, H.; Futterer, J.; Portugaller, H.R.; et al. GPU-based RFA simulation for minimally invasive cancer treatment of liver tumours. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 59–68. [Google Scholar] [CrossRef]
- Moche, M.; Busse, H.; Futterer, J.J.; Hinestrosa, C.A.; Seider, D.; Brandmaier, P.; Kolesnik, M.; Jenniskens, S.; Blanco Sequeiros, R.; Komar, G.; et al. Clinical evaluation of in silico planning and real-time simulation of hepatic radiofrequency ablation (ClinicIMPPACT Trial). Eur. Radiol. 2020, 30, 934–942. [Google Scholar] [CrossRef]
- Ooi, E.H.; Lee, K.W.; Yap, S.; Khattab, M.A.; Liao, I.Y.; Ooi, E.T.; Foo, J.J.; Nair, S.R.; Mohd Ali, A.F. The effects of electrical and thermal boundary condition on the simulation of radiofrequency ablation of liver cancer for tumours located near to the liver boundary. Comput. Biol. Med. 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Payne, S.; Flanagan, R.; Pollari, M.; Alhonnoro, T.; Bost, C.; O’Neill, D.; Peng, T.; Stiegler, P. Image-based multi-scale modelling and validation of radio-frequency ablation in liver tumours. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4233–4254. [Google Scholar] [CrossRef][Green Version]
- Singh, S.; Melnik, R. Coupled thermo-electro-mechanical models for thermal ablation of biological tissues and heat relaxation time effects. Phys. Med. Biol. 2019, 64, 245008. [Google Scholar] [CrossRef]
- Subramanian, S.; Mast, T.D. Optimization of tissue physical parameters for accurate temperature estimation from finite-element simulation of radiofrequency ablation. Phys. Med. Biol. 2015, 60, N345–N355. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Soltani, M.; Kashkooli, F.M.; Raahemifar, K. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes-A computational approach. PLoS ONE 2020, 15, e0233219. [Google Scholar] [CrossRef]
- Tucci, C.; Trujillo, M.; Berjano, E.; Iasiello, M.; Andreozzi, A.; Vanoli, G.P. Pennes’ bioheat equation vs. porous media approach in computer modeling of radiofrequency tumor ablation. Sci. Rep. 2021, 11, 5272. [Google Scholar] [CrossRef]
- Tucci, C.; Trujillo, M.; Berjano, E.; Iasiello, M.; Andreozzi, A.; Vanoli, G.P. Mathematical modeling of microwave liver ablation with a variable-porosity medium approach. Comput. Methods Programs Biomed. 2022, 214, 106569. [Google Scholar] [CrossRef]
- Vaidya, N.; Baragona, M.; Lavezzo, V.; Maessen, R.; Veroy, K. Simulation study of the cooling effect of blood vessels and blood coagulation in hepatic radio-frequency ablation. Int. J. Hyperth. 2021, 38, 95–104. [Google Scholar] [CrossRef]
- Voglreiter, P.; Mariappan, P.; Pollari, M.; Flanagan, R.; Blanco Sequeiros, R.; Portugaller, R.H.; Fütterer, J.; Schmalstieg, D.; Kolesnik, M.; Moche, M. RFA Guardian: Comprehensive Simulation of Radiofrequency Ablation Treatment of Liver Tumors. Sci. Rep. 2018, 8, 787. [Google Scholar] [CrossRef]
- Wang, X.R.; Gao, H.J.; Wu, S.C.; Jiang, T.; Zhou, Z.H.; Bai, Y.P. Numerical evaluation of ablation zone under different tip temperatures during radiofrequency ablation. Math. Biosci. Eng. 2019, 16, 2514–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Wu, Z.; Gao, H.; Huang, S. Influences of blood flow parameters on temperature distribution during liver tumor microwave ablation. Front. Biosci. (Landmark Ed.) 2021, 26, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Huang, S.Y.; Gao, H.J.; Liu, J.; Zhang, Y.B.; Wu, S.C. Computer Simulations of Dual-Antenna Microwave Ablation and Comparison to Experimental Measurements. Appl. Sci. 2022, 13, 26. [Google Scholar] [CrossRef]
- Welp, C.; Siebers, S.; Ermert, H.; Werner, J. Investigation of the influence of blood flow rate on large vessel cooling in hepatic radiofrequency ablation. Biomed. Tech. 2006, 51, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.N.; Li, P.; Shao, Q.; Hong, J.; Yang, L.; Wu, S.C. A simulation-experiment method to characterize the heat transfer in ex-vivo porcine hepatic tissue with a realistic microwave ablation system. Numer. Heat Transf. Part A-Appl. 2013, 64, 729–743. [Google Scholar] [CrossRef]
- Zhai, W.; Xu, J.; Zhao, Y.; Song, Y.; Sheng, L.; Jia, P. Preoperative surgery planning for percutaneous hepatic microwave ablation. Med. Image Comput. Comput. Assist. Interv. 2008, 11, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Z.; Dong, L.; Zhang, G.; Wang, Y.; Liang, P. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: Results in ex vivo porcine livers. Eur. J. Radiol. 2012, 81, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Amabile, C.; Ahmed, M.; Solbiati, L.; Meloni, M.F.; Solbiati, M.; Cassarino, S.; Tosoratti, N.; Nissenbaum, Y.; Ierace, T.; Goldberg, S.N. Microwave ablation of primary and secondary liver tumours: Ex vivo, in vivo, and clinical characterisation. Int. J. Hyperth. 2017, 33, 34–42. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Stein, M.C.; Gazelle, G.S.; Sheiman, R.G.; Kruskal, J.B.; Clouse, M.E. Percutaneous radiofrequency tissue ablation: Optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J. Vasc. Interv. Radiol. 1999, 10, 907–916. [Google Scholar] [CrossRef]
- Pop, M.; Molckovsky, A.; Chin, L.; Kolios, M.C.; Jewett, M.A.; Sherar, M.D. Changes in dielectric properties at 460 kHz of kidney and fat during heating: Importance for radio-frequency thermal therapy. Phys. Med. Biol. 2003, 48, 2509–2525. [Google Scholar] [CrossRef]
- Prakash, P. Theoretical modeling for hepatic microwave ablation. Open Biomed. Eng. J. 2010, 4, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Brace, C.L. Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys. Med. Biol. 2011, 56, 5249–5264. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T., Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21, S192–S203. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ahmed, M.; Weinstein, Y.; Yi, M.; Mahajan, R.L.; Goldberg, S.N. Characterization of the RF ablation-induced ‘oven effect’: The importance of background tissue thermal conductivity on tissue heating. Int. J. Hyperth. 2006, 22, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Servin, F.; Collins, J.A.; Heiselman, J.S.; Frederick-Dyer, K.C.; Planz, V.B.; Geevarghese, S.K.; Brown, D.B.; Miga, M.I. Fat Quantification Imaging and Biophysical Modeling for Patient-Specific Forecasting of Microwave Ablation Therapy. Front. Physiol. 2021, 12, 820251. [Google Scholar] [CrossRef]
- Deshazer, G.; Merck, D.; Hagmann, M.; Dupuy, D.E.; Prakash, P. Physical modeling of microwave ablation zone clinical margin variance. Med. Phys. 2016, 43, 1764. [Google Scholar] [CrossRef]
- Hendriks, P.; Boel, F.; Oosterveer, T.T.; Broersen, A.; de Geus-Oei, L.F.; Dijkstra, J.; Burgmans, M.C. Ablation margin quantification after thermal ablation of malignant liver tumors: How to optimize the procedure? A systematic review of the available evidence. Eur. J. Radiol. Open 2023, 11, 100501. [Google Scholar] [CrossRef]
- ClinicIMPPACT. Available online: https://www.clinicimppact.eu/ (accessed on 8 May 2023).
- Oosterveer, T.T.M.; van Erp, G.C.M.; Hendriks, P.; Broersen, A.; Overduin, C.G.; van Rijswijk, C.S.P.; van Erkel, A.R.; van der Meer, R.W.; Tushuizen, M.E.; Moelker, A.; et al. Study Protocol PROMETHEUS: Prospective Multicenter Study to Evaluate the Correlation Between Safety Margin and Local Recurrence After Thermal Ablation Using Image Co-registration in Patients with Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2022, 45, 606–612. [Google Scholar] [CrossRef]
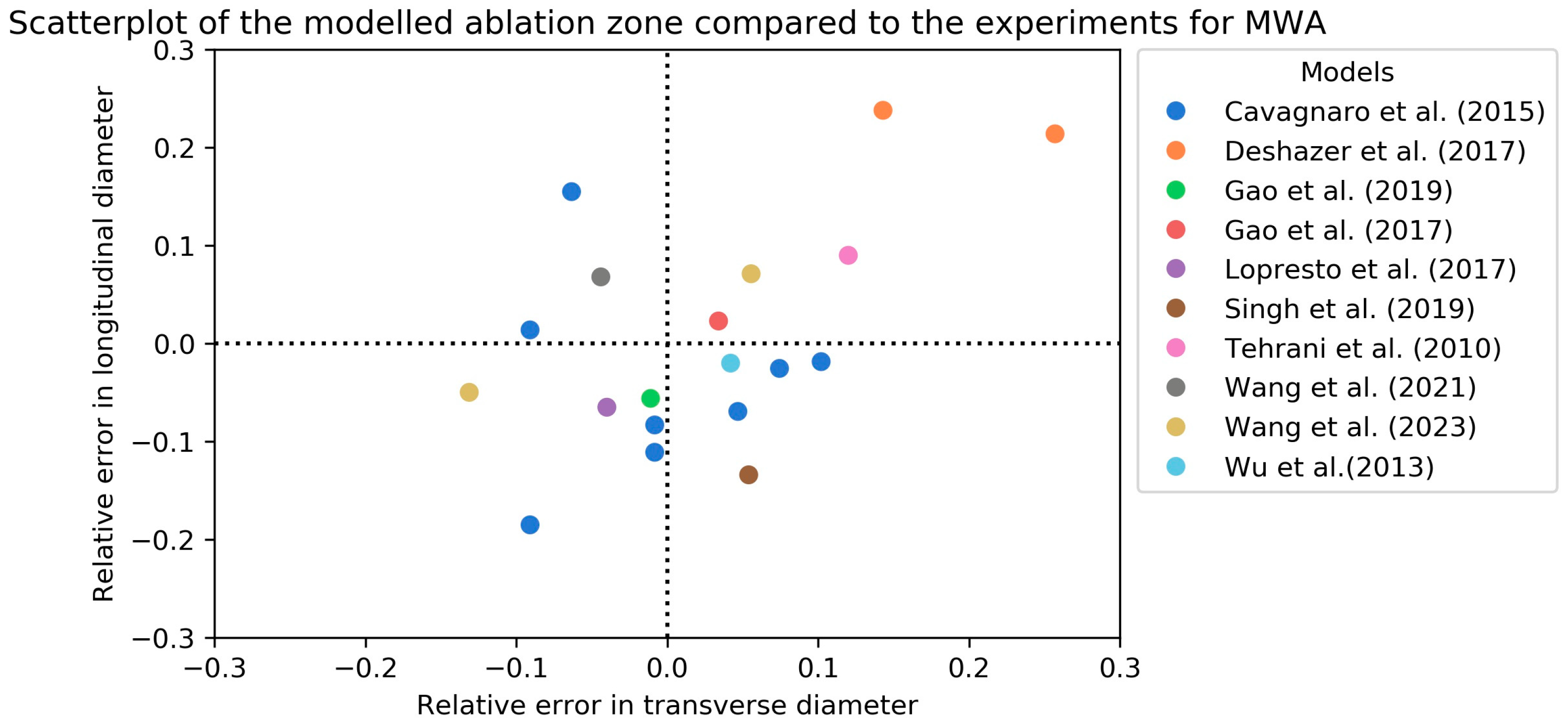

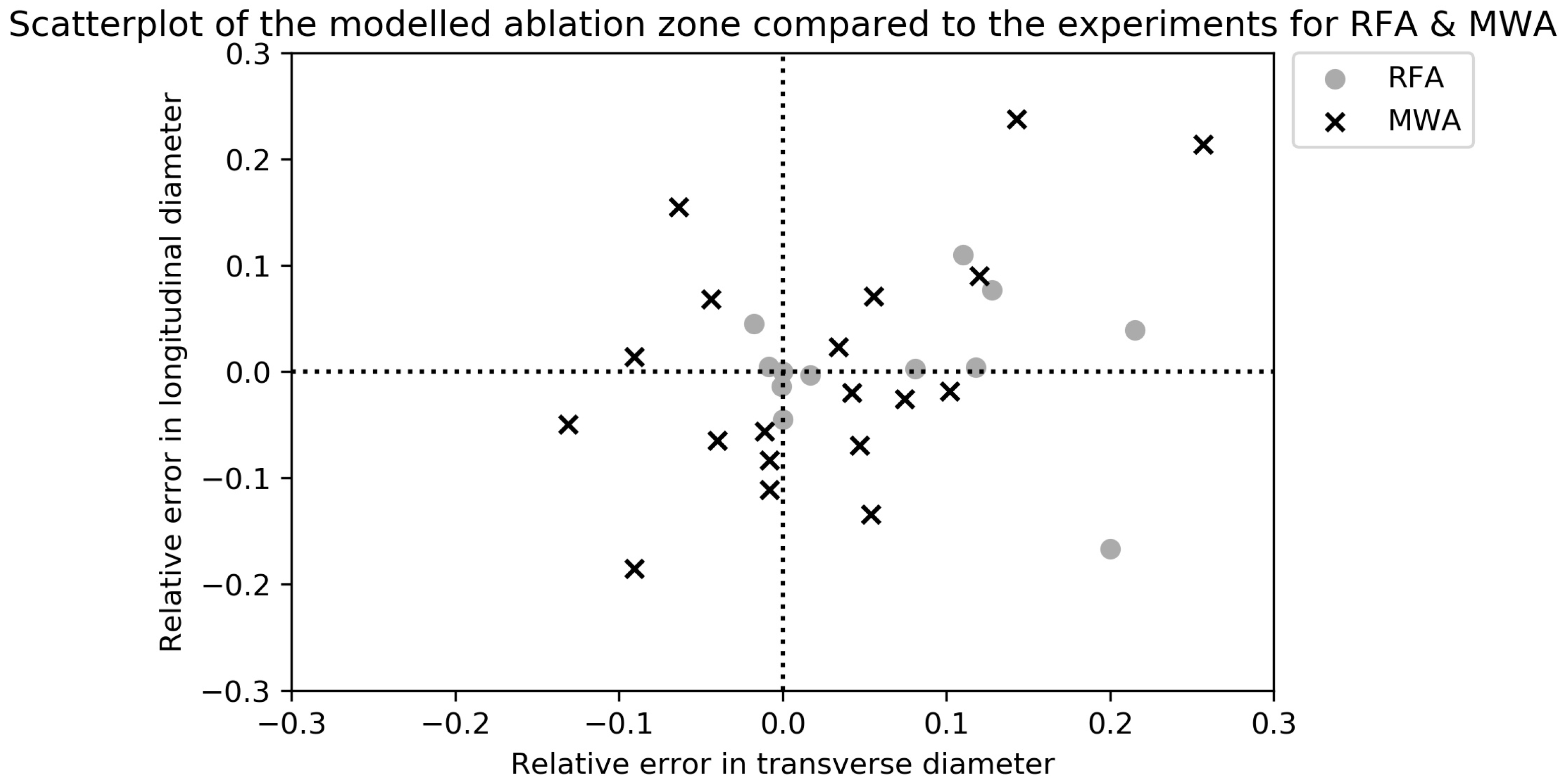
| Author (Year) | Bioheat Model | Cell Death Model | Numerical Method * | Perfusion | Blood Vessels | Water Vaporization | Temperature-Dependent Tissue Parameters | CT-Based Anatomic Model | Model Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Cavagnaro et al. [26] (2015) | Pennes’ BHE | 60 °C isothermal contour | FDTD | BHE-S: Standard BHE | |||||
| Pennes’ BHE | 60 °C isothermal contour | x | BHE-V: Standard BHE including water vaporization | ||||||
| Pennes’ BHE | 60 °C isothermal contour | x | BHE-ST_B and BHE-ST (two different equations for temperature-dependent parameters) | ||||||
| Pennes’ BHE | 60 °C isothermal contour | x | x | BHE-V-ST_B and BHE-V-ST. (two different equations for temperature-dependent parameters), only conductivity is temperature-dependent | |||||
| Pennes’ BHE | 60 °C isothermal contour | x | x | SAR-T-1min_B and SAR-T-1min (two different equations for temperature-dependent parameters). Temperature-dependency of conductivity as well as dielectric parameters | |||||
| Collins et al. [29] (2020) | Pennes’ BHE | Arrhenius thermal damage model | FEM | Determine dielectric properties based on MRI fat quantification with inverse-modeling strategy | |||||
| Deshazer et al. [30] (2017) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 63%) and 52 °C isothermal contour | FEM | x, but not in experiments | x | x | Damage-dependent blood perfusion rate. Two different models tested (A and B); they only differ in dielectric parameter dependency of temperature | ||
| Deshazer et al. [31] (2017) | Own heat-transfer model | 60 °C isothermal contour | FEM | x | x | Investigated the option of intra-procedural SAR measurement to model ablation zone | |||
| Faridi et al. [33] (2020) | Transient heat-transfer equation | Arrhenius thermal damage model (isocontour 63%) | FEM | x | x | Added the Morris method to determine the sensitivity of the ablation zones to uncertainty in tissue physical properties | |||
| Gao et al. [35] (2017) | Pennes’ BHE | 54 °C isothermal contour | FEM | Used experiments to determine phantom parameters and SAR distribution, which is the basis of the FEM model | |||||
| Gao et al. [36] (2019) | Pennes’ BHE | 54 °C isothermal contour | FEM | x | x | x | Tried to model coagulation zone over time and incorporate tumor geometry to assess tumor coverage | ||
| Gao et al. [37] (2019) | Pennes’ BHE | 54 °C isothermal contour | FEM | x | x | Used parameter sensitivity analysis to optimize the temperature-based parameters | |||
| Lopresto et al. [38] (2017) | Pennes’ BHE | 60 °C isothermal contour | FDTD | x | x | Evaluate the effect of ±25% variations in dielectric and thermal parameters using the combined expanded uncertainty | |||
| Singh et al. [44] (2019) | Pennes’ BHE with Dual phase lag model | Three-state cell death model | FEM | x, but not in experiments | x | x | Incorporates lot of complexities: damage-dependent blood perfusion rate, mechanical deformation (shrinkage) and heat-flux model. Modeled RFA as well as MWA. However, only validated MWA with experiments | ||
| Tehrani et al. [46] (2010) | Pennes’ BHE | Three-state cell death model | FEM | x | x | Used a multicompartment model including tissue, tumor and blood. Added a model for tumor shrinkage | |||
| Tucci et al. [48] (2022) | Local thermal non-equilibrium equation | Arrhenius thermal damage model (isocontour 99%) | FEM | x | x, 4 different diameters | x | x | Damage-dependent blood perfusion rate. Two compartment model with difference in porosity (and other factors) in tumor and surrounding liver tissue. Also, within the tumor, the difference in porosity in the tumor core toward the tumor rim (increasing porosity) is modeled | |
| Wang et al. [52] (2021) | Pennes’ BHE | 54 °C isothermal contour | FEM | x | x | x | x | Incorporated convection heat-transfer condition and Newton formula for heat transfer between blood vessel and tissue | |
| Wang et al. [53] (2023) | Pennes’ BHE | 54 °C isothermal contour and Arrhenius thermal damage model (isocontour 63%) | FEM | x, but not in experiments | x | x | Modeled dual-antenna MWA, different distances between antennas | ||
| Wu et al. [55] (2013) | Pennes’ BHE | 55 °C isothermal contour | FDTD | x | x | Used GPUs to simulate in 3D. Did not quantify the electrical field, but determined its contribution based on experiments. | |||
| Zhai et al. [56] (2008) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 63%) | FEM | x | x | x | GPU-accelerated model for preoperative 3D simulation of necrotic zone in clinical setting. Incorporated effect of necrosis on blood perfusion |
| Author (Year) | Bioheat Model | Cell Death Model | Numerical Method * | Perfusion | Blood Vessels | Water Vaporization | Temperature-Dependent Tissue Parameters | CT-Based Anatomic Model | Model Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Audigier et al. [22] (2013) | Combination of Pennes’ BHE and Wulff–Klinger model | Three-state cell death model | Lattice Boltzmann solver | x | x | x | Computational fluid dynamics and Darcy’s equation are coupled to the bioheat equation to model blood circulation and blood flow | ||
| Audigier et al. [23] (2015) | Combination of Pennes’ BHE and Wulff–Klinger model | Three-state cell death model | Lattice Boltzmann solver | x | x | x | Computational fluid dynamics and Darcy’s equation are coupled to the bioheat equation to model blood circulation and blood flow, two-compartment model (blood vessels and liver tissue) | ||
| Audigier et al. [24] (2017) | Combination of Pennes’ BHE and Wulff–Klinger model | Three-state cell death model | Lattice Boltzmann solver | x | x | x | Navier-stokes equation and computational fluid dynamics solver used to model blood flow. Blood flow is determined using preoperative MRI, blood pressures are measured invasively, and porosity map created on CT image. Used intra-operative measurements to validate parameter values used. Used lower conductivity for cirrhotic livers | ||
| Audigier et al. [25] (2022) | Pennes’ BHE | 50 °C isothermal contour | Lattice Boltzmann solver | x | x | Also used a spherical model and Eikonal model for comparison. Used a GPU for acceleration, multi-probe modeling | |||
| Chang et al. [27] (2004) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 63%) | FEM | x, but not in experiments | x | Damage-dependent blood perfusion ratel | |||
| Chen et al. [28] (2021) | Simplified Pennes’ BHE | 55 °C isothermal contour | Simplified toward analytical solution | Ignored the heat source of the electrical current flow in the model | |||||
| Duan et al. [32] (2016) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 63%) | FEM | x, but not in experiments | x | Using a pre-procedural determined probe position; the probability of several ablation zones is displayed by the model. Damage-dependent blood perfusion ratel | |||
| Fang et al. [34] (2022) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 99%) | FEM | x, but not in experiments | x | x | x | Used the Navier–Stokes equation for blood flow modeling | |
| Hoffer et al. [39] (2022) | Pennes’ BHE | Arrhenius thermal damage model (isocontour < 63%) | FEM and FDM | x | x | x | Used a GPU to accelerate FEM, able to model single and multi-probe ablations, focused on clinical application | ||
| Mariappan et al. [40] (2017) | Pennes’ BHE | Three-state cell death model | FEM | x | x | x | Used a GPU to accelerate FEM, focused on clinical application | ||
| Moche et al. [41] (2020) | Pennes’ BHE | Three-state cell death model | FEM | x | x | x | Used a GPU, more focused on clinical application. Simulation parameters involved a proportional integral derivative | ||
| Ooi et al. [42] (2019) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 99%) | FEM | x, but not in experiments | x | x | x | Modeled different boundary conditions | |
| Payne et al. [43] (2011) | Split-volume bioheat equation (own model) | Three-state cell death model | FEM | x | x | x | Incorporated Newton’s cooling law to model heat transfer between vessels and tissue and Darcy’s law for blood velocity | ||
| Subramanian et al. [45] (2015) | Pennes’ BHE | Own thermal damage formula | FEM | x | Experimental-based values of the specific heat, thermal conductivity, and electrical conductivity | ||||
| Tucci et al. [47] (2021) | Pennes’ BHE | Arrhenius thermal damage model (isocontour 99%) | FEM | x | x | Damage-dependent blood perfusion rate | |||
| Local thermal equilibrium equation | 60 °C isothermal contour | x | x | Porous media-based model, damage-dependent blood perfusion rate; assumes equilibrium in temperature between blood and tissue | |||||
| Local thermal non-equilibrium equation | 60 °C isothermal contour | x | x | x | Porous media-based model, damage-dependent blood perfusion rate; separates vaporization phase for water, tissue, and blood | ||||
| Vaidya et al. [49] (2021) | Pennes’ BHE | Arrhenius thermal damage model | FVM | x | x | x | Multicompartment model incorporating tissue, tumor, blood, and probe. Damage-dependent blood perfusion rate | ||
| Voglreiter et al. [50] (2018) | Pennes’ BHE | Three-state cell death model | FEM | x | x | x | Used a GPU to accelerate FEM; focused on clinical application | ||
| Wang et al. [51] (2019) | Pennes’ BHE | 54 °C isothermal contour | FEM | x | |||||
| Welp et al. [54] (2006) | Heat transfer equation | Arrhenius thermal damage model (isocontour 99%) | FEM | x | x | x | Incorporated the heat transfer between blood and tissue |
| Author (Year) | Model | In Vivo or Ex Vivo Validation | Number of Experiments | Ground Truth | Ablation Settings (Time of Ablation and Power) | Outcome Measure/Metric | Performance | Validation Remarks |
|---|---|---|---|---|---|---|---|---|
| Cavagnaro et al. [26] (2015) | BHE-S | Ex vivo, bovine livers | 6 | Sectioning sample and measure ablation zone | 10 min, 40 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: −8.31% T: −0.83% | |
| BHE-V | L: −18.5% T: −9.09% | |||||||
| BHE-ST_B | L: −1.85% T: 10.2% | |||||||
| BHE-ST | L: −2.54%, T: 7.44% | |||||||
| BHE-V-ST_B | L: −6.93%, T: 4.68% | |||||||
| BHE-V-ST | L: −11.1%, T: −0.83% | |||||||
| SAR-T-1min_B | L: 15.5%, T: −6.34% | |||||||
| SAR-T-1min | L: 1.39%, T: −9.09% | |||||||
| Collins et al. [29] (2020) | Fat phantoms | Ex vivo, phantom | 15 | Sectioning sample, photographed and 2D segmentation of ablation zone | 15 min, 60 W, 915 MHz | Jaccard similarity index | 0.866 ± 0.053 | For each phantom, the electrical and thermal conductivity were reconstructed to best fit the model |
| Non-fat phantom | 6 | Jaccard similarity index | 0.934 ± 0.022 | |||||
| Deshazer et al. [30] (2017) | Model A | Ex vivo, bovine livers | 4 | Sectioning sample and measure ablation zone | 10 min, 30 W, 915 MHz | Longitudinal (L) and transverse (T) RDD * | L: 2.9%, T: 24.0% | A: linear temperature dependency of dielectric properties, B: similar to model A but added linear decrease in electrical conductivity above 95 °C |
| Model B | 4 | L: 5.7%, T: 12.0% | ||||||
| Model A | 8 | 15 min, 60 W, 915 MHz | L: 21.4%, T: 25.7% | |||||
| Model B | 8 | L: 23.8%, T: 14.3% | ||||||
| Deshazer et al. [31] (2017) | Short-tip, 1000 W/kg iso-SAR | Ex vivo, porcine livers | 3 | Segmentation on infrared camera temperature measurements | 6 min, 15 W, 915 MHz | DSC | 0.74 ± 0.01 | |
| Short-tip, 500 W/kg iso-SAR | 0.82 ± 0.04 | |||||||
| Long-tip, 1000 W/kg iso-SAR | 3 | 0.77 ± 0.03 | ||||||
| Long-tip, 500 W/kilo iso-SAR | 0.76 ± 0.01 | |||||||
| Faridi et al. [33] (2020) | Ex vivo, bovine livers | 4 | Segmentation on MRT-derived Arrhenius thermal damage 3D maps | 10 min, 30 W, 2450 MHz | DSC | 0.8 ± 0.0 | ||
| 8 | 5 min, 30 W, 2450 MHz | 0.8 ± 0.08 | ||||||
| 3 | 5 min, 50 W, 2450 MHz | 0.75 ± 0.06 | ||||||
| Gao et al. [35] (2017) | Ex vivo, phantom | Sectioning sample and measure ablation zone | 10 min, 60 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: −5.6%, T: −1.1% | |||
| Advancement | 0.341 vs. 0.3 ± 0.05 cm | |||||||
| Gao et al. [36] (2019) | Ex vivo, porcine livers | 20 | Sectioning sample and measure ablation zone | 40, 45, 50, 55 and 60 W, 2450 MHz | Error of transverse radius, advancement and backward longitudinal length | ±5% | ||
| Gao et al. [37] (2019) | Ex vivo, porcine livers | 20 | Sectioning sample and measure ablation zone | 6 min, 60 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: 2.3%, T 3.4% | Optimized thermo-dependent parameters based on experiments | |
| Lopresto et al. [38] (2017) | Ex vivo, bovine livers | 4 | Sectioning sample and measure ablation zone | 10 min, 60 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: −6.5%, T: −4.0% | ||
| Advancement | 7.4 mm (model) versus 7.5 ± 2.1 mm | |||||||
| Singh et al. [44] (2019) | Ex vivo, porcine livers | 10 | Sectioning sample and measure ablation zone | 2 min, 40 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: −13.4% T: 5.4% | Used the experimental results of Wu et al. [52] | |
| Tehrani et al. [46] (2010) | Ex vivo, porcine livers | 56 | Sectioning sample and measure ablation zone | 10 min, 50 and 60 W, 2450 MHz and 80 W, 915 MHz | Longitudinal (L) and transverse (T) RDD * | L: 9%, T: 12% | Used the experimental results of Sun et al. [57] | |
| Wang et al. [52] (2021) | Ex vivo, porcine liver | 11 | Sectioning sample and measure ablation zone | 6 min, 60 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: 6.8%, T: −4.4% | Used a peristaltic pump to simulate blood circulation and soft plastic tubes for blood vessels | |
| Wang et al. [53] (2023) | 54 °C isothermal contour | Ex vivo, porcine liver | 5 | Sectioning sample and measure ablation zone | 8 min, 50 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: 7.12%, T: 5.56% | Results with 30 mm spacing between antennas |
| Arrhenius model | Longitudinal (L) and transverse (T) RDD * | L: −4.98%, T: −13.21% | ||||||
| Wu et al. [55] (2013) | Ex vivo, porcine livers | 10 | Sectioning sample and measure ablation zone | 2 min, 40 W, 2450 MHz | Longitudinal (L) and transverse (T) RDD * | L: −2.0%, T: 4.2% |
| Author (Year) | Model | In Vivo or Ex Vivo Validation | Number of Experiments | Ground Truth | Ablation Settings (Time of Ablation and Power) | Outcome Measure/Metric | Performance | Validation Remarks |
|---|---|---|---|---|---|---|---|---|
| Tucci et al. [48] (2022) | Capillaries | In vivo, patients | 32 | Segmentation on 24 h post-ablation CT | 5 and 10 min, 60 W, 2450 MHz | Transverse RDD * | +24% (5 min) +43% (10 min) | Used the experimental results of Amabile et al. [58] |
| RVD * | 31% (5 min), 93% (10 min) | |||||||
| Terminal arteries | Transverse RDD * | −4% (5 min), +8% (10 min) | ||||||
| RVD * | −32% (5 min), −8% (10 min) | |||||||
| Terminal branches | Transverse RDD * RVD * Transverse RDD * RVD * | −42% (5 min), −43% (10 min) | ||||||
| −83% (5 min), −84% (10 min) | ||||||||
| Tertiary branches | −18% (5 min), −13% (10 min) | |||||||
| −88% (5 min), −84% (10 min) | ||||||||
| Zhai et al. [56] (2008) | In vivo, patients | 9 | Segmentation on 1–2 weeks post-ablation CT | Patient-specific, 2450 MHz | RVD * | ±7.0% | Article contains only small details on experiments. Study type unknown |
| Author (Year) | Model | In Vivo or Ex Vivo Validation | Number of Experiments | Ground Truth | Ablation Settings (Time of Ablation and Power) | Outcome Measure/Metric | Performance | Validation Remarks |
|---|---|---|---|---|---|---|---|---|
| Chang et al. [27] (2004) | Ex vivo, porcine livers | 2 | Sectioning sample and placed in 2,3,5-triphenyltetrazolium chloride to color cell viability | 15 min, 20 V | Longitudinal (L) and transverse (T) RDD * | L: 0.0%, T: 0.0% | ||
| 2 | 15 min, 25 V | L: −16.7%, T: 20.0% | ||||||
| 2 | 15 min, 30 V | L: −4.5%, T: 0.0% | ||||||
| Chen et al. [28] (2021) | single probe | Ex vivo, porcine livers | 5 | Sectioning sample and measure ablation zone | Longitudinal (L) and transverse (T) RDD * | L: −0.35%, T: 1.68% | ||
| Switching probe (10 mm) | 5 | Longitudinal midline (Lm), longitudinal probeline (Lp), and transverse (T) RDD * | Lm: −1.38%, Lp: −1.82%, T: −0.08% | |||||
| Switching probe (15 mm) | 5 | 12 min | Lm: 0.47%, Lp: 0.05%, T: −0.87% | |||||
| Switching probe (20 mm) | 5 | Lm: 4.54%, Lp: 0.64%, T: −1.76% | ||||||
| Duan et al. [32] (2016) | Ex vivo, porcine livers | 20 | Sectioning sample and measure ablation zone | 5 min, temperature-controlled (105 °C) | Longitudinal (L) and transverse (T) RDD and relative area deviation (A) * | L: 11.1%, T:10.9%, A:1% | ||
| Fang et al. [34] (2022) | Ex vivo, bovine livers | 3 | Sectioning sample and measure ablation zone | 12 min, impedance-controlled, 1800 mA | Transverse RDD * | −2.83% | Used the experimental results of Goldberg et al. [59] | |
| Ooi et al. [42] (2019) | Ex vivo, bovine livers | 3 | Sectioning sample and measure ablation zone | 12 min, impedance-controlled, 1800 mA | Transverse RDD * | −20.9% | Used the experimental results of Goldberg et al. [59] | |
| Subramanian et al. [45] (2015) | Ex vivo, bovine livers | 15 | Segmentation on image of flatbed scanner after sectioning sample | 500 KHz, 1–6 min, 31–34 V 60–80 W | Relative area deviation * | −2.63% | Optimized tissue parameters based on experiments | |
| Vaidya et al. [49] (2021) | Ex vivo, phantom | 1 | Sectioning phantom, using temperature-sensitive ink to measure ablation zone | 10 min, temperature-controlled (103 °C), max power of 35 W | Relative area deviation * | 17.03% | Used ink which colors irreversibly above threshold T > 70 °C | |
| Wang et al. [51] (2019) | Ex vivo, porcine livers | 3 | Sectioning sample and measure ablation zone | Temperature-controlled (80 °C), 330 kHz | Longitudinal (L) and transverse (T) RDD * | L: 7.7%, T: 12.8% | Used a peristaltic pump to simulate blood circulation and soft plastic tubes to simulate blood vessels | |
| 3 | temperature-controlled (95 °C), 330 kHz | L: 3.9%, T: 21.5% | ||||||
| 3 | Temperature-controlled (90 °C), 330 kHz | L: 0.4%, T: 11.8% | ||||||
| 3 | Temperature-controlled (95 °C), 330 kHz | L: 0.3%, T: 8.1% | ||||||
| Welp et al. [54] (2006) | Vessel ⌀ = 4 mm, flow 25 mL/min | Ex vivo, porcine livers | 10 | Sectioning sample and measure ablation zone | 12 min, impedance-controlled, 25 W | Transverse RDD * | −5.7% | Used glass tubes to simulate blood vessels |
| Vessel ⌀ = 4 mm, flow 50 mL/min | −2.4% | |||||||
| Vessel ⌀ = 4 mm, flow 75 mL/min | −8.7% | |||||||
| Vessel ⌀ = 6 mm, flow 75 mL/min | 1.9% | |||||||
| Vessel ⌀ = 6 mm, flow 150 mL/min | 1.9% | |||||||
| Vessel ⌀ = 6 mm, flow 300 mL/min | 1.9% |
| Author (Year) | Model | In Vivo or Ex Vivo Validation | Number of Experiments | Ground Truth | Ablation Settings (Time of Ablation and Power) | Outcome Measure/Metric | Performance | Validation Remarks |
|---|---|---|---|---|---|---|---|---|
| Audigier et al. [22] (2013) | In vivo, patients | 5 patients, 7 ablations | Segmentation on post-ablation CT scan | Patient-specific | Surface deviation | 8.67 mm | Retrospective study | |
| Audigier et al. [23] (2015) | In vivo, patients | 10 patients, 14 tumors | Segmentation on post-ablation CT scan | Patient-specific | DSC | 0.418 | Retrospective study | |
| Sensitivity | 66.94% | |||||||
| PPV | 38.30% | |||||||
| Audigier et al. [24] (2017) | In vivo, porcine livers | 5 swine, 12 ablations | Segmentation on post-ablation CT scan | 6 min, temperature-controlled (105 °C), two iterations for large tumors | Surface deviation | 5.3 ± 3.6 mm | Surrogate tumors implanted | |
| DSC | 0.44 | |||||||
| Sensitivity | 47% | |||||||
| PPV | 53% | |||||||
| Audigier et al. [25] (2022) | Biophysics-based model | In vivo, patients | 11 patients, 12 ablations | Segmentation on post-ablation CT scan | Patient-specific | DSC, surface deviation, and RVD | Best | Retrospective study. Did not express their results numerical, but ranking extracted out of graphs |
| Spherical model | ||||||||
| Eikonal model | ||||||||
| Hoffer et al. [39] (2021) | Computational model | In vivo, porcine livers | 2 swine, 6 ablations | Segmentation on post-ablation CT scan | Mean surface deviation | 1.1 mm | ||
| Max surface deviation | 5.2 mm | |||||||
| Manufacturer’s cart | Mean surface deviation | 2.5 mm | ||||||
| Max surface deviation | 7.8 mm | |||||||
| Mariappan et al. [40] (2017) | Unknown CT perfusion values | In vivo, patients | 6 patients, 10 ablations | Segmentation on 1-month post-ablation CT scan | Patient-specific, temperature-controlled | DSC | 0.7286 | Retrospective study |
| RVD | 5.11% | |||||||
| Surface deviation | 2.55 mm | |||||||
| Known CT perfusion values | 12 patients, 23 ablations | DSC | 0.691 | |||||
| RVD | 17.93% | |||||||
| Surface deviation | 2.50 mm | |||||||
| Moche et al. [41] (2020) | In vivo, patients | 46 patients, 51 ablations | Segmentation on 1-month post-ablation CT scan | Patient-specific, temperature-controlled | DSC | 0.62 ± 0.14 | Prospective study | |
| Sensitivity | 0.70 ± 0.21 | |||||||
| PPV | 0.66 ± 0.25 | |||||||
| Surface deviation | 3.4 ± 1.7 mm | |||||||
| Payne et al. [43] (2011) | In vivo, porcine livers | 2 swine | Segmentation on post-ablation CT scan | Temperature-controlled | RVD | 39.6% | ||
| Tucci et al. [47] (2021) | Pennes | In vivo, porcine livers | 8 swine | Sectioning sample and measure ablation zone | 12 min, 90 V, 500 KHz, impedance-controlled | Transverse RDD * | −32.4% | Compared to experiments of Goldberg et al. [59] |
| LTE | −7.57% | |||||||
| LTNE | −7.57% | |||||||
| Voglreiter et al. [50] (2018) | In vivo, patients | 21 patients | Segmentation on post-ablation CT scan | Patient-specific | DSC | 0.7003 ± 0.0937 | Retrospective study | |
| RVD | 13.77 ± 12.96% | |||||||
| Sensitivity | 69.70 ± 10.94% | |||||||
| PPV | 71.73 ± 12.00% | |||||||
| Surface deviation | 2.44 ± 0.84 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Erp, G.C.M.; Hendriks, P.; Broersen, A.; Verhagen, C.A.M.; Gholamiankhah, F.; Dijkstra, J.; Burgmans, M.C. Computational Modeling of Thermal Ablation Zones in the Liver: A Systematic Review. Cancers 2023, 15, 5684. https://doi.org/10.3390/cancers15235684
van Erp GCM, Hendriks P, Broersen A, Verhagen CAM, Gholamiankhah F, Dijkstra J, Burgmans MC. Computational Modeling of Thermal Ablation Zones in the Liver: A Systematic Review. Cancers. 2023; 15(23):5684. https://doi.org/10.3390/cancers15235684
Chicago/Turabian Stylevan Erp, Gonnie C. M., Pim Hendriks, Alexander Broersen, Coosje A. M. Verhagen, Faeze Gholamiankhah, Jouke Dijkstra, and Mark C. Burgmans. 2023. "Computational Modeling of Thermal Ablation Zones in the Liver: A Systematic Review" Cancers 15, no. 23: 5684. https://doi.org/10.3390/cancers15235684
APA Stylevan Erp, G. C. M., Hendriks, P., Broersen, A., Verhagen, C. A. M., Gholamiankhah, F., Dijkstra, J., & Burgmans, M. C. (2023). Computational Modeling of Thermal Ablation Zones in the Liver: A Systematic Review. Cancers, 15(23), 5684. https://doi.org/10.3390/cancers15235684






