Gastro-Esophageal Junction Precancerosis: Histological Diagnostic Approach and Pathogenetic Insights
Abstract
:Simple Summary
Abstract
1. Introduction
2. On the Existence of Cardiac Mucosa
3. Barrett’s Esophagus
3.1. Guidelines and Definitions: A Long Journey to Standardization
3.2. Intestinal Metaplasia, or Not: Still a Diagnostic Requirement?
3.3. Molecular Pathway: From Normal Tissue to Metaplasia: Transdifferentiation, Transcommitment and Cell of Origin
4. Dysplasia in Barrett’s Esophagus
4.1. Definition
4.2. Type of Dysplasia
4.2.1. Intestinal-Type Dysplasia
4.2.2. Non-Intestinal Dysplasia
4.3. Diagnostic Categories
- Negative for dysplasia. This diagnosis is made when the biopsy represents either columnar epithelium with no cell atypia or reactive (hyperplastic/regenerative) changes.
- Indefinite for dysplasia. This category reflects the uncertainty of the diagnosis. As the real nature of the lesion cannot be assessed, follow-up should be suggested in the report [50]. In some settings, biopsy interpretation can be highly challenging for pathologists. Active inflammation, ulceration, or post-ulcer healing may determine profound changes in tissue. This descriptive, provisional category should apply only to cases where the pathologist cannot clearly decide whether the lesion is negative for dysplasia (hyperplastic/regenerative) or genuinely dysplastic. The grade of uncertainty may be due to inadequate biopsy sampling or cytological atypia and structural alterations with equivocal interpretation. This diagnosis must be followed by short-term resampling and second opinion and not be used as a “waste-basket” category.
- Low-grade dysplasia—LGD. The cells in LGD display nuclear enlargement, elongation, hyperchromasia, and stratification, but their nuclear polarity is retained (Figure 4). Although the dysplastic crypts show minimal architectural changes, the lamina propria between them is still visible. The nuclei are slightly enlarged, and the number of goblet cells present may range from a few scattered ones to numerous.
- High-grade dysplasia—HGD. HGD is characterized by striking cytological atypia and wider architectural changes. The cells have markedly enlarged nuclei, nuclear pleomorphism, irregular nuclear contours, and loss of polarity. Mitoses are increased in number and are often atypical. The crypts may appear crowded, and/or may contain marked budding or angulation, back-to-back growth, and cribriforming.
4.4. Ancillary Techniques
4.5. Molecular Pathway: From Metaplasia to Adenocarcinoma
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayeck, T.J.; Kong, C.Y.; Spechler, S.J.; Gazelle, G.S.; Hur, C. The prevalence of Barrett’s esophagus in the US: Estimates from a simulation model confirmed by SEER data. Dis. Esophagus 2010, 23, 451–457. [Google Scholar] [CrossRef]
- Sawas, T.; Killcoyne, S.; Iyer, P.G.; Wang, K.K.; Smyrk, T.C.; Kisiel, J.B.; Qin, Y.; Ahlquist, D.A.; Rustgi, A.K.; Costa, R.J.; et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology 2018, 155, 1720–1728.e4. [Google Scholar] [CrossRef] [PubMed]
- Naini, B.V.; Souza, R.F.; Odze, R.D. Barrett’s Esophagus: A Comprehensive and Contemporary Review for Pathologists. Am. J. Surg. Pathol. 2016, 40, e45–e66. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Vantanasiri, K.; Mohan, B.P.; Goyal, R.; Garg, N.; Gerberi, D.; Kisiel, J.B.; Singh, S.; Iyer, P.G. Prevalence of Barrett’s Esophagus and Adenocarcinoma with and without Gastroesophageal Reflux: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Naik, A.D.; Duan, Z.; Shakhatreh, M.; Helm, A.; Pathak, A.; Hinojosa-Lindsey, M.; Hou, J.; Nguyen, T.; Chen, J.; et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut 2016, 65, 1252–1260. [Google Scholar] [CrossRef]
- Vennalaganti, P.; Kanakadandi, V.; Goldblum, J.R.; Mathur, S.C.; Patil, D.T.; Offerhaus, G.J.; Meijer, S.L.; Vieth, M.; Odze, R.D.; Shreyas, S.; et al. Discordance Among Pathologists in the United States and Europe in Diagnosis of Low-Grade Dysplasia for Patients with Barrett’s Esophagus. Gastroenterology 2017, 152, 564–570.e4. [Google Scholar] [CrossRef]
- Sharma, P.; Falk, G.W.; Weston, A.P.; Reker, D.; Johnston, M.; Sampliner, R.E. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 2006, 4, 566–572. [Google Scholar] [CrossRef]
- de Jonge, P.J.; van Blankenstein, M.; Looman, C.W.; Casparie, M.K.; Meijer, G.A.; Kuipers, E.J. Risk of malignant progression in patients with Barrett’s oesophagus: A Dutch nationwide cohort study. Gut 2010, 59, 1030–1036. [Google Scholar] [CrossRef]
- Khoshiwal, A.M.; Frei, N.F.; Pouw, R.E.; TissueCypher SURF LGD Study Pathologists Consortium; Smolko, C.; Arora, M.; Siegel, J.J.; Duits, L.C.; Critchley-Thorne, R.J.; Bergman, J. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients with Low-Grade Dysplasia. Gastroenterology 2023, 165, 1168–1179.e6. [Google Scholar] [CrossRef]
- Duits, L.C.; van der Wel, M.J.; Cotton, C.C.; Phoa, K.N.; Ten Kate, F.J.W.; Seldenrijk, C.A.; Offerhaus, G.J.A.; Visser, M.; Meijer, S.L.; Mallant-Hent, R.C.; et al. Patients with Barrett’s Esophagus and Confirmed Persistent Low-Grade Dysplasia Are at Increased Risk for Progression to Neoplasia. Gastroenterology 2017, 152, 993–1001.e1. [Google Scholar] [CrossRef]
- Sangle, N.A.; Taylor, S.L.; Emond, M.J.; Depot, M.; Overholt, B.F.; Bronner, M.P. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: A multicenter, international study. Mod. Pathol. 2015, 28, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Mills, S. Histology for Pathologists; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Odze, R.D. Unraveling the mystery of the gastroesophageal junction: A pathologist’s perspective. Am. J. Gastroenterol. 2005, 100, 1853–1867. [Google Scholar] [CrossRef]
- Yung, E.; Li, X.; Chandrasoma, P. Intestinal Metaplasia of the “Cardia”: Accurate Differentiation of Gastric or Esophageal Origin with an Expanded Biopsy Protocol. Am. J. Surg. Pathol. 2021, 45, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; Doyle, L.A.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef]
- Borgmann, M.; Quante, M. Impact of the Tumor Microenvironment for Esophageal Tumor Development—An Opportunity for Prevention? Cancers 2022, 14, 2246. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, A.S.; Stabenau, K.A.; Altman, K.W.; Johnston, N. Cancer Risk in Barrett’s Esophagus: A Clinical Review. Int. J. Mol. Sci. 2023, 24, 6018. [Google Scholar] [CrossRef]
- Allison, P.R. Peptic ulcer of the esophagus. J. Thorac. Surg. 1946, 15, 308–317. [Google Scholar] [CrossRef]
- Allison, P.R.; Johnstone, A.S. The oesophagus lined with gastric mucous membrane. Thorax 1953, 8, 87–101. [Google Scholar] [CrossRef]
- Barrett, N.R. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br. J. Surg. 1950, 38, 175–182. [Google Scholar] [CrossRef]
- Barrett, N.R. The oesophagus lined by columnar epithelium. Gastroenterologia 1956, 86, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Tanoglu, A.; Sakin, Y.S.; Akyol, T.; Oncu, K.; Kara, M.; Yazgan, Y. Landmark reading alterations in patients with gastro-oesophageal reflux symptoms undergoing diagnostic gastroscopy. Arab. J. Gastroenterol. 2016, 17, 176–180. [Google Scholar] [CrossRef] [PubMed]
- De Hertogh, G.; Van Eyken, P.; Ectors, N.; Geboes, K. On the origin of cardiac mucosa: A histological and immunohistochemical study of cytokeratin expression patterns in the developing esophagogastric junction region and stomach. World J. Gastroenterol. 2005, 11, 4490–4496. [Google Scholar] [CrossRef] [PubMed]
- Chandrasoma, P.; Wijetunge, S.; Demeester, S.R.; Hagen, J.; Demeester, T.R. The histologic squamo-oxyntic gap: An accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am. J. Surg. Pathol. 2010, 34, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Chandrasoma, P.T.; Der, R.; Dalton, P.; Kobayashi, G.; Ma, Y.; Peters, J.; Demeester, T. Distribution and significance of epithelial types in columnar-lined esophagus. Am. J. Surg. Pathol. 2001, 25, 1188–1193. [Google Scholar] [CrossRef]
- Chandrasoma, P.T.; Der, R.; Ma, Y.; Peters, J.; Demeester, T. Histologic classification of patients based on mapping biopsies of the gastroesophageal junction. Am. J. Surg. Pathol. 2003, 27, 929–936. [Google Scholar] [CrossRef]
- Chandrasoma, P.T.; Lokuhetty, D.M.; Demeester, T.R.; Bremmer, C.G.; Peters, J.H.; Oberg, S.; Groshen, S. Definition of histopathologic changes in gastroesophageal reflux disease. Am. J. Surg. Pathol. 2000, 24, 344–351. [Google Scholar] [CrossRef]
- Asge Standards of Practice Committee; Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef]
- di Pietro, M.; Fitzgerald, R.C.; BSG Barrett’s Guidelines Working Group. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut 2018, 67, 392–393. [Google Scholar] [CrossRef]
- Kusano, C.; Singh, R.; Lee, Y.Y.; Soh, Y.S.A.; Sharma, P.; Ho, K.Y.; Gotoda, T. Global variations in diagnostic guidelines for Barrett’s esophagus. Dig. Endosc. 2022, 34, 1320–1328. [Google Scholar] [CrossRef]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus with Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020, 158, 760–769. [Google Scholar] [CrossRef]
- Saftoiu, A.; Hassan, C.; Areia, M.; Bhutani, M.S.; Bisschops, R.; Bories, E.; Cazacu, I.M.; Dekker, E.; Deprez, P.H.; Pereira, S.P.; et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 293–304. [Google Scholar] [CrossRef]
- Fock, K.M.; Talley, N.; Goh, K.L.; Sugano, K.; Katelaris, P.; Holtmann, G.; Pandolfino, J.E.; Sharma, P.; Ang, T.L.; Hongo, M.; et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: An update focusing on refractory reflux disease and Barrett’s oesophagus. Gut 2016, 65, 1402–1415. [Google Scholar] [CrossRef]
- Sugano, K.; Spechler, S.J.; El-Omar, E.M.; McColl, K.E.L.; Takubo, K.; Gotoda, T.; Fujishiro, M.; Iijima, K.; Inoue, H.; Kawai, T.; et al. Kyoto international consensus report on anatomy, pathophysiology and clinical significance of the gastro-oesophageal junction. Gut 2022, 71, 1488–1514. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.M. Epithelial metaplasia and the second anatomy. Lancet 1986, 2, 268–271. [Google Scholar] [CrossRef]
- Slack, J.M. Metaplasia and somatic cell reprogramming. J. Pathol. 2009, 217, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Burke, Z.D.; Tosh, D. Barrett’s metaplasia as a paradigm for understanding the development of cancer. Curr. Opin. Genet. Dev. 2012, 22, 494–499. [Google Scholar] [CrossRef] [PubMed]
- De Hertogh, G.; Van Eyken, P.; Ectors, N.; Tack, J.; Geboes, K. On the existence and location of cardiac mucosa: An autopsy study in embryos, fetuses, and infants. Gut 2003, 52, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Park, H.J.; Kang, G.H.; Kim, C.J.; Chi, J.G. Histology of gastroesophageal junction in fetal and pediatric autopsy. Arch. Pathol. Lab. Med. 2003, 127, 451–455. [Google Scholar] [CrossRef]
- Zhou, H.; Greco, M.A.; Daum, F.; Kahn, E. Origin of cardiac mucosa: Ontogenic consideration. Pediatr. Dev. Pathol. 2001, 4, 358–363. [Google Scholar] [CrossRef]
- Que, J.; Garman, K.S.; Souza, R.F.; Spechler, S.J. Pathogenesis and Cells of Origin of Barrett’s Esophagus. Gastroenterology 2019, 157, 349–364.e1. [Google Scholar] [CrossRef]
- Dunn, L.J.; Jankowski, J.A.; Griffin, S.M. Trefoil Factor Expression in a Human Model of the Early Stages of Barrett’s Esophagus. Dig. Dis. Sci. 2015, 60, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Odze, R. Barrett’s Dysplasia. In Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1. [Google Scholar]
- Oyama, T. Endoscopic diagnosis and treatment of superficial Barrett’s esophageal adenocarcinoma: Japanese perspective. Dig. Endosc. 2022, 34 (Suppl. S2), 27–30. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.G.; Chak, A. Surveillance in Barrett’s Esophagus: Challenges, Progress, and Possibilities. Gastroenterology 2023, 164, 707–718. [Google Scholar] [CrossRef]
- Brown, I.S.; Whiteman, D.C.; Lauwers, G.Y. Foveolar type dysplasia in Barrett esophagus. Mod. Pathol. 2010, 23, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.T.; Bennett, A.E.; Mahajan, D.; Bronner, M.P. Distinguishing Barrett gastric foveolar dysplasia from reactive cardiac mucosa in gastroesophageal reflux disease. Hum. Pathol. 2013, 44, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.S.; Alfaro, E.E.; Ooi, E.M.; Li, Y.; Srivastava, A.; Fujita, H.; Park, Y.; Kumarasinghe, M.P.; Lauwers, G.Y. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: Is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett Esophagus? Am. J. Surg. Pathol. 2012, 36, 331–342. [Google Scholar] [CrossRef]
- Reid, B.J.; Haggitt, R.C.; Rubin, C.E.; Roth, G.; Surawicz, C.M.; Van Belle, G.; Lewin, K.; Weinstein, W.M.; Antonioli, D.A.; Goldman, H.; et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum. Pathol. 1988, 19, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Flejou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef]
- Choi, W.T.; Lauwers, G.Y.; Montgomery, E.A. Utility of ancillary studies in the diagnosis and risk assessment of Barrett’s esophagus and dysplasia. Mod. Pathol. 2022, 35, 1000–1012. [Google Scholar] [CrossRef]
- Tomaszewski, K.J.; Neyaz, A.; Sauder, K.; Rickelt, S.; Zhang, M.L.; Yilmaz, O.; Crotty, R.; Shroff, S.; Odze, R.; Mattia, A.; et al. Defining an abnormal p53 immunohistochemical stain in Barrett’s oesophagus-related dysplasia: A single-positive crypt is a sensitive and specific marker of dysplasia. Histopathology 2023, 82, 555–566. [Google Scholar] [CrossRef]
- Qiu, Q.; Guo, G.; Guo, X.; Hu, X.; Yu, T.; Liu, G.; Zhang, H.; Chen, Y.; She, J. P53 Deficiency Accelerate Esophageal Epithelium Intestinal Metaplasia Malignancy. Biomedicines 2023, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Spechler, S.J. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J. Clin. 2005, 55, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Spechler, S.J. Oesophagus: A new candidate for the progenitor cell of Barrett metaplasia. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J. Gastroenterol. 2017, 52, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F. From Reflux Esophagitis to Esophageal Adenocarcinoma. Dig. Dis. 2016, 34, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F. The role of acid and bile reflux in oesophagitis and Barrett’s metaplasia. Biochem. Soc. Trans. 2010, 38, 348–352. [Google Scholar] [CrossRef]
- Feagins, L.A.; Zhang, H.Y.; Zhang, X.; Hormi-Carver, K.; Thomas, T.; Terada, L.S.; Spechler, S.J.; Souza, R.F. Mechanisms of oxidant production in esophageal squamous cell and Barrett’s cell lines. Am. J. Physiol. Gastrointest. Liver. Physiol. 2008, 294, G411–G417. [Google Scholar] [CrossRef]
- Ling, F.C.; Khochfar, J.; Baldus, S.E.; Brabender, J.; Drebber, U.; Bollschweiler, E.; Hoelscher, A.H.; Schneider, P.M. HIF-1alpha protein expression is associated with the environmental inflammatory reaction in Barrett’s metaplasia. Dis. Esophagus 2009, 22, 694–699. [Google Scholar] [CrossRef]
- Kazumori, H.; Ishihara, S.; Rumi, M.A.; Kadowaki, Y.; Kinoshita, Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett’s epithelium. Gut 2006, 55, 16–25. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, H.Y.; Zhang, X.I.; Lynch, J.P.; Strauch, E.D.; Wang, J.Y.; Melton, S.D.; Genta, R.M.; Wang, D.H.; Spechler, S.J.; et al. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett’s esophagus. Gastroenterology 2010, 139, 194–203.e1. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Pich, O.; Devonshire, G.; Zamani, S.A.; Katz-Summercorn, A.; Killcoyne, S.; Cheah, C.; Nutzinger, B.; Grehan, N.; Lopez-Bigas, N.; et al. Mutational signature dynamics shaping the evolution of oesophageal adenocarcinoma. Nat. Commun. 2023, 14, 4239. [Google Scholar] [CrossRef]
- Grillo, F.; Fassan, M.; Ceccaroli, C.; Giacometti, C.; Curto, M.; Zagonel, V.; Ceppa, P.; Nitti, D.; Castoro, C.; Fiocca, R.; et al. The Reliability of Endoscopic Biopsies in Assessing HER2 Status in Gastric and Gastroesophageal Junction Cancer: A Study Comparing Biopsies with Surgical Samples. Transl. Oncol. 2013, 6, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Fassan, M.; Brignola, S.; Pennelli, G.; Alberti, G.; Angerilli, V.; Bressan, A.; Pellino, A.; Lanza, C.; Salmaso, R.; Lonardi, S.; et al. PD-L1 expression in gastroesophageal dysplastic lesions. Virchows. Arch. 2020, 477, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Businello, G.; Angerilli, V.; Lonardi, S.; Bergamo, F.; Valmasoni, M.; Farinati, F.; Savarino, E.; Spolverato, G.; Fassan, M. Current molecular biomarkers evaluation in gastric/gastroesophageal junction adenocarcinoma: Pathologist does matter. Updates Surg. 2023, 75, 291–303. [Google Scholar] [CrossRef]
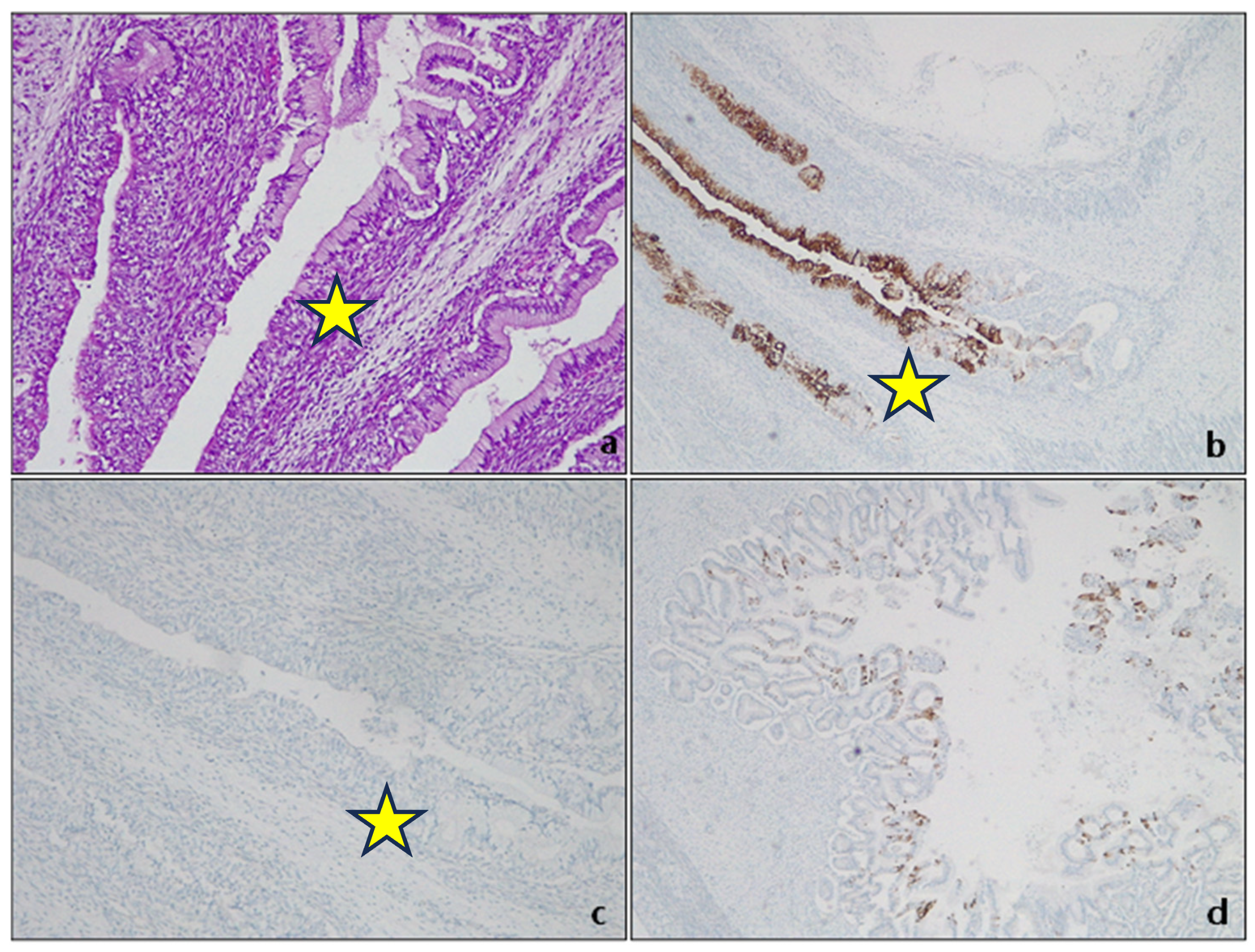
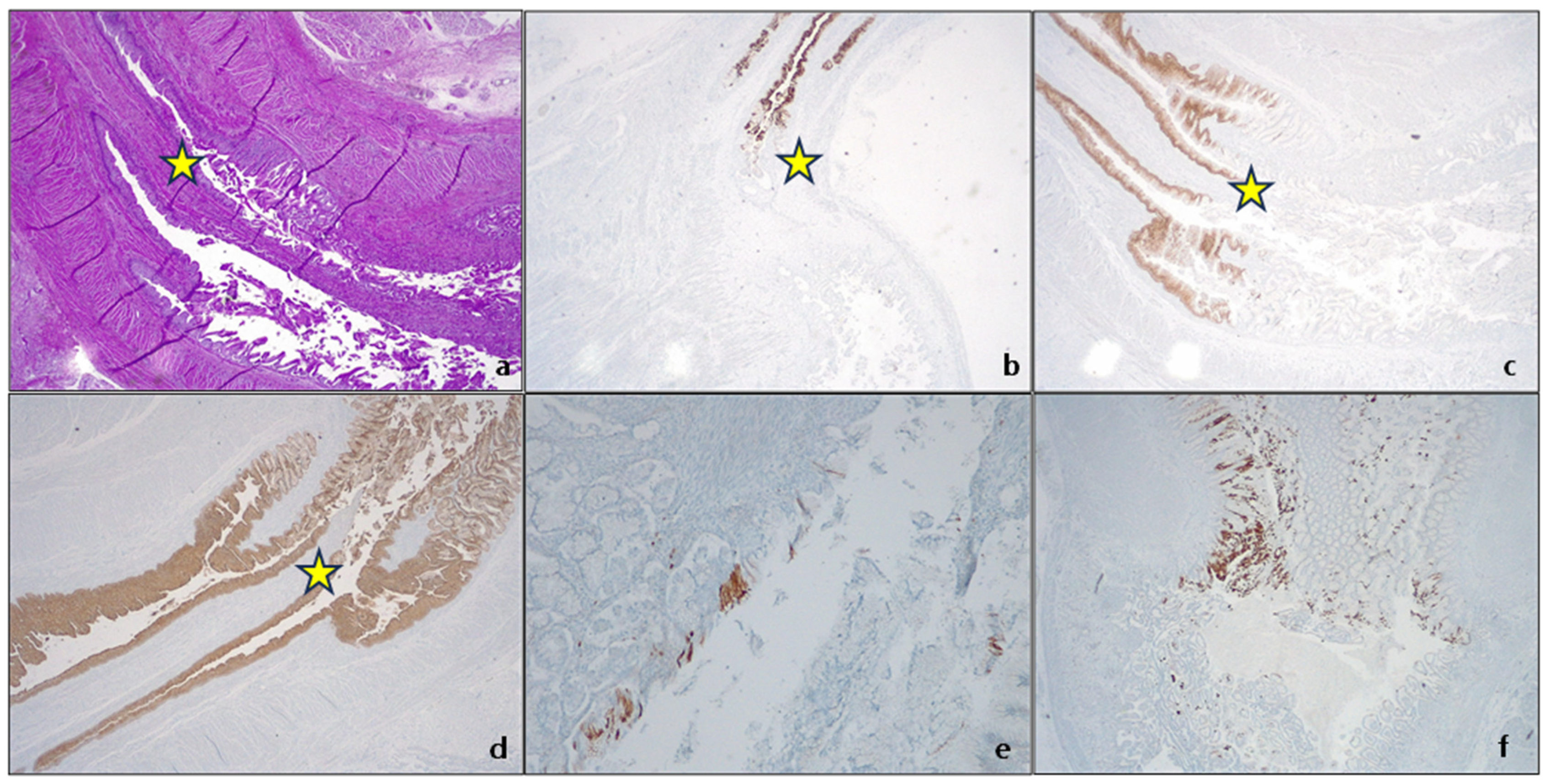

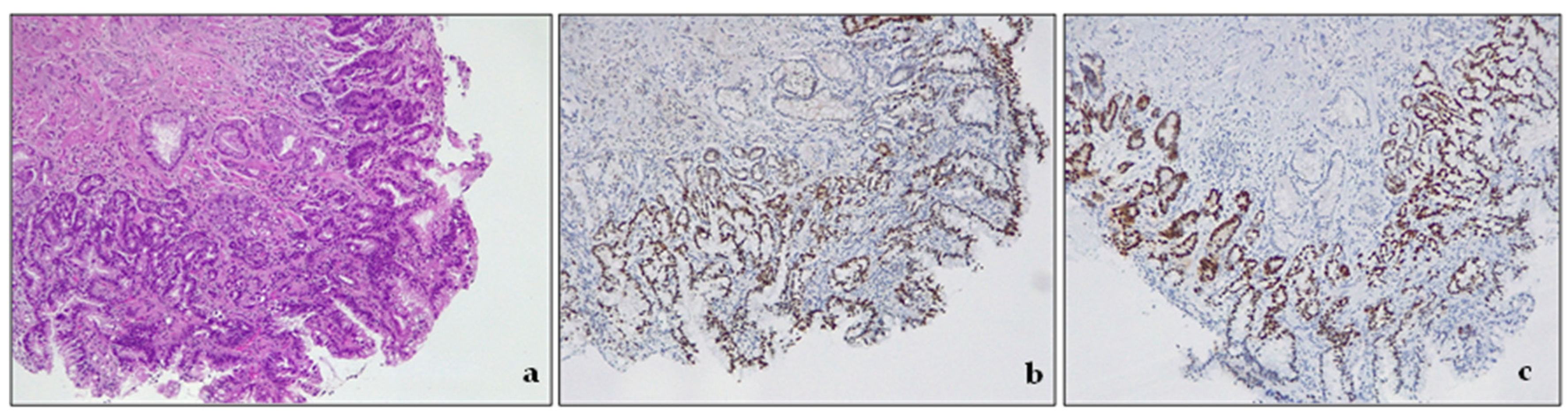
| Society | Length Criteria | Landmark | Intestinal Metaplasia |
|---|---|---|---|
| ASGE | Any | PMGF | Required |
| ACG | ≥1 cm | PMGF | Required |
| AGA | Any | PMGF | Required |
| ESGE | ≥1 cm | PMGF | Required |
| BSG | ≥1 cm | PMGF | Not Required |
| APAGE | ≥1 cm | PMGF | Not Required |
| JES | Any | DEPV | Not Required |
| Vienna | Reid |
|---|---|
| Negative for neoplasia/dysplasia | Negative for dysplasia |
| Indefinite for neoplasia/dysplasia | Indefinite for dysplasia |
| Non-invasive low-grade neoplasia (low-grade adenoma/dysplasia) | Low-grade dysplasia |
| Non-invasive high-grade neoplasia | High-grade dysplasia |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacometti, C.; Gusella, A.; Cassaro, M. Gastro-Esophageal Junction Precancerosis: Histological Diagnostic Approach and Pathogenetic Insights. Cancers 2023, 15, 5725. https://doi.org/10.3390/cancers15245725
Giacometti C, Gusella A, Cassaro M. Gastro-Esophageal Junction Precancerosis: Histological Diagnostic Approach and Pathogenetic Insights. Cancers. 2023; 15(24):5725. https://doi.org/10.3390/cancers15245725
Chicago/Turabian StyleGiacometti, Cinzia, Anna Gusella, and Mauro Cassaro. 2023. "Gastro-Esophageal Junction Precancerosis: Histological Diagnostic Approach and Pathogenetic Insights" Cancers 15, no. 24: 5725. https://doi.org/10.3390/cancers15245725
APA StyleGiacometti, C., Gusella, A., & Cassaro, M. (2023). Gastro-Esophageal Junction Precancerosis: Histological Diagnostic Approach and Pathogenetic Insights. Cancers, 15(24), 5725. https://doi.org/10.3390/cancers15245725







