A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Predictive Tools of Long-Term Prognosis in Gastric Cancer Patients
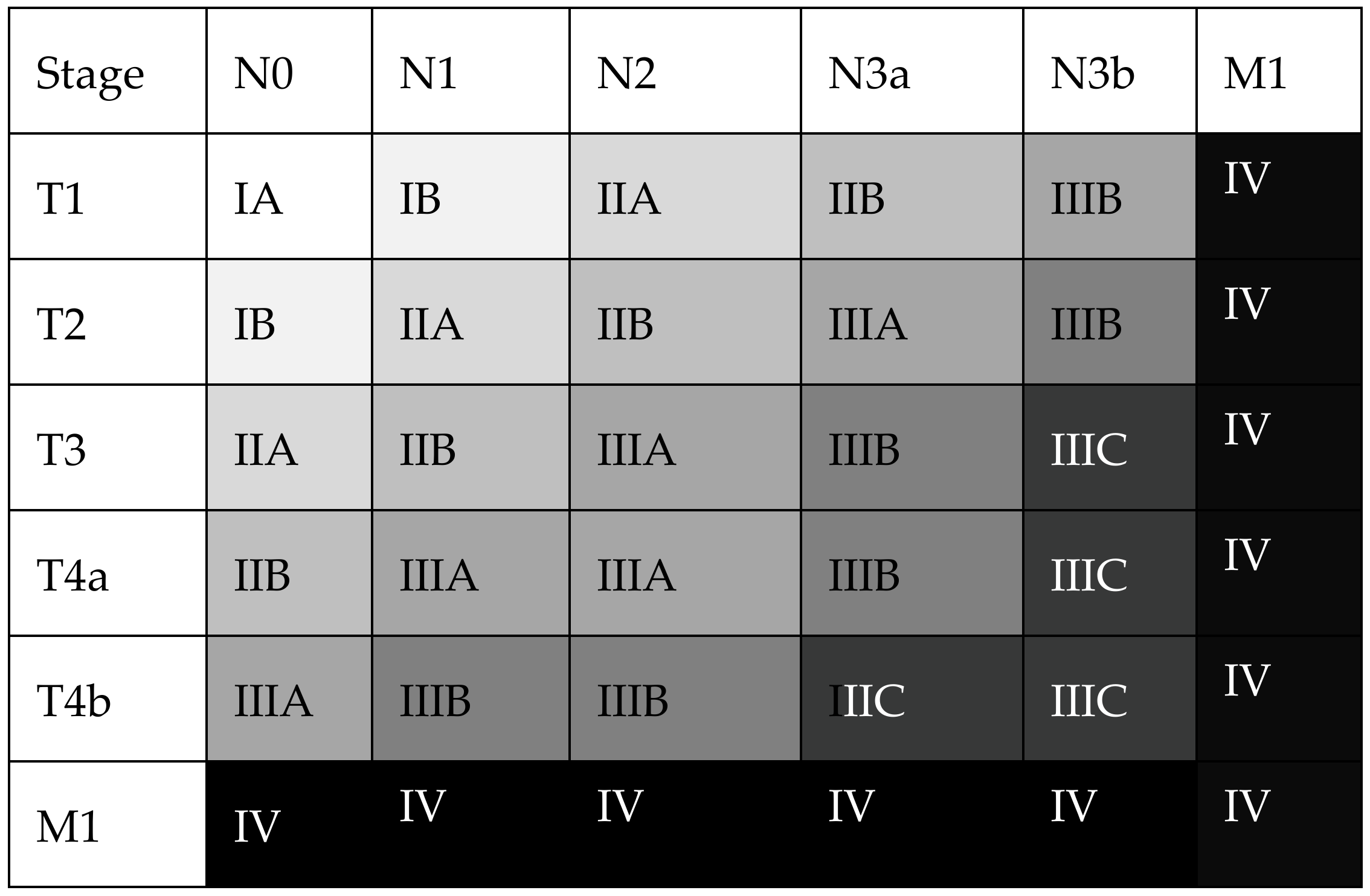
3.1.1. The TNM/UICC Staging System
3.1.2. Alternative Prognostic Tools beyond the TNM
3.2. Biological Features of Gastric Cancer and Their Impact on Long-Term Prognosis
3.2.1. Ethnicity
3.2.2. Sex-Related Differences
3.2.3. Histological Subtype
3.2.4. Tumor Micro-Environment, Genetic Phenotypes
3.3. Improvement of Gastric Cancer Prognosis over Time; Evolution of Treatment Strategies for Resectable Disease
3.3.1. Surgery
3.3.2. Systemic Chemotherapy
3.3.3. External Beam Radiation
3.3.4. Advanced Disease, Peritoneal Carcinomatosis
3.3.5. Targeted Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GLOBOCAN Cancer Data. The Global Cancer Observatory. 2018. Available online: http://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 15 January 2023).
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N.; et al. The Global, Regional, and National burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- GLOBOCAN Cancer Data. The Global Cancer Observatory. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 15 January 2023).
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Obermannova, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, D.; Yang, S.; Zhang, G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 801228. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008, 19, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.I.; Lim, D.H.; Lee, J.; Kang, W.K.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kim, S.T.; Lee, S.J.; et al. Comparison of the 7th and the 8th AJCC Staging System for Non-metastatic D2-Resected Lymph Node-Positive Gastric Cancer Treated with Different Adjuvant Protocols. Cancer Res. Treat. 2019, 51, 876–885. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.; Sano, T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- Graziosi, L.; Marino, E.; Donini, A. Survival comparison in gastric cancer patients between 7th and 8th edition of the AJCC TNM staging system: The first western single center experience. Eur. J. Surg. Oncol. 2019, 45, 1105–1108. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, Z.F.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; Huang, C.M.; et al. Is the 8th Edition of the AJCC TNM Staging System Sufficiently Reasonable for All Patients with Noncardia Gastric Cancer? A 12,549-Patient International Database Study. Ann. Surg. Oncol. 2018, 25, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, Z.F.; Wang, W.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. A novel TNM staging system for gastric cancer based on the metro-ticket paradigm: A comparative study with the AJCC-TNM staging system. Gastric Cancer 2019, 22, 759–768. [Google Scholar] [CrossRef]
- Park, H.S.; Lloyd, S.; Decker, R.H.; Wilson, L.D.; Yu, J.B. Overview of the Surveillance, Epidemiology, and End Results database: Evolution, data variables, and quality assurance. Curr. Probl. Cancer 2012, 36, 183–190. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Q.; Xue, N.; Wu, M.; Liang, Y.; Xu, Q.; Feng, L.; Xing, S.; Chen, S. A Nomogram Based on Aspartate Aminotransferase/Alanine Aminotransferase (AST/ALT) Ratio to Predict Prognosis After Surgery in Gastric Cancer Patients. Cancer Control 2020, 27, 1073274820954458. [Google Scholar] [CrossRef]
- Wang, T.; Wen, W.; Liu, H.; Zhang, J.; Zhang, X.; Wang, Y. Development and Validation of a Novel Prognosis Prediction Model for Patients with Stomach Adenocarcinoma. Front. Med. 2021, 8, 793401. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Zheng, Z.; Wen, Y.; Shi, L.; Xu, S.; Wang, X.; Zhou, Y.; Fu, B.; Li, X.; Deng, Z.; et al. Construction and validation of a TP53-associated immune prognostic model for gastric cancer. Genomics 2020, 112, 4788–4795. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ji, X.; Jin, C.; Ji, K.; Jia, Z.; Wu, X.; Zhang, J.; Bu, Z. A Practical Nomogram for Predicting the Prognosis of Elderly Patients with Gastric Adenocarcinoma After Gastrectomy. Int. J. Gen. Med. 2022, 15, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; An, J.; Wang, S.; Dong, X.; Zhao, H. A Prognostic Model for Patients with Gastric Signet Ring Cell Carcinoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211027912. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yoshikawa, T.; Taguri, M.; Hayashi, T.; Aoyama, T.; Sue-Ling, H.M.; Bonam, K.; Hayden, J.D.; Grabsch, H.I. The survival difference between gastric cancer patients from the UK and Japan remains after weighted propensity score analysis considering all background factors. Gastric Cancer 2016, 19, 479–489. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Strong, V.E.; Song, K.Y.; Park, C.H.; Jacks, L.M.; Gonen, M.; Shah, M.; Coit, D.G.; Brennan, M. F Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann. Surg. 2010, 251, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.R.; Karthikesalingam, A.; Jackson, D.; Hanna, G.B. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: Comparison between West and East. Ann. Surg. Oncol. 2013, 20, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Bertagnolli, M.M. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: Results from the Surveillance Epidemiology and End Results (SEER) database. Ann. Surg. Oncol. 2015, 22, 2965–2971. [Google Scholar] [CrossRef]
- Tee, M.C.; Pirozzi, N.; Brahmbhatt, R.D.; Raman, S.; Franko, J. Oncologic and surgical outcomes for gastric cancer patients undergoing gastrectomy differ by race in the United States. Eur. J. Surg. Oncol. 2020, 46 10 Pt A, 1941–1947. [Google Scholar] [CrossRef]
- Wagner, A.D.; Oertelt-Prigione, S.; Adjei, A.; Buclin, T.; Cristina, V.; Csajka, C.; Coukos, G.; Dafni, U.; Dotto, G.P.; Ducreux, M.; et al. Gender medicine and oncology: Report and consensus of an ESMO workshop. Ann. Oncol. 2019, 30, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Wagner, A.D.; Kouvelakis, K.; Nanji, H.; Starling, N.; Chau, I.; Watkins, D.; Rao, S.; Peckitt, C.; Cunningham, D. Influence of sex on chemotherapy efficacy and toxicity in oesophagogastric cancer: A pooled analysis of four randomised trials. Eur. J. Cancer 2019, 121, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kalff, M.C.; Wagner, A.D.; Verhoeven, R.H.; Lemmens, V.E.; Van Laarhoven, H.W.; Gisbertz, S.S.; Van Berge Henegouwen, M.I.; Dutch Upper GI Cancer Audit Group. Sex differences in tumor characteristics, treatment, and outcomes of gastric and esophageal cancer surgery: Nationwide cohort data from the Dutch Upper GI Cancer Audit. Gastric Cancer 2022, 25, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Ni, M.; Zhu, H.; Cao, J.; Zhou, L.; Shen, S.; Peng, C.; Lv, Y.; Xu, G.; Wang, L.; et al. Differential prognostic implications of gastric adenocarcinoma based on Lauren’s classification: A Surveillance, Epidemiology, and End Results (SEER)-based cohort study. Ann. Transl. Med. 2021, 9, 646. [Google Scholar] [CrossRef]
- Taghavi, S.; Jayarajan, S.N.; Davey, A.; Willis, A.I. Prognostic significance of signet ring gastric cancer. J. Clin. Oncol. 2012, 30, 3493–3498. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Zhang, W.H.; Chen, X.Z.; Liu, K.; Yang, K.; Chen, X.L.; Zhao, L.Y.; Chen, Z.X.; Zhou, Z.G.; et al. Difference Between Signet Ring Cell Gastric Cancers and Non-Signet Ring Cell Gastric Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 618477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lv, W.; Zhang, J.; Zhang, J.; Huang, B.; Lin, J. Different prognostic significance of signet ring cell histology for early and advanced gastric cancer patients: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; Arnaud, J.P.; Balon, J.M.; Bonnetain, F.; Borie, F.; et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: A multicenter comparative study. Ann. Surg. 2011, 254, 684–693. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Sohn, B.H.; Hwang, J.E.; Jang, H.J.; Lee, H.S.; Oh, S.C.; Shim, J.J.; Lee, K.W.; Kim, E.H.; Yim, S.Y.; Lee, S.H.; et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin. Cancer Res. 2017, 23, 4441–4449. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability as a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Steinberg, M.L.; Hwang, B.J.; Tang, L.; Shah, M.A. E-cadherin gene alterations in gastric cancers in different ethnic populations. Ethn. Dis. 2008, 18 (Suppl. 2), 70–74. [Google Scholar]
- Park, D.J.; Seo, A.N.; Yoon, C.; Ku, G.Y.; Coit, D.G.; Strong, V.E.; Suh, Y.S.; Lee, H.S.; Yang, H.K.; Kim, H.H.; et al. Serum VEGF-A and Tumor Vessel VEGFR-2 Levels Predict Survival in Caucasian but Not Asian Patients Undergoing Resection for Gastric Adenocarcinoma. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Wanebo, H.J.; Kennedy, B.J.; Chmiel, J.; Steele, G., Jr.; Winchester, D.; Osteen, R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann. Surg. 1993, 218, 583–592. [Google Scholar] [CrossRef]
- Bria, E.; De Manzoni, G.; Beghelli, S.; Tomezzoli, A.; Barbi, S.; Di Gregorio, C.; Scardoni, M.; Amato, E.; Frizziero, M.; Sperduti, I.; et al. A clinical-biological risk stratification model for resected gastric cancer: Prognostic impact of Her2, Fhit, and APC expression status. Ann. Oncol. 2013, 24, 693–701. [Google Scholar] [CrossRef]
- Mariette, C.; Castel, B.; Balon, J.M.; Van Seuningen, I.; Triboulet, J.P. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur. J. Surg. Oncol. 2003, 29, 588–593. [Google Scholar] [CrossRef]
- Postlewait, L.M.; Squires, M.H., 3rd; Kooby, D.A.; Poultsides, G.A.; Weber, S.M.; Bloomston, M.; Fields, R.C.; Pawlik, T.M.; Votanopoulos, K.I.; Schmidt, C.R.; et al. The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: A multi-institutional study of the US Gastric Cancer Collaborative. J. Surg. Oncol. 2015, 112, 203–207. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; Van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; McCulloch, P.; Kazi, H.; Gama-Rodrigues, J.J.; Yuan, Y.; Nitti, D. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst. Rev. 2015, 8, CD001964. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Moore, J.L.; Kumar, S.; Santaolalla, A.; Patel, P.H.; Kapiris, M.; Van Hemelrijck, M.; Maisey, N.; Hill, M.; Lagergren, J.; Gossage, J.A.; et al. Effect of peri-operative chemotherapy regimen on survival in the treatment of locally advanced oesophago-gastric adenocarcinoma—A comparison of the FLOT and ‘MAGIC’ regimens. Eur. J. Cancer 2022, 163, 180–188. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef]
- Thomassen, I.; Van Gestel, Y.R.; Van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; De Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef]
- Alyami, M.; Hubner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Khomyakov, V.; Ryabov, A.; Ivanov, A.; Bolotina, L.; Utkina, A.; Volchenko, N.; Kaprin, A. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: An open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 2016, 1, 159–166. [Google Scholar] [CrossRef]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Hubner, M.; Teixeira Farinha, H.; Grass, F.; Wolfer, A.; Mathevet, P.; Hahnloser, D.; Demartines, N. Feasibility and Safety of Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 6852749. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mei, Q.; Wang, Z.; Zhao, L.; Zhang, D.; Liao, D.; Zuo, J.; Xie, H.; Jia, Y.; Kong, F. Research Progress of Antibody-Drug Conjugate Therapy for Advanced Gastric Cancer. Front. Oncol. 2022, 12, 889017. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Coit, D.G.; Kim, H.H.; Roviello, F.; Kassab, P.; Wittekind, C.; Yamamoto, Y.; Ohashi, Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017, 20, 217–225. [Google Scholar] [CrossRef] [PubMed]


| Tumor Stage (8th TNM ed., [8]) | American NCDB Registry [10] | 5-Year OS (%) | Median Survival (95%CI) | European Monocentric Registry [11] | 5-Year OS (%) | American SEER Registry [12] | 5-Year OS (%) | Asian Monocentric Registry [13] | 5-Year OS (%) |
|---|---|---|---|---|---|---|---|---|---|
| IA | 1501 | 81.0 | 129.8 (129.8–133) | 2170 | 77.5 | 618 | 94.8 | ||
| IB | 1095 | 68.5 | 112.8 (100.0-NA) | 74 | 69.8 | 1065 | 63.3 | 313 | 89 |
| IIA | 1245 | 59.3 | 91.6 (79.1–103.1) | 1241 | 50.8 | 345 | 84.6 | ||
| IIB | 1432 | 46.4 | 50.5 (46.6–58.2) | 60 | 51.6 | 1404 | 35.3 | 455 | 76.1 |
| IIIA | 2310 | 30.5 | 25.0 (23.3–26.9) | 30 | 25.9 | 2113 | 20.5 | 791 | 60.3 |
| IIIB | 1896 | 20.1 | 17.4 (16.4–18.7) | 32 | 32.7 | 1466 | 13.5 | 920 | 40.9 |
| IIIC | 1067 | 8.3 | 11.8 (10.9–12.7) | 28 | 9.8 | 735 | 5.3 | 825 | 27.5 |
| IV | 1449 | 5.6 | 8.9 (8.3–9.7) | 17 | 4.5 | 0 | NA | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantziari, S.; St Amour, P.; Abboretti, F.; Teixeira-Farinha, H.; Gaspar Figueiredo, S.; Gronnier, C.; Schizas, D.; Demartines, N.; Schäfer, M. A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma. Cancers 2023, 15, 1628. https://doi.org/10.3390/cancers15051628
Mantziari S, St Amour P, Abboretti F, Teixeira-Farinha H, Gaspar Figueiredo S, Gronnier C, Schizas D, Demartines N, Schäfer M. A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma. Cancers. 2023; 15(5):1628. https://doi.org/10.3390/cancers15051628
Chicago/Turabian StyleMantziari, Styliani, Penelope St Amour, Francesco Abboretti, Hugo Teixeira-Farinha, Sergio Gaspar Figueiredo, Caroline Gronnier, Dimitrios Schizas, Nicolas Demartines, and Markus Schäfer. 2023. "A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma" Cancers 15, no. 5: 1628. https://doi.org/10.3390/cancers15051628
APA StyleMantziari, S., St Amour, P., Abboretti, F., Teixeira-Farinha, H., Gaspar Figueiredo, S., Gronnier, C., Schizas, D., Demartines, N., & Schäfer, M. (2023). A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma. Cancers, 15(5), 1628. https://doi.org/10.3390/cancers15051628







