How to Manage Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnostic Barriers in LMIC
3. Genetic Evaluation of Ph+ ALL
4. Frontline Induction in Ph+ ALL
5. Availability of TKIs
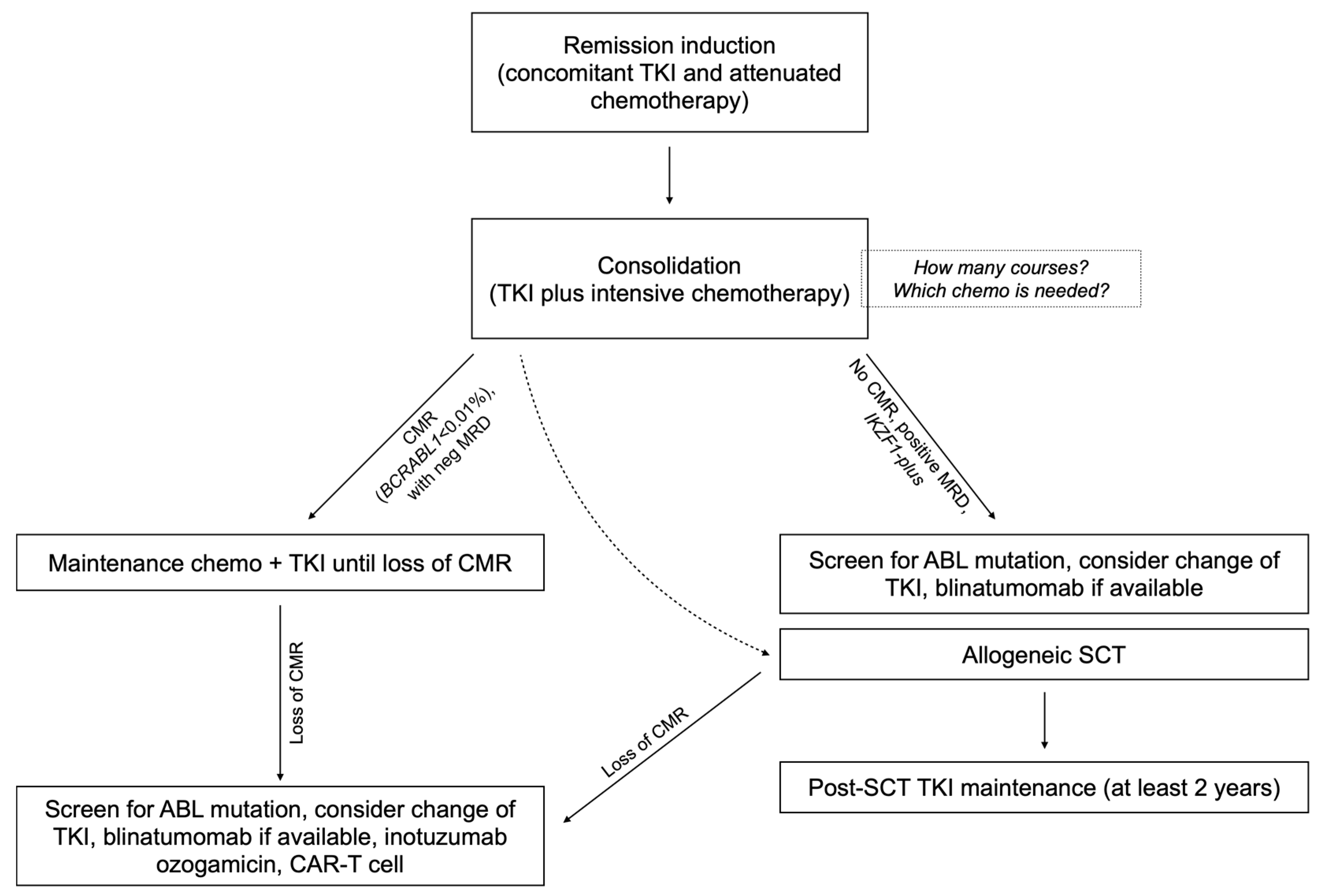
6. Consolidation and Maintenance Therapy
7. MRD Monitoring in Ph+ ALL
8. ABL Mutations and Management of TKI
9. CNS Prophylaxis
10. Allogeneic Stem-Cell Transplantation and Alternative Approaches
11. Conclusions
12. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Foà, R.; Chiaretti, S. Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2022, 386, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Gleibetaner, B.; Gleißner, B.; Gökbuget, N.; Bartram, C.R.; Janssen, B.; Rieder, H.; Janssen, J.W.G.; Fonatsch, C.; Heyll, A.; Voliotis, D.; et al. Leading Prognostic Relevance of the BCR-ABL Translocation in Adult Acute B-Lineage Lymphoblastic Leukemia: A Prospective Study of the German Multicenter Trial Group and Confirmed Polymerase Chain Reaction Analysis. Blood 2002, 99, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.-M.; Oriol, A.; González, M.; Vidriales, B.; Brunet, S.; Esteve, J.; del Potro, E.; Rivas, C.; Moreno, M.-J.; Tormo, M.; et al. Concurrent Intensive Chemotherapy and Imatinib before and after Stem Cell Transplantation in Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Final Results of the CSTIBES02 Trial. Haematologica 2010, 95, 87–95. [Google Scholar] [CrossRef]
- Bleckmann, K.; Schrappe, M. Advances in Therapy for Philadelphia-Positive Acute Lymphoblastic Leukaemia of Childhood and Adolescence. Br. J. Haematol. 2016, 172, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Thomas, D.; Ravandi, F.; Cortes, J.; Garris, R.; Jabbour, E.; Garcia-Manero, G.; Borthakur, G.; Kadia, T.; Rytting, M.; et al. Final Report of a Phase II Study of Imatinib Mesylate with Hyper-CVAD for the Front-Line Treatment of Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Haematologica 2015, 100, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults With Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia—From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens. JAMA Oncol. 2022, 8, 1340–1348. [Google Scholar] [CrossRef]
- Cazzaniga, G.; De Lorenzo, P.; Alten, J.; Röttgers, S.; Hancock, J.; Saha, V.; Castor, A.; Madsen, H.O.; Gandemer, V.; Cavé, H.; et al. Predictive Value of Minimal Residual Disease in Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Imatinib in the European Intergroup Study of Post-Induction Treatment of Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia, Based on Immunoglobulin/T-Cell Receptor and BCR/ABL1 Methodologies. Haematologica 2018, 103, 107–115. [Google Scholar] [CrossRef]
- Short, N.J.; Jabbour, E.; Macaron, W.; Ravandi, F.; Jain, N.; Kanagal-Shamanna, R.; Patel, K.P.; Loghavi, S.; Haddad, F.G.; Yilmaz, M.; et al. Ultrasensitive NGS MRD Assessment in Ph+ ALL: Prognostic Impact and Correlation with RT-PCR for BCR::ABL1. Am. J. Hematol. 2023, 98, 1196–1203. [Google Scholar] [CrossRef]
- Ribera, J.-M.; Chiaretti, S. Modern Management Options for Ph+ ALL. Cancers 2022, 14, 4554. [Google Scholar] [CrossRef]
- Short, N.J.; Jabbour, E.; Jain, N.; Macaron, W.; Huang, X.; Montalban-Bravo, G.; Kadia, T.M.; Daver, N.G.; Haddad, F.; Zoghbi, M.; et al. A Phase II Trial of Ponatinib and Blinatumomab in Adults with Newly Diagnosed Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia (Ph+ ALL). J. Clin. Oncol. 2023, 41, e19013. [Google Scholar] [CrossRef]
- Jabbour, E.; Short, N.J.; Jain, N.; Huang, X.; Montalban-Bravo, G.; Banerjee, P.; Rezvani, K.; Jiang, X.; Kim, K.H.; Kanagal-Shamanna, R.; et al. Ponatinib and Blinatumomab for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia: A US, Single-Centre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2023, 10, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.F.; Silverio, A.; Duarte, B.K.L.; Aguiar, T.F.; Bendlin, R.M.; Massaut, I.H.B.; Pagnano, K.B.B.; Velloso, E.D.R.P.; Rocha, V.; Rego, E.M. Philadelphia-Positive B-Lymphoblastic Leukemia in a Middle-Income Country—A Real-World Multicenter Cohort. Leuk. Res. 2021, 110, 106666. [Google Scholar] [CrossRef] [PubMed]
- Fernandes da Silva Junior, W.; Medina, A.B.; Yamakawa, P.E.; Buccheri, V.; Velloso, E.D.R.P.; Rocha, V. Treating Adult Acute Lymphoblastic Leukemia in Brazil—Increased Early Mortality Using a German Multicenter Acute Lymphoblastic Leukemia-Based Regimen. Clin. Lymphoma Myeloma Leuk. 2018, 18, e255–e259. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Huante, E.; Espinosa-Bautista, K.; Rangel-Patiño, J.; Demichelis-Gómez, R. Comparison of Two Pediatric-Inspired Regimens to Hyper-CVAD in Hispanic Adolescents and Young Adults With Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, 55–62.e2. [Google Scholar] [CrossRef]
- Bajel, A.; George, B.; Mathews, V.; Viswabandya, A.; Kavitha, M.L.; Srivastava, A.; Chandy, M. Adult ALL: Treatment Outcome and Prognostic Factors in an Indian Population Using a Modified German ALL (GMALL) Protocol. Leukemia 2007, 21, 2230–2233. [Google Scholar] [CrossRef]
- Silva, W.F.; Amano, M.T.; Perruso, L.L.; Cordeiro, M.G.; Kishimoto, R.K.; de Medeiros Leal, A.; Nardinelli, L.; Bendit, I.; Velloso, E.D.; Rego, E.M.; et al. Adult Acute Lymphoblastic Leukemia in a Resource-Constrained Setting: Outcomes after Expansion of Genetic Evaluation. Hematology 2022, 27, 396–403. [Google Scholar] [CrossRef]
- Hoelzer, D.; Bassan, R.; Dombret, H.; Fielding, A.; Ribera, J.-M.; Buske, C. Acute Lymphoblastic Leukaemia in Adult Patients: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v69–v82. [Google Scholar] [CrossRef]
- Cox, M.C.; Maffei, L.; Buffolino, S.; Del Poeta, G.; Venditti, A.; Cantonetti, M.; Aronica, G.; Aquilina, P.; Masi, M.; Amadori, S. A Comparative Analysis of FISH, RT-PCR, and Cytogenetics for the Diagnosis of Bcr-Abl Positive Leukemias. Am. J. Clin. Pathol. 1998, 109, 24–31. [Google Scholar] [CrossRef]
- Brown, P.A.; Shah, B.; Advani, A.; Aoun, P.; Boyer, M.W.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1079–1109. [Google Scholar] [CrossRef]
- Aguiar, T.F.; da Conceição Barbosa, T.; Maciel, A.L.T.; Blunck, C.B.; Sellos-Laclette, J.; de Melo, A.C.; Mansur, M.B.; Emerenciano, M. Outcome of Adolescents and Young Adults with Acute Lymphoblastic Leukemia in a Single Center in Brazil. Hematol. Transfus. Cell Ther. 2023, 45, S108–S112. [Google Scholar] [CrossRef]
- Crespo-Solis, E.; Espinosa-Bautista, K.; Alvarado-Ibarra, M.; Rozen-Fuller, E.; Pérez-Rocha, F.; Nava-Gómez, C.; Ortiz-Zepeda, M.; Álvarez-Vera, J.L.; Ramos-Peñafiel, C.O.; Meillón-García, L.A.; et al. Survival Analysis of Adult Patients with ALL in Mexico City: First Report from the Acute Leukemia Workgroup (ALWG) (GTLA). Cancer Med. 2018, 7, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, B.; Pfeifer, H.; Goekbuget, N.; Beelen, D.W.; Beck, J.; Stelljes, M.; Bornhäuser, M.; Reichle, A.; Perz, J.; Haas, R.; et al. Alternating versus Concurrent Schedules of Imatinib and Chemotherapy as Front-Line Therapy for Philadelphia-Positive Acute Lymphoblastic Leukemia (Ph+ALL). Blood 2006, 108, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.K.; Rowe, J.M.; Buck, G.; Foroni, L.; Gerrard, G.; Litzow, M.R.; Lazarus, H.; Luger, S.M.; Marks, D.I.; McMillan, A.K.; et al. UKALLXII/ECOG2993: Addition of Imatinib to a Standard Treatment Regimen Enhances Long-Term Outcomes in Philadelphia Positive Acute Lymphoblastic Leukemia. Blood 2014, 123, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Gu, J.; Kanagal-Shamanna, R.; Tang, Z.; Patel, K.P.; Yao, H.; Fang, L.; Bao, H.-Y.; Liu, C.-H.; Lin, P.; et al. Clinical Implications of Cytogenetic Heterogeneity in Philadelphia Chromosome Positive (Ph+) Adult B Cell Acute Lymphoblastic Leukemia Following Tyrosine Kinase Inhibitors and Chemotherapy Regimens. Leuk. Res. 2019, 84, 106176. [Google Scholar] [CrossRef]
- Heerema, N.A.; Harbott, J.; Galimberti, S.; Camitta, B.M.; Gaynon, P.S.; Janka-Schaub, G.; Kamps, W.; Basso, G.; Pui, C.-H.; Schrappe, M.; et al. Secondary Cytogenetic Aberrations in Childhood Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Are Nonrandom and May Be Associated with Outcome. Leukemia 2004, 18, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, M.; Dodge, R.K.; Mrózek, K.; Stewart, C.C.; Carroll, A.J.; Tantravahi, R.; Vardiman, J.W.; Larson, R.A.; Bloomfield, C.D. Additional Cytogenetic Abnormalities in Adults with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia: A Study of the Cancer and Leukaemia Group B. Br. J. Haematol. 2004, 124, 275–288. [Google Scholar] [CrossRef]
- Aldoss, I.; Stiller, T.; Cao, T.M.; Palmer, J.M.; Thomas, S.H.; Forman, S.J.; Pullarkat, V. Impact of Additional Cytogenetic Abnormalities in Adults with Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1326–1329. [Google Scholar] [CrossRef]
- Akahoshi, Y.; Mizuta, S.; Shimizu, H.; Uchida, N.; Fukuda, T.; Kanamori, H.; Onizuka, M.; Ozawa, Y.; Ohashi, K.; Ohta, S.; et al. Additional Cytogenetic Abnormalities with Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia on Allogeneic Stem Cell Transplantation in the Tyrosine Kinase Inhibitor Era. Biol. Blood Marrow Transplant. 2018, 24, 2009–2016. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.-H.; et al. BCR–ABL1 Lymphoblastic Leukaemia Is Characterized by the Deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef]
- van der Veer, A.; Zaliova, M.; Mottadelli, F.; De Lorenzo, P.; te Kronnie, G.; Harrison, C.J.; Cavé, H.; Trka, J.; Saha, V.; Schrappe, M.; et al. IKZF1 Status as a Prognostic Feature in BCR-ABL1–Positive Childhood ALL. Blood 2014, 123, 1691–1698. [Google Scholar] [CrossRef]
- Deboer, R.; Koval, G.; Mulkey, F.; Wetzler, M.; Devine, S.; Marcucci, G.; Stone, R.M.; Larson, R.A.; Bloomfield, C.D.; Geyer, S.; et al. Clinical Impact of ABL1 Kinase Domain Mutations and IKZF1 Deletion in Adults under Age 60 with Philadelphia Chromosome-Positive (Ph+) Acute Lymphoblastic Leukemia (ALL): Molecular Analysis of CALGB (Alliance) 10001 and 9665. Leuk. Lymphoma 2016, 57, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Fedullo, A.L.; Messina, M.; Elia, L.; Piciocchi, A.; Gianfelici, V.; Lauretti, A.; Soddu, S.; Puzzolo, M.C.; Minotti, C.; Ferrara, F.; et al. Prognostic Implications of Additional Genomic Lesions in Adult Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Haematologica 2019, 104, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.; Raum, K.; Markovic, S.; Nowak, V.; Fey, S.; Obländer, J.; Pressler, J.; Böhm, V.; Brüggemann, M.; Wunderle, L.; et al. Genomic CDKN2A/2B Deletions in Adult Ph+ ALL Are Adverse despite Allogeneic Stem Cell Transplantation. Blood 2018, 131, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Ansuinelli, M.; Vitale, A.; Elia, L.; Matarazzo, M.; Piciocchi, A.; Fazi, P.; Di Raimondo, F.; Santoro, L.; Fabbiano, F.; et al. A Multicenter Total Therapy Strategy for de Novo Adult Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Patients: Final Results of the GIMEMA LAL1509 Protocol. Haematologica 2021, 106, 1828–1838. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kantarjian, H.M.; Short, N.J.; Wang, F.; Furudate, K.; Uryu, H.; Garris, R.; Jain, N.; Sasaki, K.; Ravandi, F.; et al. Genetic Correlates in Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Hyper-CVAD plus Dasatinib or Ponatinib. Leukemia 2022, 36, 1253–1260. [Google Scholar] [CrossRef]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef]
- Maciel, A.L.T.; Barbosa, T.d.C.; Blunck, C.B.; Wolch, K.; Machado, A.d.A.L.; da Costa, E.S.; Bergier, L.L.; Schramm, M.T.; Ikoma-Coltutato, M.R.V.; Lins, M.M.; et al. IKZF1 Deletions Associate with CRLF2 Overexpression Leading to a Poor Prognosis in B-Cell Precursor Acute Lymphoblastic Leukaemia. Transl. Oncol. 2022, 15, 101291. [Google Scholar] [CrossRef]
- Ravandi, F. How I Treat Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. Blood 2019, 133, 130–136. [Google Scholar] [CrossRef]
- Chalandon, Y.; Thomas, X.; Hayette, S.; Cayuela, J.-M.; Abbal, C.; Huguet, F.; Raffoux, E.; Leguay, T.; Rousselot, P.; Lepretre, S.; et al. Randomized Study of Reduced-Intensity Chemotherapy Combined with Imatinib in Adults with Ph-Positive Acute Lymphoblastic Leukemia. Blood 2015, 125, 3711–3719. [Google Scholar] [CrossRef]
- Frisch, A.; Ofran, Y. How I Diagnose and Manage Philadelphia Chromosome-like Acute Lymphoblastic Leukemia. Haematologica 2019, 104, 2135–2143. [Google Scholar] [CrossRef]
- Kantarjian, H.; Short, N.J.; Jain, N.; Sasaki, K.; Huang, X.; Haddad, F.G.; Khouri, I.; DiNardo, C.D.; Pemmaraju, N.; Wierda, W.; et al. Frontline Combination of Ponatinib and Hyper-CVAD in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: 80-Months Follow-Up Results. Am. J. Hematol. 2023, 98, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Vitale, A.; Vignetti, M.; Piciocchi, A.; Fazi, P.; Elia, L.; Falini, B.; Ronco, F.; Ferrara, F.; De Fabritiis, P.; et al. A Sequential Approach with Imatinib, Chemotherapy and Transplant for Adult Ph+ Acute Lymphoblastic Leukemia: Final Results of the GIMEMA LAL 0904 Study. Haematologica 2016, 101, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Papayannidis, C.; Piciocchi, A.; Robustelli, V.; Soverini, S.; Terragna, C.; Marconi, G.; Lemoli, R.M.; Guolo, F.; Fornaro, A.; et al. INCB84344-201: Ponatinib and Steroids in Frontline Therapy for Unfit Patients with Ph+ Acute Lymphoblastic Leukemia. Blood Adv. 2022, 6, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Jabbour, E.; Sasaki, K.; Patel, K.; O’Brien, S.M.; Cortes, J.E.; Garris, R.; Issa, G.C.; Garcia-manero, G.; Luthra, R.; et al. Impact of Complete Molecular Response on Survival in Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Blood 2016, 128, 504–507. [Google Scholar] [CrossRef]
- Pajares, B.; Torres, E.; Trigo, J.M.; Sáez, M.I.; Ribelles, N.; Jiménez, B.; Alba, E. Tyrosine Kinase Inhibitors and Drug Interactions: A Review with Practical Recommendations. Clin. Transl. Oncol. 2012, 14, 94–101. [Google Scholar] [CrossRef]
- Marks, D.I.; Kirkwood, A.A.; Rowntree, C.J.; Aguiar, M.; Bailey, K.E.; Beaton, B.; Cahalin, P.; Castleton, A.Z.; Clifton-Hadley, L.; Copland, M.; et al. Addition of Four Doses of Rituximab to Standard Induction Chemotherapy in Adult Patients with Precursor B-Cell Acute Lymphoblastic Leukaemia (UKALL14): A Phase 3, Multicentre, Randomised Controlled Trial. Lancet Haematol. 2022, 9, e262–e275. [Google Scholar] [CrossRef]
- Pfeifer, H.; Lang, F.; Fiedler, W.; Stelljes, M.; Schneller, F.; Steffen, B.; Böll, B.; Viardot, A.; Faul, C.; Lars Teichmann, L.; et al. P355: Favorable Outcome of Philadelphia-Positive Acute Lymphoblastic Leukemia with Imatinib, Dose-Reduced Induction Followed by Allogeneic Stem Cell Transplantation—Results from the GMALL Trial 08/13. HemaSphere 2023, 7, e33834d6. [Google Scholar] [CrossRef]
- Rousselot, P.; Coudé, M.M.; Goekbuget, N.; Gambacorti Passerini, C.; Hayette, S.; Cayuela, J.-M.; Huguet, F.; Leguay, T.; Chevallier, P.; Salanoubat, C.; et al. Dasatinib and Low-Intensity Chemotherapy in Elderly Patients with Philadelphia Chromosome-Positive ALL. Blood 2017, 128, 774–782. [Google Scholar] [CrossRef]
- Ottmann, O.G.; Pfeifer, H.; Cayuela, J.-M.; Spiekermann, K.; Jung, W.; Beck, J.; Raffoux, E.; Turlure, P.; Himberlin, C.; Huguet, F.; et al. Nilotinib (Tasigna®) and Low Intensity Chemotherapy for First-Line Treatment of Elderly Patients with BCR-ABL1-Positive Acute Lymphoblastic Leukemia: Final Results of a Prospective Multicenter Trial (EWALL-PH02). Blood 2018, 132, 31. [Google Scholar] [CrossRef]
- Rousselot, P.; Chalandon, Y.; Chevret, S.; Cayuela, J.-M.; Huguet, F.; Chevallier, P.; Graux, C.; Thiebaut-Bertrand, A.; Chantepie, S.; Thomas, X.; et al. The Omission of High-Dose Cytarabine during Consolidation Therapy of Ph-Positive ALL Patients Treated with Nilotinib and Low-Intensity Chemotherapy Results in an Increased Risk of Relapses Despite Non-Inferior Levels of Late BCR-ABL1 MRD Response. First Results of the Randomized Graaph-2014 Study. Blood 2021, 138, 512. [Google Scholar]
- Malhotra, H.; Radich, J.; Garcia-Gonzalez, P. Meeting the Needs of CML Patients in Resource-Poor Countries. Hematology 2019, 2019, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Moye-Holz, D.; Vogler, S. Comparison of Prices and Affordability of Cancer Medicines in 16 Countries in Europe and Latin America. Appl. Health Econ. Health Policy 2022, 20, 67–77. [Google Scholar] [CrossRef]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of Chronic Myeloid Leukemia in 2023—Common Ground and Common Sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as First-Line Treatment for Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Blood 2011, 118, 6521–6528. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R.; Rohatiner, A.Z.; Lerede, T.; Di Bona, E.; Rambaldi, A.; Pogliani, E.; Rossi, G.; Fabris, P.; Morandi, S.; Casula, P.; et al. Role of Early Anthracycline Dose-Intensity According to Expression of Philadelphia Chromosome/BCR–ABL Rearrangements in B-Precursor Adult Acute Lymphoblastic Leukemia. Hematol. J. 2000, 1, 226–234. [Google Scholar] [CrossRef]
- Soverini, S.; Bassan, R.; Lion, T. Treatment and Monitoring of Philadelphia Chromosome-Positive Leukemia Patients: Recent Advances and Remaining Challenges. J. Hematol. Oncol. 2019, 12, 39. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Cai, J.; Yu, J.; Gao, J.; Hu, S.; Zhai, X.; Liang, C.; Ju, X.; Jiang, H.; et al. Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 358–366. [Google Scholar] [CrossRef]
- Sasaki, K.; Jabbour, E.J.; Ravandi, F.; Short, N.J.; Thomas, D.A.; Garcia-Manero, G.; Daver, N.G.; Kadia, T.M.; Konopleva, M.Y.; Jain, N.; et al. Hyper-CVAD plus Ponatinib versus Hyper-CVAD plus Dasatinib as Frontline Therapy for Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Propensity Score Analysis. Cancer 2016, 122, 3650–3656. [Google Scholar] [CrossRef]
- Ravandi, F.; O’Brien, S.M.; Cortes, J.E.; Thomas, D.M.; Garris, R.; Faderl, S.; Burger, J.A.; Rytting, M.E.; Ferrajoli, A.; Wierda, W.G.; et al. Long-Term Follow-up of a Phase 2 Study of Chemotherapy plus Dasatinib for the Initial Treatment of Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Cancer 2015, 121, 4158–4164. [Google Scholar] [CrossRef]
- Ahmed, U.; Ahmed, D.; Awan, M.N.; Ahmad, U.; Ahsan, B.; Iftikhar, R.; Mir, M.A.; Bokhari, S.W. Outcomes of Philadelphia Positive Acute Lymphoblastic Leukemia in Adolescent and Young Adults. Cureus 2022, 14, e32467. [Google Scholar] [CrossRef]
- Rocha, V.; Fatobene, G.; Niederwieser, D. Increasing Access to Allogeneic Hematopoietic Cell Transplant: An International Perspective. Hematology 2021, 2021, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.R.; Jabbour, E.J.; Kantarjian, H.M.; Kadia, T.M. Optimizing the Use of the HyperCVAD Regimen: Clinical Vignettes and Practical Management. Cancer 2020, 126, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Samra, B.; Kantarjian, H.M.; Sasaki, K.; Alotaibi, A.S.; Konopleva, M.; O’Brien, S.; Ferrajoli, A.; Garris, R.; Nunez, C.A.; Kadia, T.M.; et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Outside of Transplant. Acta Haematol. 2021, 144, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Zhou, S.; Higley, H.; Mukundan, L.; Fu, S.; Reaman, G.H.; Wood, B.L.; Kelloff, G.J.; Jessup, J.M.; Radich, J.P. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia. JAMA Oncol. 2017, 3, e170580. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.J.M.; Van Der Velden, V.H.J.; Brüggemann, M.; Orfao, A. Minimal Residual Disease Diagnostics in Acute Lymphoblastic Leukemia: Need for Sensitive, Fast, and Standardized Technologies. Blood 2015, 125, 3996–4009. [Google Scholar] [CrossRef]
- Pfeifer, H.; Cazzaniga, G.; van der Velden, V.H.J.; Cayuela, J.M.; Schäfer, B.; Spinelli, O.; Akiki, S.; Avigad, S.; Bendit, I.; Borg, K.; et al. Standardisation and Consensus Guidelines for Minimal Residual Disease Assessment in Philadelphia-Positive Acute Lymphoblastic Leukemia (Ph + ALL) by Real-Time Quantitative Reverse Transcriptase PCR of E1a2 BCR-ABL1. Leukemia 2019, 33, 1910–1922. [Google Scholar] [CrossRef]
- KIM, R.; Rousselot, P.; Cayuela, J.-M.; Chalandon, Y.; Passet, M.; Thomas, X.; Straetmans, N.; Chevallier, P.; Huguet, F.; Berthon, C.; et al. Frequency and Outcome of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia with BCR-ABL1 Clonal Hematopoiesis after Blast Clearance: Results from the Graaph-2014 Trial. Blood 2021, 138, 3478. [Google Scholar] [CrossRef]
- Webster, J.A.; Luznik, L.; Tsai, H.-L.; Imus, P.H.; DeZern, A.E.; Pratz, K.W.; Levis, M.J.; Gojo, I.; Showel, M.M.; Prince, G.; et al. Allogeneic Transplantation for Ph+ Acute Lymphoblastic Leukemia with Posttransplantation Cyclophosphamide. Blood Adv. 2020, 4, 5078–5088. [Google Scholar] [CrossRef]
- Ikoma-Colturato, M.R.V.; Beltrame, M.P.; Furtado, F.M.; Pimenta, G.; da Costa, E.S.; Azambuja, A.P.; Malvezzi, M.; Yamamoto, M. Minimal Residual Disease Assessment in Acute Lymphoblastic Leukemia by 4-Color Flow Cytometry: Recommendations from the MRD Working Group of the Brazilian Society of Bone Marrow Transplantation. Hematol. Transfus. Cell Ther. 2021, 43, 332–340. [Google Scholar] [CrossRef]
- Patkar, N.; Abu Alex, A.; Bargavi, B.; Ahmed, R.; Abraham, A.; George, B.; Vishwabandya, A.; Srivastava, A.; Mathews, V. Standardizing Minimal Residual Disease by Flow Cytometry for Precursor B Lineage Acute Lymphoblastic Leukemia in a Developing Country. Cytom. Part B Clin. Cytom. 2012, 82B, 252–258. [Google Scholar] [CrossRef]
- Ikoma-Colturato, M.R.V.; Bertolucci, C.M.; Conti-Spilari, J.E.; Oliveira, E.; Simioni, A.J.; Figueredo-Pontes, L.L.; Furtado, F.M.; Alegretti, A.P.; Azambuja, A.P.; Gevert, F.; et al. Multicentric Standardization of Minimal/Measurable Residual Disease in B-cell Precursor Acute Lymphoblastic Leukaemia Using Next-generation Flow Cytometry in a Low/Middle-level Income Country. Br. J. Haematol. 2023, 200, 381–384. [Google Scholar] [CrossRef]
- Short, N.J.; Jabbour, E. Minimal Residual Disease in Acute Lymphoblastic Leukemia: How to Recognize and Treat It. Curr. Oncol. Rep. 2017, 19, 6. [Google Scholar] [CrossRef]
- Coustan-Smith, E.; Sancho, J.; Hancock, M.L.; Razzouk, B.I.; Ribeiro, R.C.; Rivera, G.K.; Rubnitz, J.E.; Sandlund, J.T.; Pui, C.-H.; Campana, D. Use of Peripheral Blood Instead of Bone Marrow to Monitor Residual Disease in Children with Acute Lymphoblastic Leukemia. Blood 2002, 100, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, V.; Jacobs, D.; Wijkhuijs, A.; Comans-Bitter, W.; Willemse, M.; Hählen, K.; Kamps, W.; van Wering, E.; van Dongen, J. Minimal Residual Disease Levels in Bone Marrow and Peripheral Blood Are Comparable in Children with T Cell Acute Lymphoblastic Leukemia (ALL), but Not in Precursor-B-ALL. Leukemia 2002, 16, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Kotrova, M.; Volland, A.; Kehden, B.; Trautmann, H.; Ritgen, M.; Wäsch, R.; Faul, C.; Viardot, A.; Schwartz, S.; Baldus, C.D.; et al. Comparison of Minimal Residual Disease Levels in Bone Marrow and Peripheral Blood in Adult Acute Lymphoblastic Leukemia. Leukemia 2020, 34, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.; Wassmann, B.; Pavlova, A.; Wunderle, L.; Oldenburg, J.; Binckebanck, A.; Lange, T.; Hochhaus, A.; Wystub, S.; Brück, P.; et al. Kinase Domain Mutations of BCR-ABL Frequently Precede Imatinib-Based Therapy and Give Rise to Relapse in Patients with de Novo Philadelphia-Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood 2007, 110, 727–734. [Google Scholar] [CrossRef]
- Soverini, S.; Vitale, A.; Poerio, A.; Gnani, A.; Colarossi, S.; Iacobucci, I.; Cimino, G.; Elia, L.; Lonetti, A.; Vignetti, M.; et al. Philadelphia-Positive Acute Lymphoblastic Leukemia Patients Already Harbor BCR-ABL Kinase Domain Mutations at Low Levels at the Time of Diagnosis. Haematologica 2011, 96, 552–557. [Google Scholar] [CrossRef]
- Short, N.J.; Kantarjian, H.; Kanagal-Shamanna, R.; Sasaki, K.; Ravandi, F.; Cortes, J.; Konopleva, M.; Issa, G.C.; Kornblau, S.M.; Garcia-Manero, G.; et al. Ultra-Accurate Duplex Sequencing for the Assessment of Pretreatment ABL1 Kinase Domain Mutations in Ph+ ALL. Blood Cancer J. 2020, 10, 61. [Google Scholar] [CrossRef]
- Soverini, S.; Hochhaus, A.; Nicolini, F.E.; Gruber, F.; Lange, T.; Saglio, G.; Pane, F.; Müller, M.C.; Ernst, T.; Rosti, G.; et al. BCR-ABL Kinase Domain Mutation Analysis in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors: Recommendations from an Expert Panel on Behalf of European LeukemiaNet. Blood 2011, 118, 1208–1215. [Google Scholar] [CrossRef]
- Ting, S.; Mixue, X.; Lixia, Z.; Xueying, L.; Wanzhuo, X.; Xiujin, Y. T315I Mutation Exerts a Dismal Prognosis on Adult BCR-ABL1-Positive Acute Lymphoblastic Leukemia, and Salvage Therapy with Ponatinib or CAR-T Cell and Bridging to Allogeneic Hematopoietic Stem Cell Transplantation Can Improve Clinical Outcomes. Ann. Hematol. 2020, 99, 829–834. [Google Scholar] [CrossRef]
- Paul, S.; Kantarjian, H.; Sasaki, K.; Marx, K.; Jain, N.; Savoy, J.M.; DiPippo, A.; Jammal, N.; Bravo, G.M.; Kadia, T.; et al. Intrathecal Prophylaxis with 12 versus 8 Administrations Reduces the Incidence of Central Nervous System Relapse in Patients with Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Am. J. Hematol. 2023, 98, E11–E14. [Google Scholar] [CrossRef] [PubMed]
- Kopmar, N.E.; Cassaday, R.D. How I Prevent and Treat Central Nervous System Disease in Adults with Acute Lymphoblastic Leukemia. Blood 2023, 141, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Porkka, K.; Koskenvesa, P.; Lundán, T.; Rimpiläinen, J.; Mustjoki, S.; Smykla, R.; Wild, R.; Luo, R.; Arnan, M.; Brethon, B.; et al. Dasatinib Crosses the Blood-Brain Barrier and Is an Efficient Therapy for Central Nervous System Philadelphia Chromosome–Positive Leukemia. Blood 2008, 112, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Slade, M.; Kantarjian, H.; Alvarenga, J.; Aldoss, I.; Mohammed, K.A.; Jabbour, E.; Faramand, R.; Shah, B.; Locke, F.; et al. The Role of Allogeneic Transplant for Adult Ph+ ALL in CR1 with Complete Molecular Remission: A Retrospective Analysis. Blood 2022, 140, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Joo, Y.-D.; Lim, S.-N.; Kim, S.-D.; Lee, J.-H.; Lee, J.-H.; Kim, D.H.; Kim, K.; Jung, C.W.; Kim, I.; et al. Nilotinib Combined with Multiagent Chemotherapy for Newly Diagnosed Philadelphia-Positive Acute Lymphoblastic Leukemia. Blood 2015, 126, 746–756. [Google Scholar] [CrossRef]
- Silva, W.F.; Cysne, D.N.; Kerbauy, M.N.; Colturato, I.; Maia, A.C.A.; Tucunduva, L.; Barros, G.M.N.; Colturato, V.A.R.; Hamerschlak, N.; Rocha, V. Predictive Factors and Outcomes after Allogeneic Stem Cell Transplantation for Adults with Acute Lymphoblastic Leukemia in Brazil. Transplant. Cell. Ther. 2022, 28, 763.e1–763.e7. [Google Scholar] [CrossRef]
- Ribera, J.-M.; Ribera, J.; Genescà, E. The Role of Stem Cell Transplantation in the Management of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Ther. Adv. Hematol. 2018, 9, 357–368. [Google Scholar] [CrossRef]
- Saini, N.; Marin, D.; Ledesma, C.; Delgado, R.; Rondon, G.; Popat, U.R.; Bashir, Q.; Hosing, C.M.; Nieto, Y.; Alousi, A.M.; et al. Impact of TKIs Post–Allogeneic Hematopoietic Cell Transplantation in Philadelphia Chromosome–Positive ALL. Blood 2020, 136, 1786–1789. [Google Scholar] [CrossRef]
- Giebel, S.; Czyz, A.; Ottmann, O.; Baron, F.; Brissot, E.; Ciceri, F.; Cornelissen, J.J.; Esteve, J.; Gorin, N.-C.; Savani, B.; et al. Use of Tyrosine Kinase Inhibitors to Prevent Relapse after Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Position Statement of the Acute Leukemia Working Party of The European Society for Blood and Marrow Transplantation. Cancer 2016, 122, 2941–2951. [Google Scholar] [CrossRef]
- Short, N.; Jabbour, E.; Jain, N.; Huang, X.; Macaron, W.; Nasr, L.; Montalban-Bravo, G.; Kadia, T.; Daver, N.; Chien, K.; et al. S118: A Chemotherapy-Free Combination of Ponatinib and Blinatumomab For Patients with Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Subgroup Analysis From a Phase II Study. HemaSphere 2023, 7, e4968152. [Google Scholar] [CrossRef]
- Puzzolo, M.C.; Radice, G.; Peragine, N.; de Propris, M.S.; Mariglia, P.; Vignetti, M.; Vitale, A.; Bassan, R.; Annunziata, M.; Gaidano, G.; et al. Host Immune System Modulation in Ph+ Acute Lymphoblastic Leukemia Patients Treated with Dasatinib and Blinatumomab. Blood 2021, 138, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- León, A.G.-D.; Mejía-Aranguré, J.M. Blinatumomab plus Hyper-CVAD: The Prelude to a New Era in Acute Lymphocytic Leukaemia. Lancet Haematol. 2022, 9, e864–e865. [Google Scholar] [CrossRef] [PubMed]
| Test to Establish the Diagnosis (Minimal) | |
|---|---|
| Complete blood count and differential count Bone marrow aspirate Bone marrow trephine biopsy * Immunophenotyping by flow cytometry RT-PCR for BCR-ABL1 (p190 and p210) | |
| Genetic analyses | Additional tests and procedures |
| Conventional karyotype MLPA for IKZF1 and correlated genes (CDKN2A/B and PAX5) Alternate methods if available: FISH for cytogenetic alterations; array for CNA alterations (e.g., IKZF1 deletions) | Complete physical examination Testicular examination, including scrotal ultrasound as indicated Performance status (ECOG/WHO score) Geriatric assessment (optional) Biochemistry, coagulation tests Hepatitis A, B, C; HIV-1 testing; CMV, EBV, HSV, VZV Serum pregnancy test Eligibility assessment for allogeneic HCT (incl. HLA-typing) Chest X-ray, 12-lead electrocardiogram, echocardiography or MUGA (on indication) Information on oocyte and sperm cryopreservation Biobanking |
| Not recommended | |
| Upfront ABL mutation testing | |
| Regimen | N | Induction Regimen | Median Age (Range) | CR, % | CMR, % | Early Mortality, % |
|---|---|---|---|---|---|---|
| GRAAPH-2005 [39] | 135 | VCR 2 mg/d IV Days 1, 8, 15, and 22 DXM 20 mg/d PO Days 1–2, 8–9, 15–16, and 22–23 Imatinib 400 mg bid PO Days 1–28 Triple intrathecal Days 1, 8, and 15 | 48 (18–59) | 98 | 29 (2 cycles) | 0.7 (2 cycles) |
| GIMEMA LAL0904 [42] | 51 | PDN 60 mg/m2/d Days 1–32 Imatinib 600 mg PO Days 1–50 MTX intrathecal Days 21 and 35 | 46 (17–59) | 96 | 3 (D50) | 0 |
| GIMEMA LAL1509 [34] | 60 | PDN 60 mg/m2/d Days 1–31 Dasatinib 140 mg/d PO Days 1–84 MTX intrathecal Days 0, 22, 45, 57, and 85 | 42 (19–59) | 100 | 18.3 | 0 |
| EWALL-PH01 [48] | 71 | VCR 2 mg/d IV Days 1, 8, 15, and 22 (>70 y: 1 mg) DXM 40 mg/d PO Days 1–2, 8–9, 15–16, and 22–23 (>70 y: 20 mg) Dasatinib 140 mg/d PO Triple intrathecal Days 1, 8, 15, and 22 | 69 (59–83) | 96 | 20 | 4.2 |
| EWALL-PH02 [49] | 72 | VCR 2 mg/d IV Days 1, 8, 15, and 22 (>70 y: 1 mg) DXM 40 mg/d PO Days 1–2, 8–9, 15–16, and 22–23 (>70 y: 20 mg) Nilotinib 400 mg/d bid PO Triple intrathecal Days 1, 8, 15, and 22 | 65 (55–85) | 94 | 14 | 1.4 |
| GRAAPH-2014 [50] | 156 | VCR 2 mg/d IV Days 1, 8, 15, and 22 DXM 20 mg/d PO Days 1–2, 8–9, 15–16, and 22–23 Nilotinib 400 mg bid PO Days 1–28 Triple intrathecal Days 1, 8, and 15 | 47 (18–60) | 100 | NR | 2 |
| INCB84344-201 [43] | 44 | PDN 60 mg/m2/d Days 1–29 Ponatinib 45 mg/d PO MTX intrathecal each month | 66 (26–85) | 91 | 47.7 (week 6) | 4.5 |
| Induction Remission (Minimal Requirements) | Induction Remission (Ideal Requirements) |
|---|---|
| Always combine TKI with chemo or low-intensity chemo Intrathecal chemotherapy Antimicrobial prophylaxis Frontline TKI: imatinib | Frontline TKI: dasatinib (improved CMR rates, potentially increased survival); ponatinib (remarkably higher survival rates). Upfront blinatumomab |
| Consolidation (minimal requirements) | Consolidation (ideal requirements) |
| Intensive chemotherapy—alternate courses, including cytarabine and methotrexate plus TKI Intrathecal chemotherapy Antimicrobial prophylaxis | Newer-generation TKI: dasatinib, ponatinib Blinatumomab for all patients or, at minimum, for those with positive MRD AlloSCT for patients with positive MRD, not achieving CMR or IKZF1-plus signature. |
| Monitoring (minimal requirements) | Monitoring (ideal requirements) |
| MRD by flow cytometry with sensitivity at minimum 10–4 (bone marrow) BCR-ABL1 by quantitative PCR ABL mutation by Sanger sequencing at relapse | MRD by IGH qPCR or NGS BCR-ABL1 by digital PCR or NGS ABL mutation by digital PCR or NGS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, W.; Rego, E. How to Manage Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. Cancers 2023, 15, 5783. https://doi.org/10.3390/cancers15245783
Silva W, Rego E. How to Manage Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. Cancers. 2023; 15(24):5783. https://doi.org/10.3390/cancers15245783
Chicago/Turabian StyleSilva, Wellington, and Eduardo Rego. 2023. "How to Manage Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings" Cancers 15, no. 24: 5783. https://doi.org/10.3390/cancers15245783
APA StyleSilva, W., & Rego, E. (2023). How to Manage Philadelphia-Positive Acute Lymphoblastic Leukemia in Resource-Constrained Settings. Cancers, 15(24), 5783. https://doi.org/10.3390/cancers15245783







