Neutralization of p40 Homodimer and p40 Monomer Leads to Tumor Regression in Patient-Derived Xenograft Mice with Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Serum Samples of Pancreatic Cancer Patients
2.3. Sandwich ELISA
2.4. Assessment of Cell Viability: MTT and LDH Assays
2.5. Fragment End Labeling DNA—TUNEL Assay
2.6. Flow Cytometry
2.7. Immunostaining
2.8. Real-Time PCR
Immunoblotting
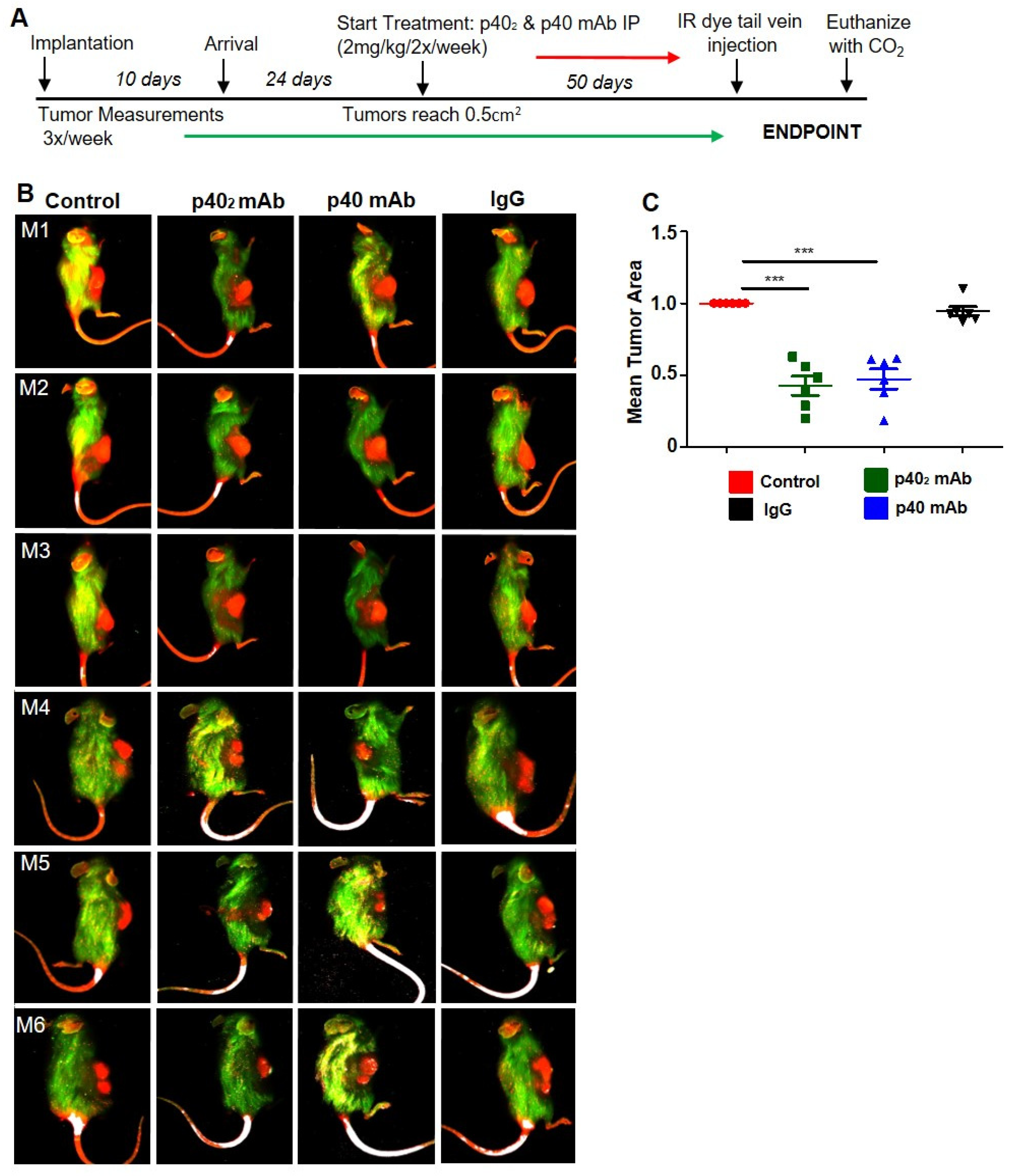
2.9. Experimental Animals
2.9.1. Histopathology
2.9.2. Statistical Analysis
3. Results
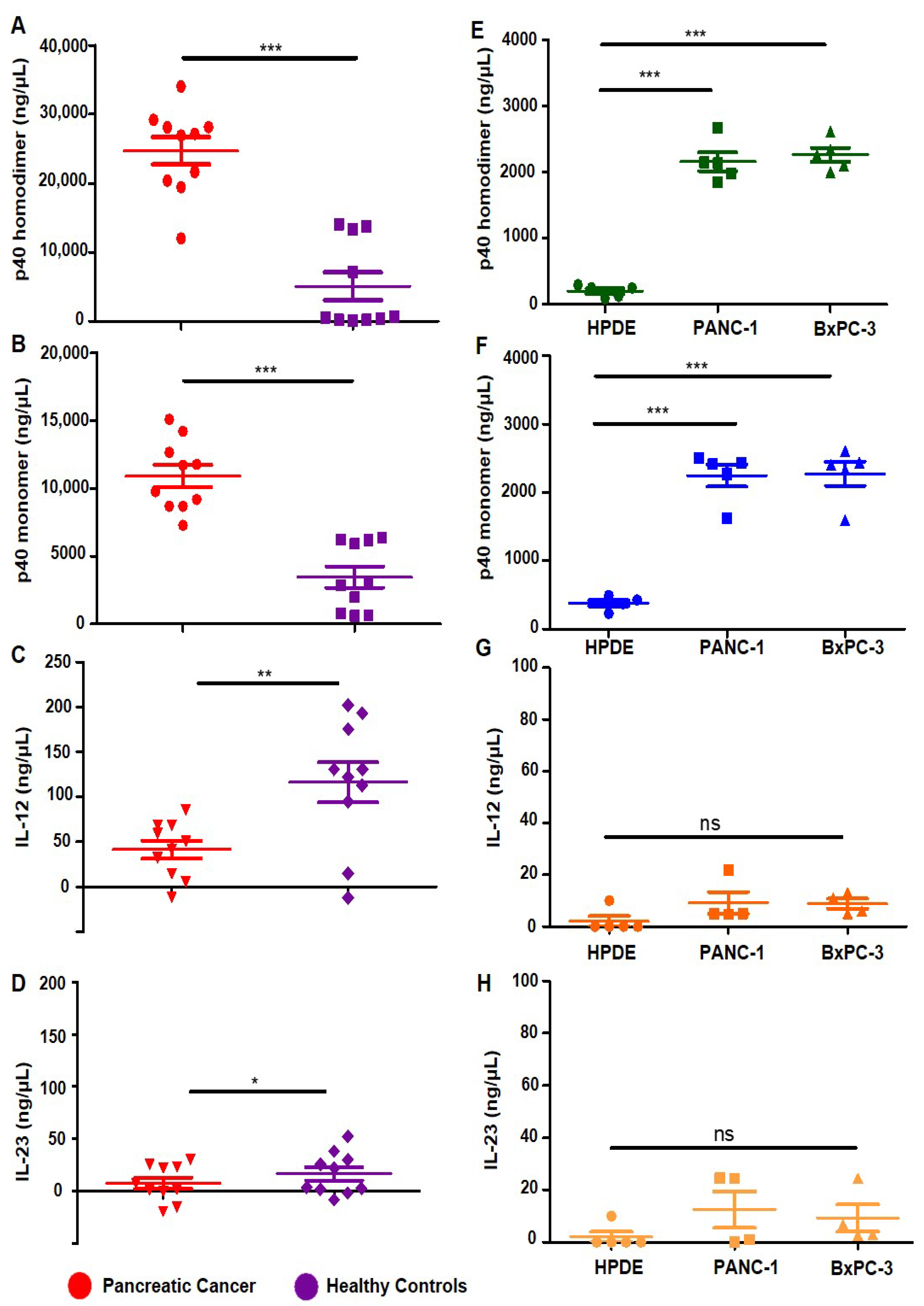
3.1. Levels of p402, p40 and IL-12, IL-23 in Serum of Pancreatic Cancer Patients
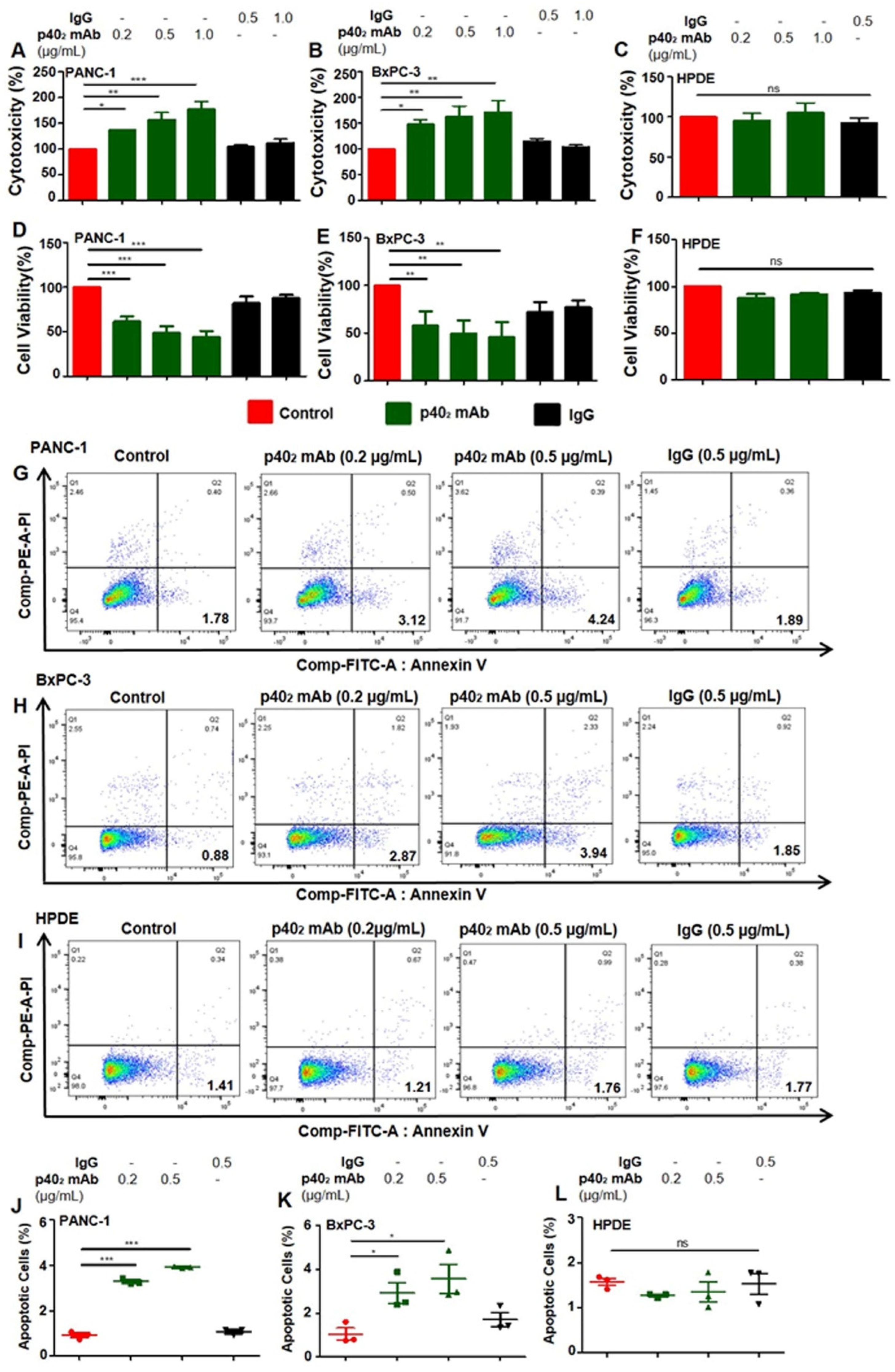
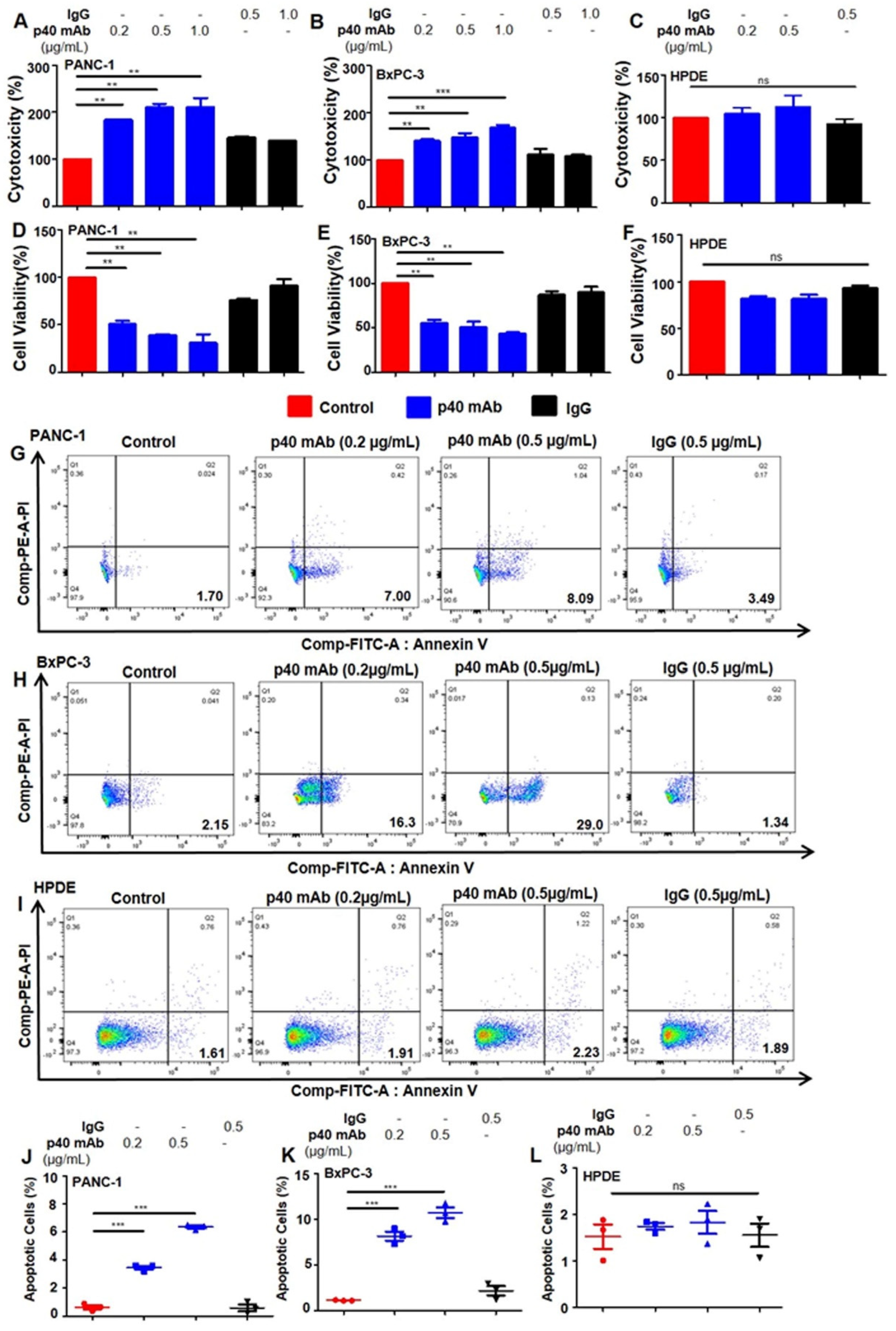
3.2. Immunotherapies with mAbs against p402 and p40 Induces Death in Human Pancreatic Cancer Cells
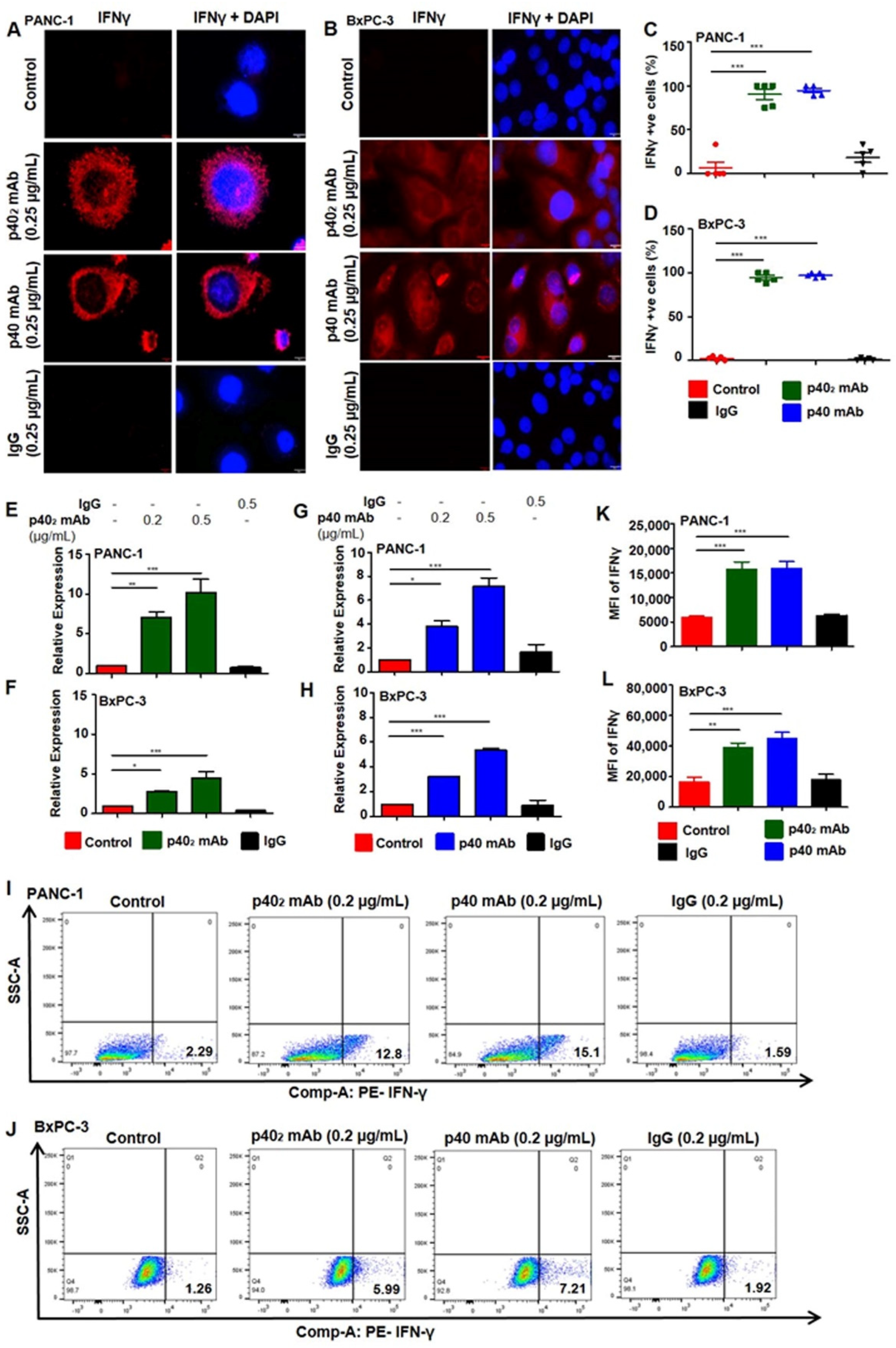
3.3. Upregulation of IFN-γ In Vitro in PDAC Cells after p402 mAb and p40 mAb Treatments
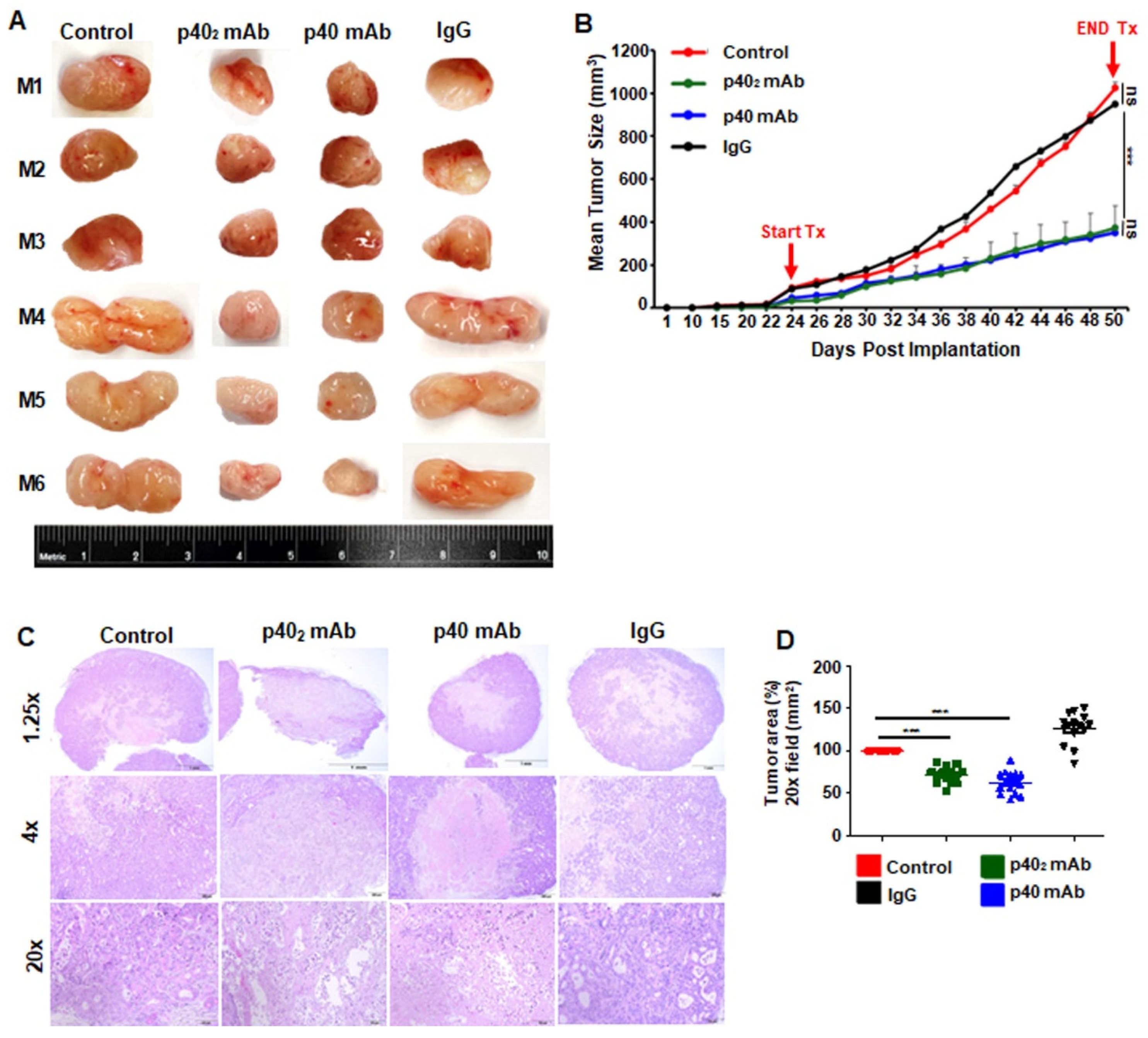
3.4. Immunotherapies with p402 mAb and p40 mAb Lead to Tumor Regression in a Patient-Derived Xenograft (PDX) Mouse Model of Pancreatic Cancer
3.5. Immunotherapies with p402 mAb and p40 mAb Induce a Death Response and Upregulation of IFN-γ in Tumor Tissues of PDX Mouse Model of Pancreatic Cancer
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Combs, S.E.; Springfeld, C.; Hartwig, W.; Hackert, T.; Buchler, M.W. Advanced-stage pancreatic cancer: Therapy options. Nat. Rev. Clin. Oncol. 2013, 10, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lasek, W.; Zagozdzon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.H.; Byrne, K.T.; Vonderheide, R.H. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018, 4, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Brahmachari, S.; Pahan, K. Role of cytokine p40 family in multiple sclerosis. Minerva Med. 2008, 99, 105–118. [Google Scholar]
- Heinzel, F.P.; Hujer, A.M.; Ahmed, F.N.; Rerko, R.M. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 1997, 158, 4381–4388. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Macatonia, S.E.; Tripp, C.S.; Wolf, S.F.; O’Garra, A.; Murphy, K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–549. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gately, M.K.; Renzetti, L.M.; Magram, J.; Stern, A.S.; Adorini, L.; Gubler, U.; Presky, D.H. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998, 16, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Fan, J.Q.; Wang, M.F.; Chen, H.L.; Shang, D.; Das, J.K.; Song, J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer 2020, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Kumar, S.; Ross, K.A.; Gautam, S.; Poelaert, B.; Nasser, M.W.; Aithal, A.; Bhatia, R.; Wannemuehler, M.J.; Narasimhan, B.; et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018, 417, 35–46. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bandopadhyay, M.; Pahan, K. Generation of functional blocking monoclonal antibodies against mouse interleukin-12 p40 homodimer and monomer. Hybridoma 2008, 27, 141–151. [Google Scholar] [CrossRef]
- Kundu, M.; Roy, A.; Pahan, K. Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-gamma. Proc. Natl. Acad. Sci. USA 2017, 114, 11482–11487. [Google Scholar] [CrossRef]
- Kundu, M.; Raha, S.; Roy, A.; Pahan, K. Regression of Triple-Negative Breast Cancer in a Patient-Derived Xenograft Mouse Model by Monoclonal Antibodies against IL-12 p40 Monomer. Cells 2022, 11, 259. [Google Scholar] [CrossRef]
- Mondal, S.; Kundu, M.; Jana, M.; Roy, A.; Rangasamy, S.B.; Modi, K.K.; Wallace, J.; Albalawi, Y.A.; Balabanov, R.; Pahan, K. IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rβ1 internalization and suppress EAE. Proc. Natl. Acad. Sci. USA 2020, 117, 21557–21567. [Google Scholar] [CrossRef]
- Sheinin, M.; Jeong, B.; Paidi, R.K.; Pahan, K. Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1. Cancers 2022, 14, 5648. [Google Scholar] [CrossRef] [PubMed]
- Sheinin, M.; Mondal, S.; Roy, A.; Gorai, S.; Rangasamy, S.B.; Poddar, J.; Pahan, K. Suppression of Experimental Autoimmune Encephalomyelitis in Mice by beta-Hydroxy beta-Methylbutyrate, a Body-Building Supplement in Humans. J. Immunol. 2023, 211, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Roh, J.; Park, C.S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef]
- Boyer, S.; Lee, H.J.; Steele, N.; Zhang, L.; Sajjakulnukit, P.; Andren, A.; Ward, M.H.; Singh, R.; Basrur, V.; Zhang, Y.; et al. Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway. eLife 2022, 11, e73796. [Google Scholar] [CrossRef] [PubMed]
- Vaziri-Gohar, A.; Cassel, J.; Mohammed, F.S.; Zarei, M.; Hue, J.J.; Hajihassani, O.; Graor, H.J.; Srikanth, Y.V.V.; Karim, S.A.; Abbas, A.; et al. Limited nutrient availability in the tumor microenvironment renders pancreatic tumors sensitive to allosteric IDH1 inhibitors. Nat. Cancer 2022, 3, 852–865. [Google Scholar] [CrossRef]
- Ouyang, H.; Mou, L.; Luk, C.; Liu, N.; Karaskova, J.; Squire, J.; Tsao, M.S. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am. J. Pathol. 2000, 157, 1623–1631. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site of SARS-CoV-2 spike protein is a key determinant for transmission due to enhanced replication in airway cells. BioRxiv. 2020. [Google Scholar] [CrossRef]
- Gollob, J.A.; Murphy, E.A.; Mahajan, S.; Schnipper, C.P.; Ritz, J.; Frank, D.A. Altered interleukin-12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1. Blood 1998, 91, 1341–1354. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNgamma receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. Corrigendum: A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2016, 531, 126. [Google Scholar] [CrossRef]
- Nastala, C.L.; Edington, H.D.; McKinney, T.G.; Tahara, H.; Nalesnik, M.A.; Brunda, M.J.; Gately, M.K.; Wolf, S.F.; Schreiber, R.D.; Storkus, W.J.; et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J. Immunol. 1994, 153, 1697–1706. [Google Scholar] [CrossRef]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022, 20, 206. [Google Scholar] [CrossRef]
- Brock, R.M.; Beitel-White, N.; Coutermarsh-Ott, S.; Grider, D.J.; Lorenzo, M.F.; Ringel-Scaia, V.M.; Manuchehrabadi, N.; Martin, R.C.G.; Davalos, R.V.; Allen, I.C. Patient Derived Xenografts Expand Human Primary Pancreatic Tumor Tissue Availability for ex vivo Irreversible Electroporation Testing. Front. Oncol. 2020, 10, 843. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Roy, S.; Varma, S.; Bose, M. Pulmonary epithelial cells are a source of interferon-gamma in response to Mycobacterium tuberculosis infection. Immunol. Cell Biol. 2007, 85, 229–237. [Google Scholar] [CrossRef]
- Jakubowska, K.; Guzinska-Ustymowicz, K.; Famulski, W.; Cepowicz, D.; Jagodzinska, D.; Pryczynicz, A. Reduced expression of caspase-8 and cleaved caspase-3 in pancreatic ductal adenocarcinoma cells. Oncol. Lett. 2016, 11, 1879–1884. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Demeter, M.R.; Ruan, H.; Comb, M.J. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J. Biol. Chem. 2000, 275, 25865–25869. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef]
- Garcia, P.L.; Miller, A.L.; Yoon, K.J. Patient-Derived Xenograft Models of Pancreatic Cancer: Overview and Comparison with Other Types of Models. Cancers 2020, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Schuster, T.; Meyer Zum Buschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Koblish, H.K.; Horton, B.; Scherle, P.A.; Newton, R.; Gajewski, T.F. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J. Immunother. Cancer 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Selleck, W.A.; Canfield, S.E.; Hassen, W.A.; Meseck, M.; Kuzmin, A.I.; Eisensmith, R.C.; Chen, S.H.; Hall, S.J. IFN-gamma sensitization of prostate cancer cells to Fas-mediated death: A gene therapy approach. Mol. Ther. 2003, 7, 185–192. [Google Scholar] [CrossRef]
- Wigginton, J.M.; Gruys, E.; Geiselhart, L.; Subleski, J.; Komschlies, K.L.; Park, J.W.; Wiltrout, T.A.; Nagashima, K.; Back, T.C.; Wiltrout, R.H. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J. Clin. Investig. 2001, 108, 51–62. [Google Scholar] [CrossRef]
- Barnett, D.; Liu, Y.; Partyka, K.; Huang, Y.; Tang, H.; Hostetter, G.; Brand, R.E.; Singhi, A.D.; Drake, R.R.; Haab, B.B. The CA19-9 and Sialyl-TRA Antigens Define Separate Subpopulations of Pancreatic Cancer Cells. Sci. Rep. 2017, 7, 4020. [Google Scholar] [CrossRef]
- Azizian, A.; Ruhlmann, F.; Krause, T.; Bernhardt, M.; Jo, P.; Konig, A.; Kleiss, M.; Leha, A.; Ghadimi, M.; Gaedcke, J. CA19-9 for detecting recurrence of pancreatic cancer. Sci. Rep. 2020, 10, 1332. [Google Scholar] [CrossRef]


| Cell Line | Diagnosis | Mutation | Cell Type | Tissue | Age | Sex | Manufacturer |
|---|---|---|---|---|---|---|---|
| HPDE-H6c7 | Normal | wt p53 wt KRAS | Epithelial | Pancreatic duct | 63 | F | Kerafast |
| PANC-1 | Epithelioid Carcinoma | mut p53 mut KRAS | Epithelial | Pancreatic duct | 56 | M | ATCC |
| BxPC-3 | Adenocarcinoma | mut p53 wt KRAS | Epithelial | Pancreas | 61 | F | ATCC |
| Patient ID | Diagnosis | Age | Sex | Ethnicity | Manufacturer |
|---|---|---|---|---|---|
| Healthy Controls | |||||
| HC-130 | Normal | 39 | F | White | DLS |
| HC-798 | Normal | 57 | F | White | DLS |
| HC-144 | Normal | 65 | F | White | DLS |
| HC-792 | Normal | 51 | F | White | DLS |
| HC-594 | Normal | 37 | F | American Indian | DLS |
| HC-806 | Normal | 34 | F | White | RUMC |
| HC-593 | Normal | 63 | M | White | RUMC |
| HC-810 | Normal | 63 | M | White | RUMC |
| HC-900 | Normal | 54 | M | Asian | RUMC |
| HC-901 | Normal | 32 | F | Asian | RUMC |
| Pancreatic Cancer | |||||
| PC-416 | Pancreatic Cancer | 65 | F | Black | DLS |
| PC-316 | Adenocarcinoma | 58 | F | White | DLS |
| PC-716 | Adenocarcinoma | 68 | M | Black | DLS |
| PC-617 | Adenocarcinoma | 78 | M | White | DLS |
| PC-717 | Pancreatic Cancer | 71 | M | White | DLS |
| PC-2617 | Adenocarcinoma | 52 | M | White | DLS |
| PC-217 | Adenocarcinoma | 58 | M | White | DLS |
| PC-417 | Adenocarcinoma | 57 | F | Black | DLS |
| PC-110 | Adenocarcinoma | 74 | F | White | DLS |
| PC-418 | Adenocarcinoma | 70 | M | Black | DLS |
| Antibody | Manufacturer | Catalog | Host | Application | Dilution |
|---|---|---|---|---|---|
| Cleaved caspase 3 | Cell Signaling | #9661 | rabbit | IF | 1:1000 |
| IFN-γ | Invitrogen | MA5-44024 | mouse | IF | 1:200 |
| CA19-9 | Invitrogen | MA5-12421 | mouse | IF | 1:200 |
| Phospho-BAD | Santa-Cruz | sc-166932 | mouse | WB | 1:200 |
| Bcl-2 | Santa-Cruz | sc-7382 | mouse | WB | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheinin, M.; Mondal, S.; Pahan, K. Neutralization of p40 Homodimer and p40 Monomer Leads to Tumor Regression in Patient-Derived Xenograft Mice with Pancreatic Cancer. Cancers 2023, 15, 5796. https://doi.org/10.3390/cancers15245796
Sheinin M, Mondal S, Pahan K. Neutralization of p40 Homodimer and p40 Monomer Leads to Tumor Regression in Patient-Derived Xenograft Mice with Pancreatic Cancer. Cancers. 2023; 15(24):5796. https://doi.org/10.3390/cancers15245796
Chicago/Turabian StyleSheinin, Monica, Susanta Mondal, and Kalipada Pahan. 2023. "Neutralization of p40 Homodimer and p40 Monomer Leads to Tumor Regression in Patient-Derived Xenograft Mice with Pancreatic Cancer" Cancers 15, no. 24: 5796. https://doi.org/10.3390/cancers15245796
APA StyleSheinin, M., Mondal, S., & Pahan, K. (2023). Neutralization of p40 Homodimer and p40 Monomer Leads to Tumor Regression in Patient-Derived Xenograft Mice with Pancreatic Cancer. Cancers, 15(24), 5796. https://doi.org/10.3390/cancers15245796





