HPV-Negative and HPV-Positive Oral Cancer Cells Stimulate the Polarization of Neutrophils towards Different Functional Phenotypes In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Supernatant Collection
2.2. Neutrophil Purification, Culture, and Stimulation
2.3. Multiplex Immunoassay
2.4. Flow Cytometry
2.5. MTT Assay
2.6. ROS Production
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
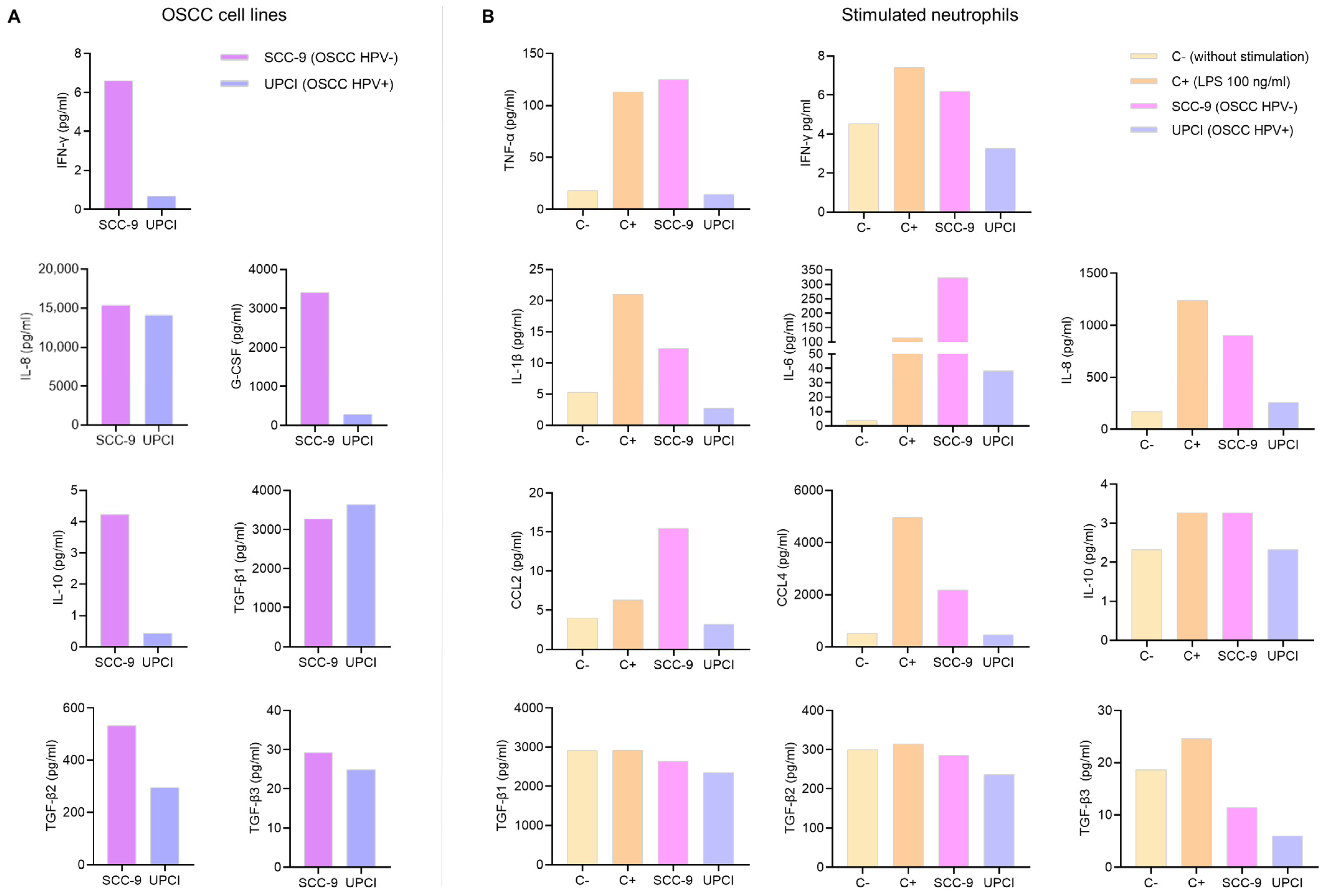
3.1. Cytokine Production by OSCC Cell Lines and Stimulated Neutrophils
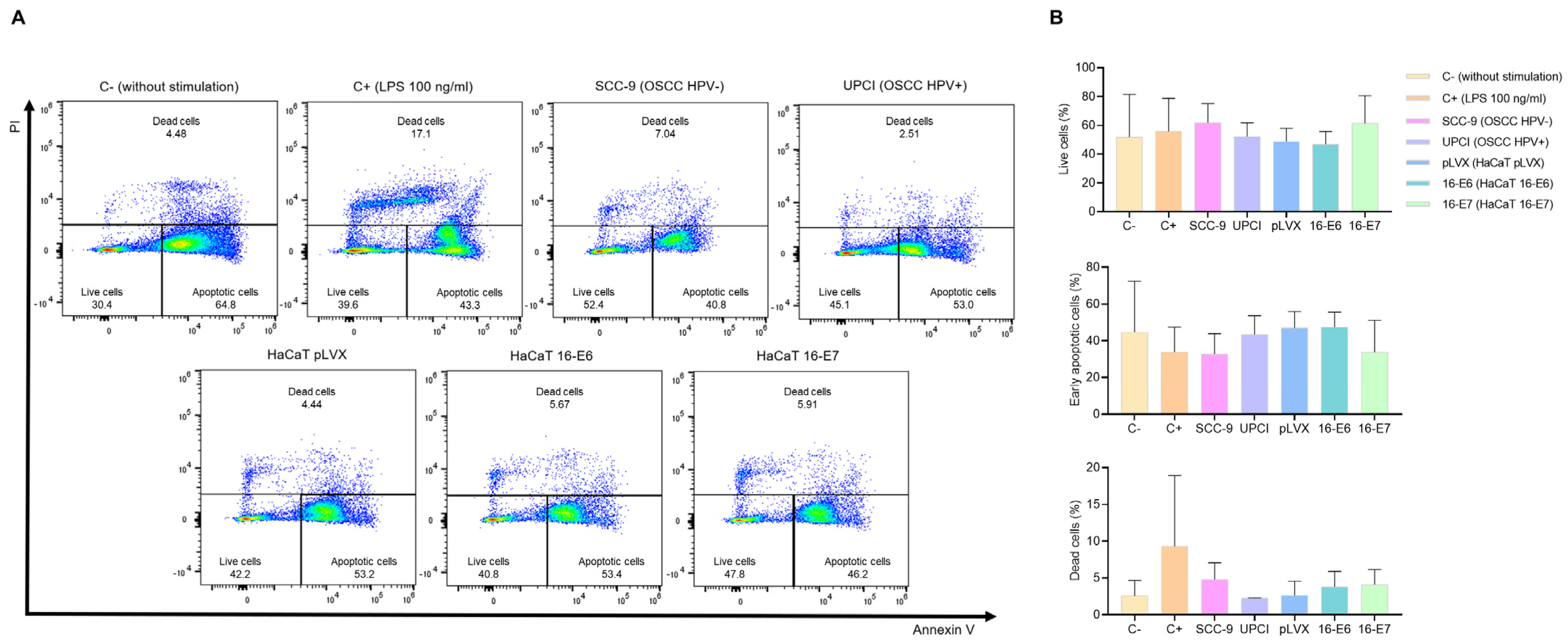
3.2. Stimulated Neutrophils’ Survival
3.3. Stimulated Neutrophils’ Metabolism
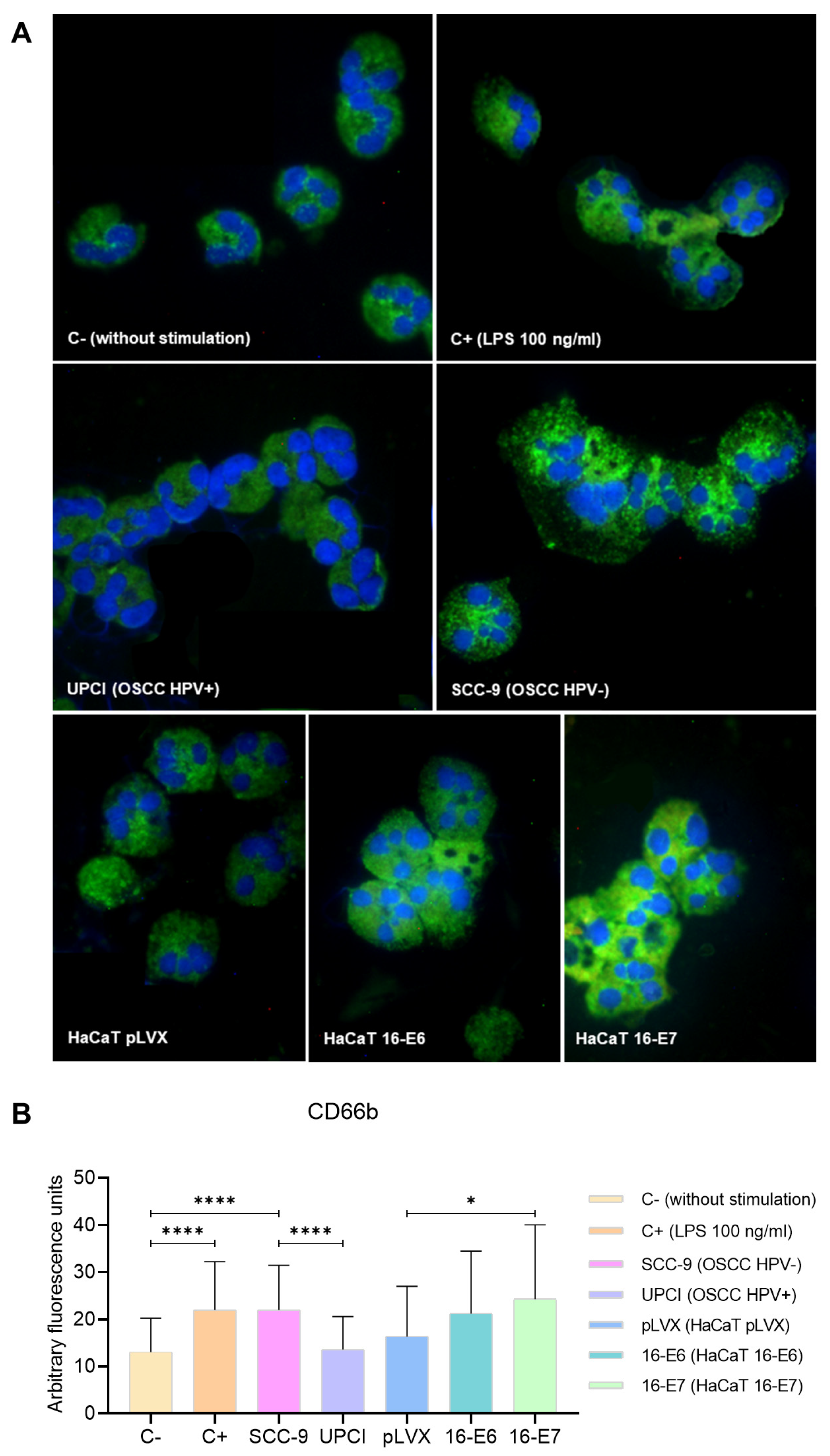
3.4. Expression of CD66b in Stimulated Neutrophils
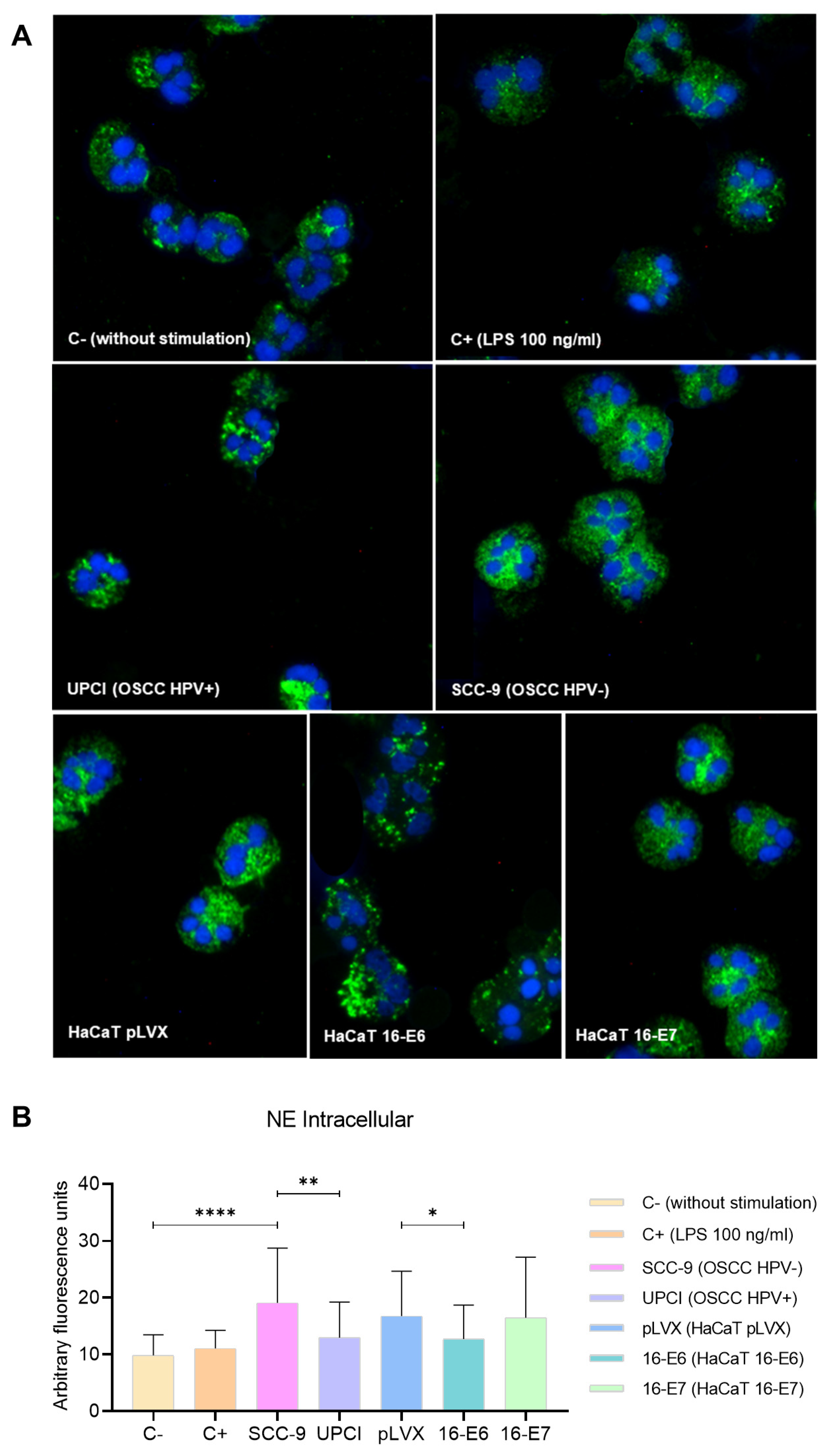
3.5. Intracellular Expression of NE in Stimulated Neutrophils
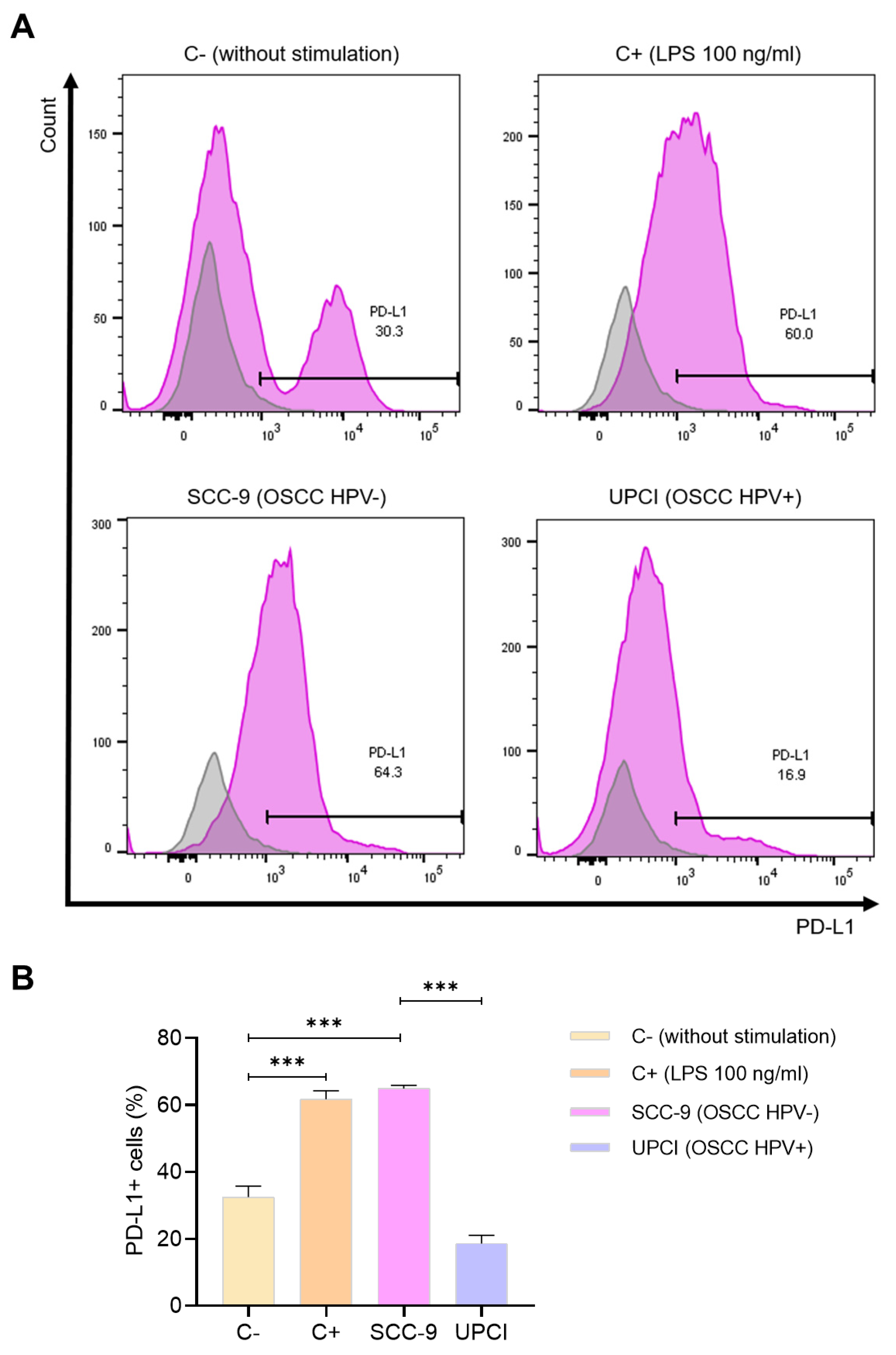
3.6. Expression of PD-L1 in Stimulated Neutrophils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Author Correction: Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 2023, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Pérez-Islas, E.; García-Carrancá, A.; Acosta-Gio, E.; Reynoso-Noverón, N.; Maldonado-Martínez, H.-A.; Guido-Jiménez, M.; Sobrevilla-Moreno, N.; Granados-García, M.; Pérez-Báez, W.-B.; Vilar-Compte, D. Prognostic Importance of DNA from Human Papillomavirus in Patients with Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e150–e158. [Google Scholar] [CrossRef]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct Patterns of Intratumoral Immune Cell Infiltrates in Patients with HPV-Associated Compared to Non-Virally Induced Head and Neck Squamous Cell Carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The Head and Neck Cancer Immune Landscape and Its Immunotherapeutic Implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-Naïve HPV+ Head and Neck Cancers Display a T-Cell-Inflamed Phenotype Distinct from Their HPV- Counterparts That Has Implications for Immunotherapy. Oncoimmunology 2018, 7, e1498439. [Google Scholar] [CrossRef]
- Chen, X.; Yan, B.; Lou, H.; Shen, Z.; Tong, F.; Zhai, A.; Wei, L.; Zhang, F. Immunological Network Analysis in HPV Associated Head and Neck Squamous Cancer and Implications for Disease Prognosis. Mol. Immunol. 2018, 96, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, L.; Wang, Q.; Ma, S.; Sun, J.; Ma, C.; Liu, J.; Jing, X.; Ai, D.; Nan, Z.; et al. Neutrophils Infiltration and Its Correlation with Human Papillomavirus Status in the Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2019, 11, 5171–5185. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Goel, P.; Prajapati, D.R.; Wang, C.; Singh, R.K. Breast Cancer Cell-Neutrophil Interactions Enhance Neutrophil Survival and Pro-Tumorigenic Activities. Cancers 2020, 12, 2884. [Google Scholar] [CrossRef]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hütte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear Granulocytes in Human Head and Neck Cancer: Enhanced Inflammatory Activity, Modulation by Cancer Cells and Expansion in Advanced Disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Qiu, S.J.; Fan, J.; Zhou, J.; Gao, Q.; Xiao, Y.S.; Xu, Y.F. Intratumoral Neutrophils: A Poor Prognostic Factor for Hepatocellular Carcinoma Following Resection. J. Hepatol. 2011, 54, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in Vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Li, H.; Torabi, S.J.; Yarbrough, W.G.; Mehra, S.; Osborn, H.A.; Judson, B. Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites with Overall Survival. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 519–525. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Henderson, S.; Thirdborough, S.M.; Ottensmeier, C.H.; Su, X.; Lechner, M.; Feber, A.; Thomas, G.J.; Fenton, T.R. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated with an Immune Response Largely Restricted to the Oropharynx. J. Clin. Oncol. 2016, 34, 4132–4141. [Google Scholar] [CrossRef]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial Profiling Technologies Illuminate the Tumor Microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Carus, A.; Ladekarl, M.; Hager, H.; Nedergaard, B.S.; Donskov, F. Tumour-Associated CD66b+ Neutrophil Count Is an Independent Prognostic Factor for Recurrence in Localised Cervical Cancer. Br. J. Cancer 2013, 108, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Beaman, B.L. Interferon-Gamma Activation of Polymorphonuclear Neutrophil Function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Sandhu, J.K.; Birnboim, H.C. Expression of Interleukin-8 Promotes Neutrophil Infiltration and Genetic Instability in Mutatect Tumors. Neoplasia 2000, 2, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Kuang, H.; Fan, W.; Chen, X.; Yu, T.; Tang, Q.; Zhou, Z.; Liang, F. Downregulation of FOXP3 in Neutrophils by IL-8 Promotes the Progression of Oral Squamous Cell Carcinoma. Oncol. Lett. 2019, 18, 4771–4777. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Loison, F.; Luo, H.R. Neutrophil Spontaneous Death Is Mediated by Down-Regulation of Autocrine Signaling through GPCR, PI3Kgamma, ROS, and Actin. Proc. Natl. Acad. Sci. USA 2010, 107, 2950–2955. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Bellevicine, C.; Lansione, T.; Ferrara, A.L.; Iannone, R.; di Somma, S.; Borriello, F.; Clery, E.; et al. Potential Involvement of Neutrophils in Human Thyroid Cancer. PLoS ONE 2018, 13, e0199740. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Wakabayashi, I.; Kimuro, S.; Takahashi, N.; Takahashi, K.; Kobayashi, M.; Maishi, N.; Podyma-Inoue, K.A.; Hida, K.; Miyazono, K.; et al. TNF-α Enhances TGF-β-Induced Endothelial-to-Mesenchymal Transition via TGF-β Signal Augmentation. Cancer Sci. 2020, 111, 2385–2399. [Google Scholar] [CrossRef]
- Li, R.; Ong, S.L.; Tran, L.M.; Jing, Z.; Liu, B.; Park, S.J.; Huang, Z.L.; Walser, T.C.; Heinrich, E.L.; Lee, G.; et al. Chronic IL-1β-Induced Inflammation Regulates Epithelial-to-Mesenchymal Transition Memory Phenotypes via Epigenetic Modifications in Non-Small Cell Lung Cancer. Sci. Rep. 2020, 10, 377. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lin, C.C.; Huang, Y.W.; Chen, J.H.; Tsou, Y.A.; Chang, L.C.; Fan, C.C.; Lin, C.Y.; Chang, W.C. Macrophage Secretory IL-1β Promotes Docetaxel Resistance in Head and Neck Squamous Carcinoma via SOD2/CAT-ICAM1 Signaling. JCI Insight 2022, 7, e157285. [Google Scholar] [CrossRef]
- Xiao, L.; Li, X.; Cao, P.; Fei, W.; Zhou, H.; Tang, N.; Liu, Y. Interleukin-6 Mediated Inflammasome Activation Promotes Oral Squamous Cell Carcinoma Progression via JAK2/STAT3/Sox4/NLRP3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2022, 41, 166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ma, H.; Chang, H.; Feng, Z.; Zhang, C.; Yang, X. The Interaction of Interleukin-8 and PTEN Inactivation Promotes the Malignant Progression of Head and Neck Squamous Cell Carcinoma via the STAT3 Pathway. Cell Death Dis. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B. Tumor Promoting Roles of IL-10, TGF-β, IL-4, and IL-35: Its Implications in Cancer Immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Chen, D.P.; Ouyang, F.Z.; Chen, M.M.; Wu, Y.; Kuang, D.M.; Zheng, L. Increased Autophagy Sustains the Survival and Pro-Tumourigenic Effects of Neutrophils in Human Hepatocellular Carcinoma. J. Hepatol. 2015, 62, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.M.; Liu, Z.G.; Zhou, X.; Song, S.H.; Weng, G.Y.; Wen, Y.; Liu, F.B.; Cao, D.L.; Liu, Y.F. Expansion of PMN-Myeloid Derived Suppressor Cells and Their Clinical Relevance in Patients with Oral Squamous Cell Carcinoma. Oral Oncol. 2019, 95, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Greten, F.R. Immune Cell—Produced ROS and Their Impact on Tumor Growth and Metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Skrzeczynska-Moncznik, J.; Zabieglo, K.; Osiecka, O.; Morytko, A.; Brzoza, P.; Drozdz, L.; Kapinska-Mrowiecka, M.; Korkmaz, B.; Pastuszczak, M.; Kosalka-Wegiel, J.; et al. Differences in Staining for Neutrophil Elastase and Its Controlling Inhibitor SLPI Reveal Heterogeneity among Neutrophils in Psoriasis. J. Invest. Dermatol. 2020, 140, 1371–1378.e3. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.; Carré, A.; Ardi, V.; Muramatsu, T.; Schmidt, J.; Pham, C.; Quigley, J.P. Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events. iScience 2020, 23, 101799. [Google Scholar] [CrossRef] [PubMed]
- Bowers, N.L.; Helton, E.S.; Huijbregts, R.P.H.; Goepfert, P.A.; Heath, S.L.; Hel, Z. Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway. PLoS Pathog. 2014, 10, e1003993. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, D.; Heng, Y.; Zhu, X.K.; Lin, H.Q.; Zhou, J.; Tao, L.; Lu, L.M. Tumor-Infiltrating PD-L1+ Neutrophils Induced by GM-CSF Suppress T Cell Function in Laryngeal Squamous Cell Carcinoma and Predict Unfavorable Prognosis. J. Inflamm. Res. 2022, 15, 1079–1097. [Google Scholar] [CrossRef]
- Khayatan, D.; Hussain, A.; Tebyaniyan, H. Exploring Animal Models in Oral Cancer Research and Clinical Intervention: A Critical Review. Vet. Med. Sci. 2023, 9, 1833–1847. [Google Scholar] [CrossRef]
- Quail, D.F.; Amulic, B.; Aziz, M.; Barnes, B.J.; Eruslanov, E.; Fridlender, Z.G.; Goodridge, H.S.; Granot, Z.; Hidalgo, A.; Huttenlocher, A.; et al. Neutrophil Phenotypes and Functions in Cancer: A Consensus Statement. J. Exp. Med. 2022, 219, e20220011. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Barajas, M.G.; Jave-Suárez, L.F.; Ramírez-López, I.G.; García-Chagollán, M.; Zepeda-Nuño, J.S.; Ramírez-de-Arellano, A.; Ortiz-Lazareno, P.C.; Villegas-Pineda, J.C.; Pereira-Suárez, A.L. HPV-Negative and HPV-Positive Oral Cancer Cells Stimulate the Polarization of Neutrophils towards Different Functional Phenotypes In Vitro. Cancers 2023, 15, 5814. https://doi.org/10.3390/cancers15245814
Martínez-Barajas MG, Jave-Suárez LF, Ramírez-López IG, García-Chagollán M, Zepeda-Nuño JS, Ramírez-de-Arellano A, Ortiz-Lazareno PC, Villegas-Pineda JC, Pereira-Suárez AL. HPV-Negative and HPV-Positive Oral Cancer Cells Stimulate the Polarization of Neutrophils towards Different Functional Phenotypes In Vitro. Cancers. 2023; 15(24):5814. https://doi.org/10.3390/cancers15245814
Chicago/Turabian StyleMartínez-Barajas, Marcela Guadalupe, Luis Felipe Jave-Suárez, Inocencia Guadalupe Ramírez-López, Mariel García-Chagollán, José Sergio Zepeda-Nuño, Adrián Ramírez-de-Arellano, Pablo César Ortiz-Lazareno, Julio César Villegas-Pineda, and Ana Laura Pereira-Suárez. 2023. "HPV-Negative and HPV-Positive Oral Cancer Cells Stimulate the Polarization of Neutrophils towards Different Functional Phenotypes In Vitro" Cancers 15, no. 24: 5814. https://doi.org/10.3390/cancers15245814
APA StyleMartínez-Barajas, M. G., Jave-Suárez, L. F., Ramírez-López, I. G., García-Chagollán, M., Zepeda-Nuño, J. S., Ramírez-de-Arellano, A., Ortiz-Lazareno, P. C., Villegas-Pineda, J. C., & Pereira-Suárez, A. L. (2023). HPV-Negative and HPV-Positive Oral Cancer Cells Stimulate the Polarization of Neutrophils towards Different Functional Phenotypes In Vitro. Cancers, 15(24), 5814. https://doi.org/10.3390/cancers15245814







