The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Sample Collection and Circulating Cell Enumeration
3. Results
3.1. Patients
3.2. Circulating Tumour Cells, Circulating Tumour Micro-Emboli and Circulating Tumour-Derived Endothelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, O.A.; Anderson, R.L. Editorial: Therapy-induced metastasis. Clin. Exp. Metastasis 2018, 35, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef]
- Thery, L.; Meddis, A.; Cabel, L.; Proudhon, C.; Latouche, A.; Pierga, J.-Y.; Bidard, F.-C. Circulating tumor cells in early breast cancer. JNCI Cancer Spectr. 2019, 3, pkz026. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.; Alix-Panabières, C.; Hofman, P.; Parks, S.K.; Chamorey, E.; Naman, H.; Hannoun-Lévi, J.M. Circulating tumor cells in prostate cancer: A potential surrogate marker of survival. Crit. Rev. Oncol. Hematol. 2012, 81, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-j.; Wang, P.; Peng, J.; Wang, X.; Yao-wu, Z.; Shen, N. Meta-analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci. Rep. 2017, 7, 905. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef]
- Jatana, K.R.; Balasubramanian, P.; Lang, J.C.; Yang, L.; Jatana, C.A.; White, E.; Agrawal, A.; Ozer, E.; Schuller, D.E.; Teknos, T.N.; et al. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: Initial results. Arch. Otolaryngol.-Head Neck Surg. 2010, 136, 1274–1279. [Google Scholar] [CrossRef]
- Gröbe, A.; Blessmann, M.; Hanken, H.; Friedrich, R.E.; Schön, G.; Wikner, J.; Effenberger, K.E.; Kluwe, L.; Heiland, M.; Pantel, K.; et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. 2014, 20, 425–433. [Google Scholar] [CrossRef]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, K.; Xue, Y.; Tong, F.; Li, S. Prognostic value of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Med. Oncol. 2015, 32, 164. [Google Scholar] [CrossRef]
- Pantel, K. Circulating tumor cells in head and neck carcinomas. Clin. Chem. 2019, 65, 1193–1195. [Google Scholar] [CrossRef]
- Lin, P.P. Aneuploid CTC and CEC. Diagnostics 2018, 8, 26. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Y.; Wang, Y.; Xie, J.; Cheng, J.; Huang, Q. Comprehensive Atlas of Circulating Rare Cells Detected by SE-iFISH and Image Scanning Platform in Patients with Various Diseases. Front. Oncol. 2022, 12, 821454. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Dianat-Moghadam, H.; Azizi, M.; Eslami-S, Z.; Cortés-Hernández, L.E.; Heidarifard, M.; Nouri, M.; Alix-Panabières, C. The Role of Circulating Tumor Cells in the Metastatic Cascade: Biology, Technical Challenges, and Clinical Relevance. Cancers 2020, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Shaked, Y.; Mancuso, P.; Kerbel, R.S. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat. Rev. Cancer 2006, 6, 835–845. [Google Scholar] [CrossRef]
- Dasgupta, A.; Lim, A.R.; Ghajar, C.M. Circulating and disseminated tumor cells: Harbingers or initiators of metastasis. Mol. Oncol. 2017, 11, 40–61. [Google Scholar] [CrossRef]
- Partridge, M.; Phillips, E.; Francis, R.; Li, S.R. Immunomagnetic separation for enrichment and sensitive detection of disseminated tumour cells in patients with head and neck SCC. J. Pathol. 1999, 189, 368–377. [Google Scholar] [CrossRef]
- Hristozova, T.; Konschak, R.; Stromberger, C.; Fusi, A.; Liu, Z.; Weichert, W.; Stenzinger, A.; Budach, V.; Keilholz, U.; Tinhofer, I. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann. Oncol. 2011, 22, 1878–1885. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Lang, J.C.; Jatana, K.R.; Miller, B.; Ozer, E.; Old, M.; Schuller, D.E.; Agrawal, A.; Teknos, T.N.; Summers, T.A., Jr.; et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS ONE 2012, 7, e42048. [Google Scholar] [CrossRef]
- Buglione, M.; Grisanti, S.; Almici, C.; Mangoni, M.; Polli, C.; Consoli, F.; Verardi, R.; Costa, L.; Paiar, F.; Pasinetti, N.; et al. Circulating tumour cells in locally advanced head and neck cancer: Preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur. J. Cancer 2012, 48, 3019–3026. [Google Scholar] [CrossRef]
- Nonaka, T.; Wong, D.T.W. Liquid Biopsy in Head and Neck Cancer: Promises and Challenges. J. Dent. Res. 2018, 97, 701–708. [Google Scholar] [CrossRef]
- Jatana, K.R.; Balasubramanian, P.; McMullen, K.P.; Lang, J.C.; Teknos, T.N.; Chalmers, J.J. Effect of surgical intervention on circulating tumor cells in patients with squamous cell carcinoma of the head and neck using a negative enrichment technology. Head Neck 2016, 38, 1799–1803. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Padmanaban, V.; Silvestri, V.; Schipper, K.; Cohen, J.D.; Fairchild, A.N.; Gorin, M.A.; Verdone, J.E.; Pienta, K.J.; Bader, J.S.; et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. USA 2016, 113, E854–E863. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017, 34, 12. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Koppaka, D.; Anand, A.; Deb, B.; Grenci, G.; Viasnoff, V.; Thompson, E.W.; Gowda, H.; Bhat, R.; Rangarajan, A.; et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci. Rep. 2019, 9, 7933. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.B.; Braun, A.C.; Nicolau, U.R.; Abdallah, E.A.; da Silva Alves, V.; de Jesus, V.H.; Calsavara, V.F.; Kowaslki, L.P.; Chinen, L.T. Prognostic impact and potential predictive role of baseline circulating tumor cells in locally advanced head and neck squamous cell carcinoma. Oral Oncol. 2021, 121, 105480. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Sagalowsky, A.; Clifford, E.; Beitsch, P.; Saboorian, H.; Euhus, D.; Meng, S.; Morrison, L.; Tucker, T.; Lane, N.; et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin. Cancer Res. 2002, 8, 2073–2084. [Google Scholar]
- Lin, P.P. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 2020, 9, 1539. [Google Scholar] [CrossRef]
- Beerepoot, L.V.; Mehra, N.; Vermaat, J.S.; Zonnenberg, B.A.; Gebbink, M.F.; Voest, E.E. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann. Oncol. 2004, 15, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kraan, J.; Sleijfer, S.; Foekens, J.A.; Gratama, J.W. Clinical value of circulating endothelial cell detection in oncology. Drug Discov. Today 2012, 17, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Strijbos, M.; Gratama, J.; Schmitz, P.; Rao, C.; Onstenk, W.; Doyle, G.; Miller, M.; de Wit, R.; Terstappen, L.; Sleijfer, S. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur. J. Cancer 2010, 46, 2027–2035. [Google Scholar] [CrossRef]
- Poudineh, M.; Sargent, E.H.; Pantel, K.; Kelley, S.O. Profiling circulating tumour cells and other biomarkers of invasive cancers. Nat. Biomed. Eng. 2018, 2, 72–84. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Characterization of single circulating tumor cells. FEBS Lett. 2017, 591, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
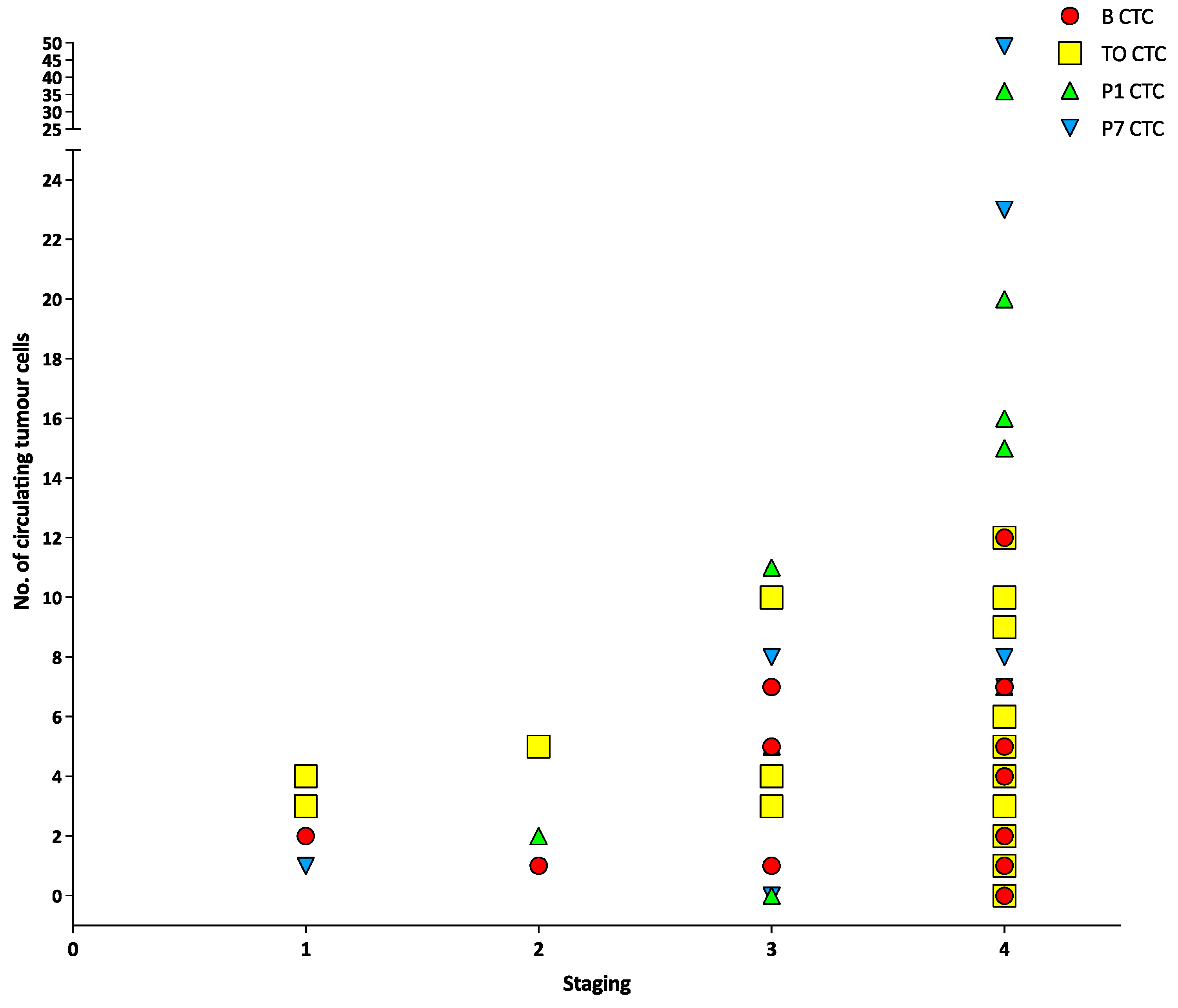


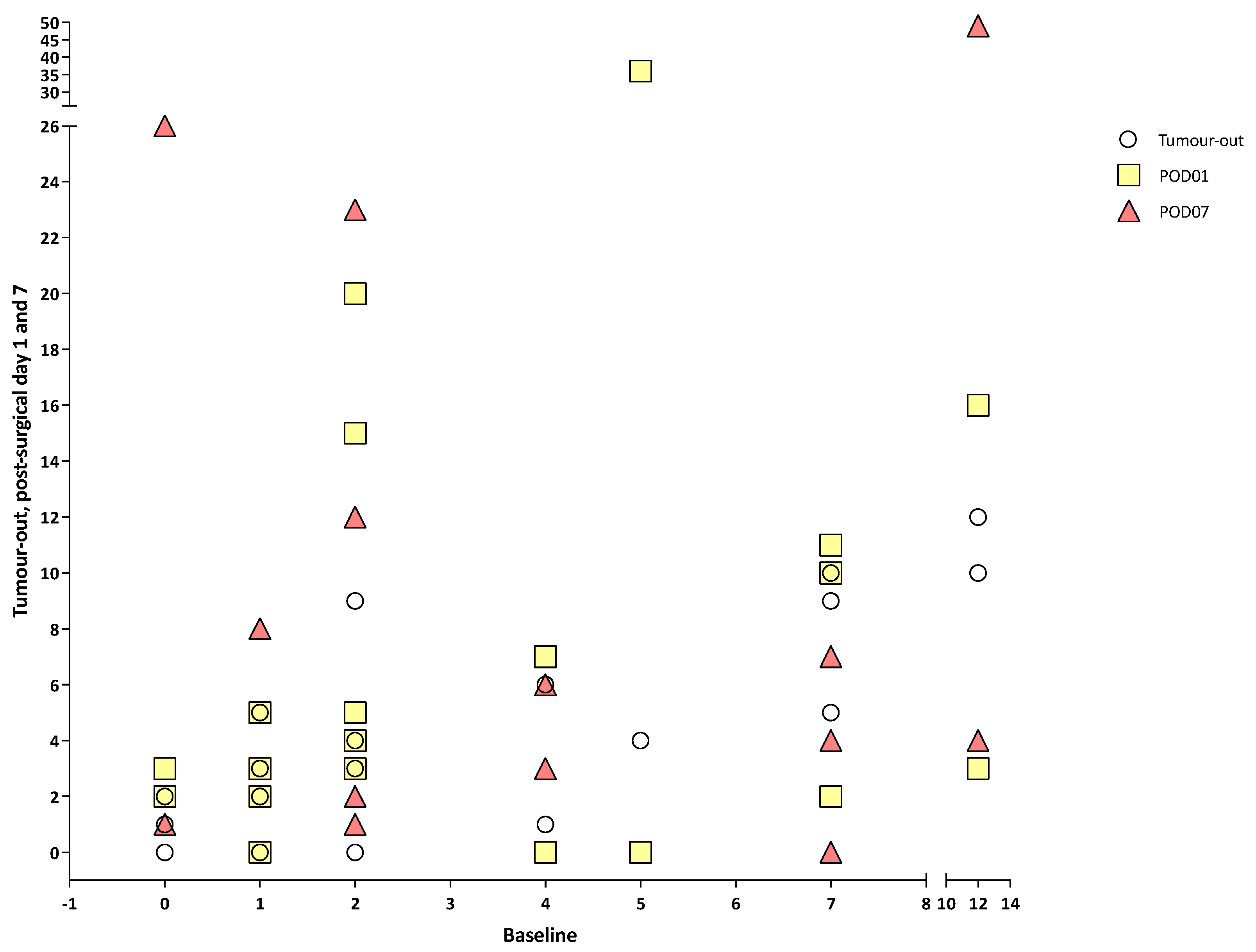
| Variable | All (n = 22) |
|---|---|
| Age in years at first diagnosis, mean (SD) | 65.52 (8.9) |
| Current status (n) | |
| Alive | 21 |
| Died | 1 |
| History of cancer other than head and neck (n) | |
| Yes | 2 |
| No | 19 |
| Missing | 1 |
| Smoking status (at time of diagnosis) (n) | |
| Non-smoker | 14 |
| Past smoker | 4 |
| Current smoker | 4 |
| Alcohol drinking status (at time of diagnosis) (n) | |
| Non-drinker | 13 |
| Past drinker | 2 |
| Current drinker | 6 |
| Unknown | 1 |
| HPV status (n) | |
| Positive | 2 |
| Negative | 5 |
| Unknown | 15 |
| EBER status (n) | |
| Positive | 0 |
| Negative | 3 |
| Unknown | 19 |
| Variable | All (n = 23) |
|---|---|
| Overall tumour site, number (%) | |
| Tongue (anterior) | 3 (13.0%) |
| Tongue (base/posterior) | 4 (17.4%) |
| Buccal mucosa | 4 (17.4%) |
| Floor of mouth | 2 (8.7%) |
| Gingiva (mandibular) | 3 (13.0%) |
| Gingiva (maxillary) | 1 (4.3%) |
| Soft palate/oropharyngeal wall | 1 (4.3%) |
| Retromolar area | 1 (4.3%) |
| Hard palate | 2 (13.0%) |
| Overlapping sites | 1 (4.3%) |
| pTNM, number (%) | |
| pT | |
| T1 | 3 (13.0%) |
| T2 | 6 (26.1%) |
| T3 | 2 (8.7%) |
| T4a | 10 (52.2%) |
| pN | |
| Nx | 1 (4.3%) |
| N0 | 6 (34.8%) |
| N1 | 4 (17.4%) |
| N2a | 1 (4.3%) |
| N2b | 2 (8.7%) |
| N2c | 2 (8.7%) |
| N3 | 1 (4.3%) |
| Missing | 2 (13.0%) |
| pM | |
| M0 | 4 (17.4%) |
| M1 | 0 (0.0%) |
| Missing | 18 (82.6%) |
| Overall stage | |
| Stage 1 | 2 |
| Stage 2 | 1 |
| Stage 3 | 3 |
| Stage 4A | 16 |
| Neck dissection | |
| No | 2 (8.7%) |
| Yes | 20 (91.3%) |
| Tumour grading | |
| Well differentiated | 4 (17.4%) |
| Moderately differentiated | 9 (39.1%) |
| Poorly differentiated | 5 (21.7%) |
| In situ | 1 (4.3%) |
| Missing | 3 (13.0%) |
| Chemo-radiotherapy scheme | |
| CTRT | 6 (26.1%) |
| RT | 10 (47.8%) |
| No treatment | 0 (0.0%) |
| Missing | 6 (26.1%) |
| Resection margin | |
| Negative | 20 (91.3%) |
| Positive | 0 (0.0%) |
| Missing | 2 (8.7%) |
| Patient Code | Circulating Tumour Cells | Circulating Tumour Microemboli | Circulating Tumour-Derived Endothelial Cels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Tumour-Out | POD01 | POD07 | Baseline | Tumour-Out | POD01 | POD07 | Baseline | Tumour-out | POD01 | POD07 | |
| A | 4 | 6 | 7 | 6 | 0 | 1 | 4 | 0 | 1 | 2 | 0 | 0 |
| B | 2 | 9 | 15 | 23 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 5 |
| C | 2 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| D | 7 | 5 | 2 | 7 | 4 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| E | 1 | 5 | 2 | NA | 0 | 1 | 0 | NA | 0 | 0 | 0 | NA |
| F | 5 | 4 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 3 | 0 | 0 |
| G | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| H | 4 | 1 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| I | 2 | 3 | 20 | 12 | 0 | 4 | 3 | 4 | 14 | 0 | 10 | 10 |
| J | 0 | 0 | NA | 3 | 0 | 0 | NA | 2 | 0 | 0 | NA | 0 |
| K | 7 | 9 | 10 | 4 | 5 | 4 | 5 | 10 | 1 | 0 | 23 | 20 |
| L | 12 | 12 | 3 | 4 | 7 | 4 | 1 | 1 | 2 | 1 | 0 | 0 |
| M | 2 | 4 | 5 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| N | 7 | 10 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| O | 12 | 10 | 16 | 49 | 3 | 1 | 0 | 2 | 5 | 9 | 6 | 16 |
| P | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Q | 5 | 4 | 36 | NA | 0 | 0 | 1 | NA | 0 | 0 | 15 | NA |
| R | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S | 2 | 4 | 4 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| T | 2 | 3 | 3 | NA | 0 | 1 | 1 | NA | 2 | 2 | 2 | NA |
| U | 1 | 0 | 0 | 8 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 |
| V | 0 | 1 | 3 | 26 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 4 |
| W | 1 | 3 | 5 | 8 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 |
| None at Baseline | None at Baseline => Tumour-Out + ve | None at Baseline => Post-Operative + ve | |
|---|---|---|---|
| CTC | 2 | 1 | 2 |
| CTM | 16 | 7 | 10 |
| CTEC | 12 | 2 | 7 |
| CTC alone at baseline | 8 (36.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtin, J.; Thomson, P.; Wong, G.; Lam, A.; Choi, S.-W. The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma. Cancers 2023, 15, 584. https://doi.org/10.3390/cancers15030584
Curtin J, Thomson P, Wong G, Lam A, Choi S-W. The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma. Cancers. 2023; 15(3):584. https://doi.org/10.3390/cancers15030584
Chicago/Turabian StyleCurtin, Justin, Peter Thomson, Gordon Wong, Alfred Lam, and Siu-Wai Choi. 2023. "The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma" Cancers 15, no. 3: 584. https://doi.org/10.3390/cancers15030584
APA StyleCurtin, J., Thomson, P., Wong, G., Lam, A., & Choi, S.-W. (2023). The Impact of Surgery on Circulating Malignant Tumour Cells in Oral Squamous Cell Carcinoma. Cancers, 15(3), 584. https://doi.org/10.3390/cancers15030584







