Pediatric Acute Myeloid Leukemia Post Cytotoxic Therapy—Retrospective Analysis of the Patients Treated in Poland from 2005 to 2022
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
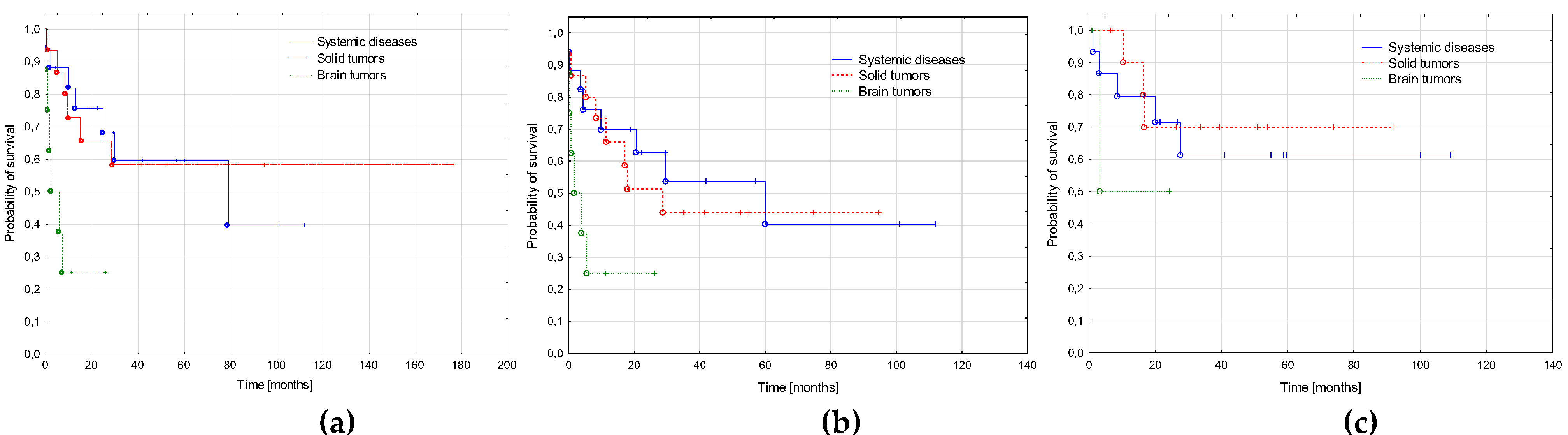
3.1. Primary Cancer
3.2. Latency Period
3.3. AML-pCT Characteristics
3.4. AML-pCT Treatement
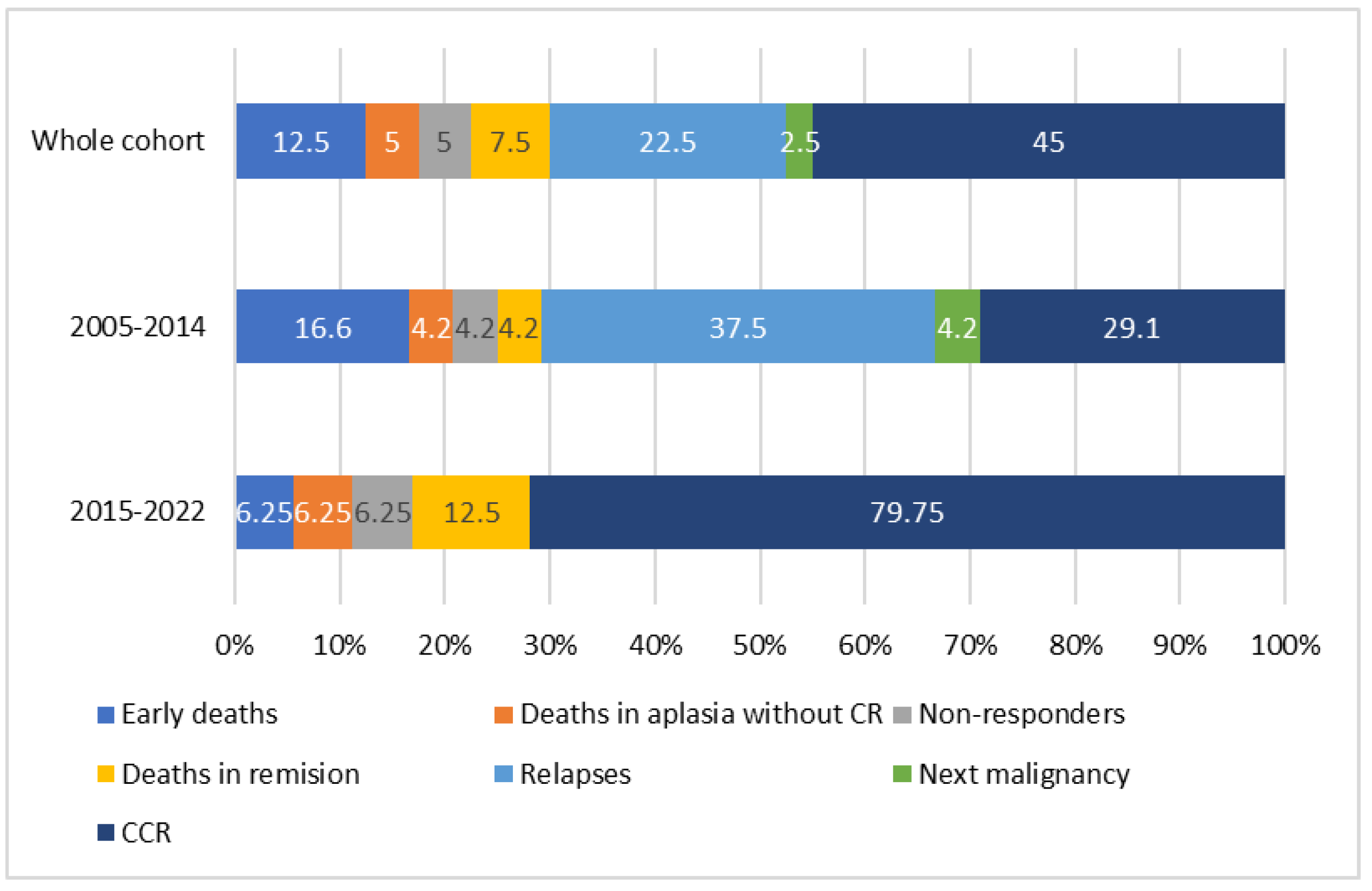
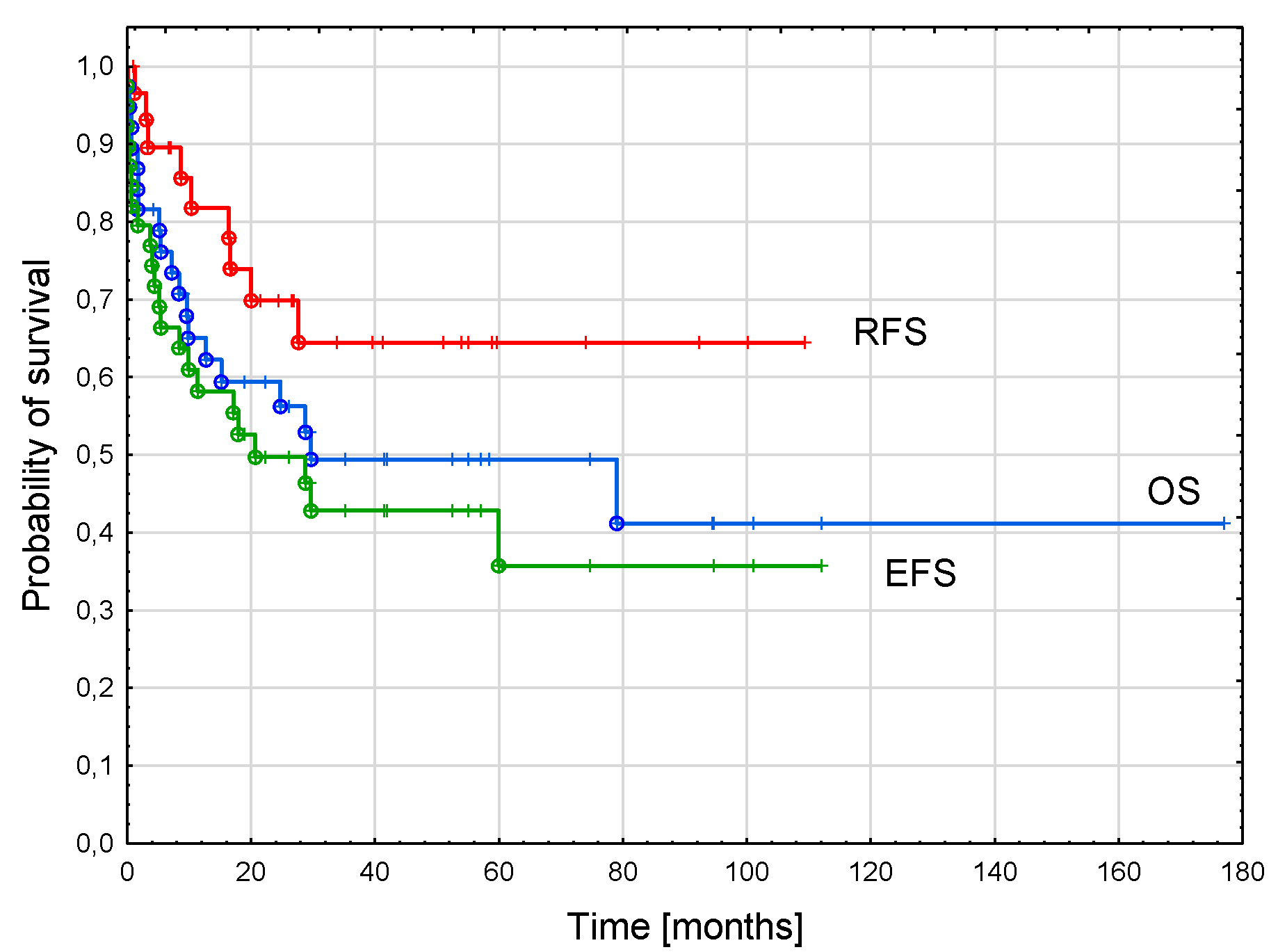
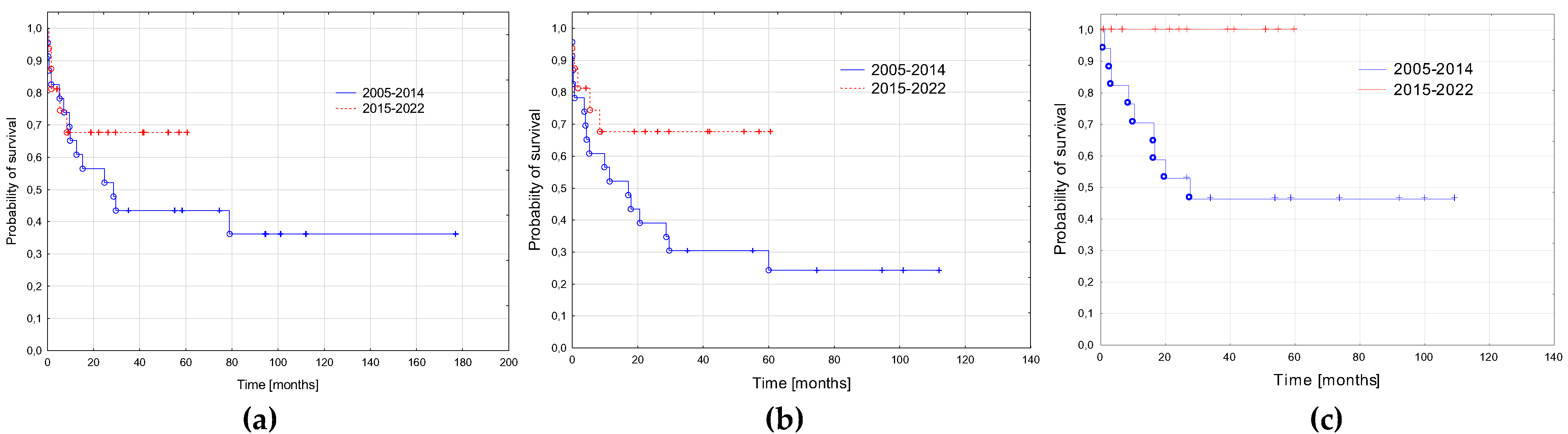
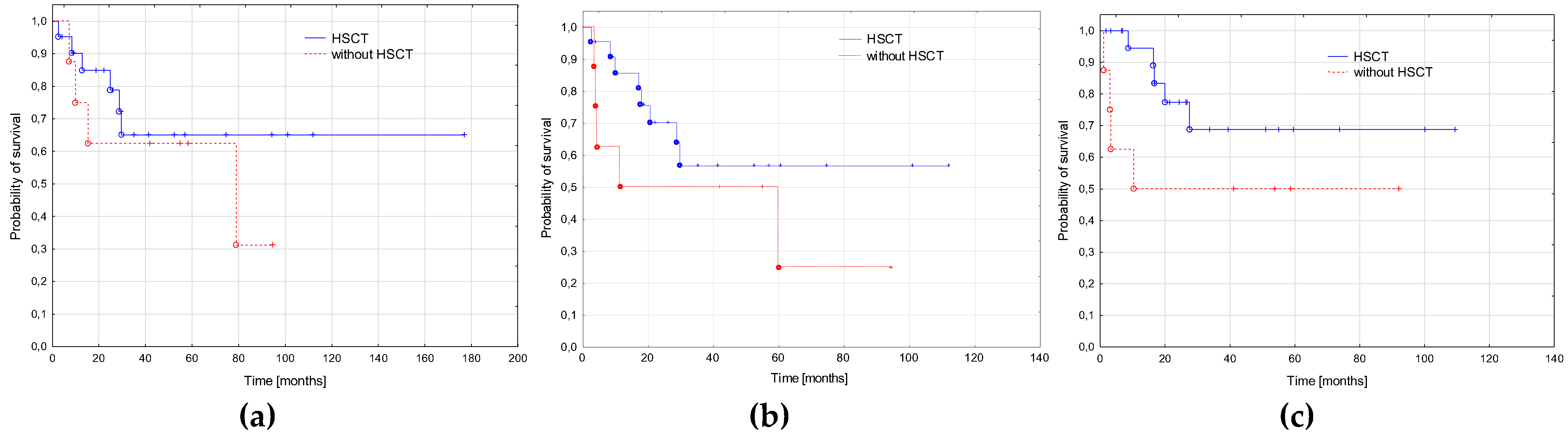
3.5. AML-pCT Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen-Bjergaard, J.; Andersen, M.T.; Andersen, M.K. Genetic pathways in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Hematology Am. Soc. Hematol. Educ. Program 2007, 2007, 392–397. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Brown, C.A.; Youlden, D.R.; Aitken, J.F.; Moore, A.S. Therapy-related acute myeloid leukemia following treatment for cancer in childhood: A population-based registry study. Pediatr. Blood Cancer 2018, 65, e27410. [Google Scholar] [CrossRef]
- Sandoval, C.; Pui, C.H.; Bowman, L.C.; Heaton, D.; Hurwitz, C.A.; Raimondi, S.C.; Behm, F.G.; Head, D.R. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J. Clin. Oncol. 1993, 11, 1039–1045. [Google Scholar] [CrossRef]
- Barnard, D.R.; Woods, W.G. Treatment-related myelodysplastic syndrome/acute myeloid leukemia in survivors of childhood cancer—An update. Leuk. Lymphoma 2005, 46, 651–663. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Ma, J.; Kamens, J.; Westover, T.; Walsh, M.P.; Brady, S.W.; Michael, J.R.; Chen, X.; Montefiori, L.; Song, G.; et al. The acquisition of molecular drivers in pediatric therapy-related myeloid neoplasms. Nat. Commun. 2021, 12, 985. [Google Scholar] [CrossRef]
- Aguilera, D.G.; Vaklavas, C.; Tsimberidou, A.M.; Wen, S.; Medeiros, L.J.; Corey, S.J. Pediatric therapy-related myelodysplastic syndrome/acute myeloid leukemia: The MD Anderson Cancer Center experience. J. Pediatr. Hematol. Oncol. 2009, 31, 803–811. [Google Scholar] [CrossRef]
- Waack, K.; Röllecke, K.; Rasche, M.; Walter, C.; Creutzig, U.; Reinhardt, D. Treatment-Related Acute Myeloid Leukemia in Children. Blood 2019, 134 (Suppl. S1), 1322. [Google Scholar] [CrossRef]
- Imamura, T.; Taga, T.; Takagi, M.; Kawasaki, H.; Koh, K.; Taki, T.; Adachi, S.; Manabe, A.; Ishida, Y.; Leukemia/Lymphoma Committee; et al. Nationwide survey of therapy-related leukemia in childhood in Japan. Int. J. Hematol. 2018, 108, 91–97. [Google Scholar] [CrossRef]
- Kowalczyk, J. Epidemiologia nowotworów złośliwych u dzieci. In Onkologia I Hematologia Dziecięca; Chybicka, A., Sawicz-Birkowska, K., Kazanowska, B., Eds.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2021; Volume 1, pp. 19–26. [Google Scholar]
- Tragiannidis, A.; Gombakis, N.; Papageorgiou, M.; Hatzipantelis, E.; Papageorgiou, T.; Hatzistilianou, M. Treatment-related myelodysplastic syndrome (t-MDS)/acute myeloid leukemia (AML) in children with cancer: A single-center experience. Int. J. Immunopathol. Pharmacol. 2016, 29, 729–730. [Google Scholar] [CrossRef]
- Cho, H.W.; Choi, J.B.; Yi, E.S.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Therapy-related myeloid neoplasms in children and adolescents. Blood Res. 2016, 51, 242–248. [Google Scholar] [CrossRef]
- Tsurusawa, M.; Manabe, A.; Hayashi, Y.; Akiyama, Y.; Kigasawa, H.; Inada, H.; Noguchi, Y.; Sawai, N.; Kobayashi, R.; Nagatoshi, Y.; et al. Therapy-related myelodysplastic syndrome in childhood: A retrospective study of 36 patients in Japan. Leuk. Res. 2005, 29, 625–632. [Google Scholar] [CrossRef]
- Strickland, S.A.; Vey, N. Diagnosis and treatment of therapy-related acute myeloid leukemia. Crit. Rev. Oncol. /Hematol. 2022, 171, 103607. [Google Scholar] [CrossRef]
- Cowell, I.G.; Austin, C.A. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int. J. Environ. Res. Public Health 2012, 9, 2075–2091. [Google Scholar] [CrossRef]
- Olney, H.J.; Mitelman, F.; Johansson, B.; Mrozek, K.; Berger, R.; Rowley, J.D. Unique balanced chromosome abnormalities in treatment-related myelodysplastic syndromes and acute myeloid leukemia: Report from an international workshop. Genes Chromosomes Cancer 2002, 33, 413–423. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Hale, G.A.; Heslop, H.E.; Bowman, L.C.; Rochester, R.A.; Pui, C.H.; Brenner, M.K.; Krance, R.A. Bone marrow transplantation for therapy-induced acute myeloid leukemia in children with previous lymphoid malignancies. Bone Marrow Transplant. 1999, 24, 735–739. [Google Scholar] [CrossRef]
- Woodard, P.; Barfield, R.; Hale, G.; Horwitz, E.; Leung, W.; Ribeiro, R.; Rubnitz, J.; Srivistava, D.K.; Tong, X.; Yusuf, U.; et al. Outcome of hematopoietic stem cell transplantation for pediatric patients with therapy-related acute myeloid leukemia or myelodysplastic syndrome. Pediatr. Blood Cancer. 2006, 47, 931–935. [Google Scholar] [CrossRef]
- Czogała, M.; Balwierz, W.; Pawińska-Wąsikowska, K.; Książek, T.; Bukowska-Strakova, K.; Czogała, W.; Sikorska-Fic, B.; Matysiak, M.; Skalska-Sadowska, J.; Wachowiak, J.; et al. Advances in the First Line Treatment of Pediatric Acute Myeloid Leukemia in the Polish Pediatric Leukemia and Lymphoma Study Group from 1983 to 2019. Cancers 2021, 13, 4536. [Google Scholar] [CrossRef]
- Schmiegelow, K.; Levinsen, M.F.; Attarbaschi, A.; Baruchel, A.; Devidas, M.; Escherich, G.; Gibson, B.; Heydrich, C.; Horibe, K.; Ishida, Y.; et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 31, 2469–2476. [Google Scholar] [CrossRef]
- Hu, Y.; Caldwell, K.J.; Onciu, M.; Federico, S.M.; Salek, M.; Lewis, S.; Lei, S.; Zhang, J.; Nichols, K.E.; Takemoto, C.M.; et al. CPX-351 induces remission in newly diagnosed pediatric secondary myeloid malignancies. Blood Adv. 2022, 6, 521–527. [Google Scholar] [CrossRef]
- Oliai, C.; Schiller, G. How to address second and therapy-related acute myelogenous leukaemia. Br. J. Haematol. 2020, 188, 116–128. [Google Scholar] [CrossRef]
- Fianchi, L.; Criscuolo, M.; Lunghi, M.; Gaidano, G.; Breccia, M.; Levis, A.; Finelli, C.; Santini, V.; Musto, P.; Oliva, E.N.; et al. Outcome of therapy-related myeloid neoplasms treated with azacitidine. J. Hematol. Oncol. 2012, 5, 44. [Google Scholar] [CrossRef]
- Reinhardt, D.; Hasle, H.; Nysom, K.; Baruchel, A.; Locatelli, F.; Benettaib, B.; Biserna, N.; Patturajan, M.; Simcoc, M.; Gaud, A.; et al. Efficacy, Safety, and Pharmacokinetics (PK) of Azacitidine (AZA) in Children and Young Adults with AcuteMyeloid Leukemia(AML) inthePhase2AZA-AML-004Trial. Blood 2020, 136 (Suppl. S1), 10–11. [Google Scholar] [CrossRef]
- Sun, W.; Triche, T., Jr.; Malvar, J.; Gaynon, P.; Sposto, R.; Yang, X.; Bittencourt, H.; Place, A.E.; Messinger, Y.; Fraser, C.; et al. A phase 1 study of azacitidine combined with chemotherapy in child hood leukemia: A report from the TACL consortium. Blood 2018, 131, 1145–1148. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Di Nardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Döhner, H.; Fenaux, P.; Recher, C.; Konopleva, M.; et al. A randomized, double-blind, placebo-controlled study of venetoclax with azacitidine vs azacitidine in treatment-naïve patients with acute myeloid leukemia ineligible for intensive therapy: VIALE-A. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Venditti, A.; Voso, M.T.; Mazzone, C.; Magro, D.; et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: A nonrandomized, open-label, phase II study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Paschka, P.; Schlenk, R.F.; Weber, D.; Benner, A.; Bullinger, L.; Heuser, M.; Gaidzik, V.; Thol, F.; Agrawal, M.; Teleanu, V.; et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia 2018, 32, 1621–1630. [Google Scholar] [CrossRef]
| Period | 2005–2014 | 2015–2022 | All Patients |
|---|---|---|---|
| Number of patients | 24 | 16 | 40 |
| Observation end-point | 31/12/2020 | 31/12/2021 | |
| Median observation time (range) [months] | 94.4 (35.2–176.9) | 29.5 (4.2–57.1) | 53.7 (7.0–176.9) |
| Gender: male/female | 10/14 | 9/7 | 19/21 |
| Age at primary cancer diagnosis—median (range) [years] | 9.4 (0.5–15.7) | 9.3 (0.7–15.9) | 9.4 (0.5–15.9) |
| Age at AML-pCT diagnosis—median (range) [years] | 13.0 (2.7–18.2) | 12.4 (2.8–18.5) | 12.8 (2.7–18.5) |
| Latency period—median (range) [years] | 3.1 (1.5–10.5) | 2.7 (0.7–12.9) | 2.9 (0.7–12.9) |
| Primary neoplasm n (%) | |||
| ALL | 8 (33.3) | 5 (31.2) | 13 (32.5)) |
| Brain tumors | 4 (16.7) | 4 (25) | 8 (20) |
| NBL | 4 (16.7) | 0 | 4 (10) |
| RMS | 3 (12.5) | 1 (6.25) | 4 (10) |
| Osteosarcoma | 2 (8.3) | 1 (6.25) | 3 (7.5)) |
| RBL | 1 (4.2) | 1 (6.25) | 2 (5) |
| HLH | 0 | 2 (12.5) | 2 (5) |
| JMML | 1 (4.2) | 0 | 1 (2.5) |
| Nephroblastoma | 0 | 1 (6.250 | 1 (2.5) |
| Immature teratoma | 1 (4.2) | 0 | 1 (2.5) |
| AML | 0 | 1 (6.25) | 1 (2.5) |
| Alkylating Agents | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | Ifosfamide | Melphalan | Busulfan | Lomustine | Temozolomide | Cisplatin | Carboplatin | |
| Number of patients (%) | 25 (62.5) | 14 (35) | 3 (7.5) | 3 (7.5) | 7 (17.5) | 1 (2.5) | 16 (4) | 18 (4.5) |
| Topoisomerase II inhibitors | ||||||||
| Daunorubicin | Doxorubicin | Idarubicin | Etoposide | |||||
| Number of patients (%) | 15 (37.5) | 19 (47.5) | 1 (2.5) | 24 (60) | ||||
| Primary Neoplasm—Number of Patients (%) | Latency Period—Median (Range) [Months] | Genetics —Number of Patients (% of Patients with Available Result) | |||
|---|---|---|---|---|---|
| Complex Karyotype | Aneuploidies | KMT2A Rearrangements | CBF Mutations | ||
| ALL—13 (32.5) | 24.8 (16.2–117.1) | 2 (25.0) | 3 (37.5) | 4 (40) | 1 (10) |
| Brain tumors—8 (20) | 30.4 (23.2–54.8) | 0 | 5 (71.4) | 2 (33.3) | 0 |
| NBL—4 (10) | 52.9 (50.6–125.9) | 1 (33.3) | 1 (33.3) | 0 | 2 (66.6) |
| RMS—4 (10) | 35.7 (22.4–45.7) | 1 (50) | 1 (50) | 0 | 1 (50) |
| Osteosarcoma—3 (7.5) | 45.6 (42.2–48.9) | 1 (50) | 1 (50) | 1 (33.3) | 0 |
| RBL—2 (5) | 95.0 (35.1–154.8) | 0 | 1 (50) | 0 | 1 (50) |
| HLH—2 (5) | 20.1 (7.3–32.8) | 0 | 1 (50) | 1 (50) | 0 |
| JMML—1 (2.5) | 52.1 | 0 | 0 | 0 | 0 |
| Nephroblastoma—1 (2.5) | 25.4 | 0 | 0 | 1 | 0 |
| Immature teratoma—1 (2.5) | 121.3 | 0 | 1 | 0 | 0 |
| AML—1 (2.5) | 77.9 | 0 | 0 | 0 | 0 |
| Period | 2005–2014 | 2015–2022 | All Patients |
|---|---|---|---|
| Number of patients | 24 | 16 | 40 |
| FAB types n (%) | |||
| M0 | 5 (20.9) | 0 | 5 (12.5) |
| M1 | 0 | 3 (18.7) | 3 (7.5) |
| M2 | 4 (16.7) | 1 (6.2) | 5 (12.5) |
| M3 | 0 | 0 | 0 |
| M4 | 4 (16.7) | 0 | 4 (10) |
| M5 | 7 (29.2) | 3 (18.7) | 10 (25) |
| M6 | 0 | 0 | 0 |
| M7 | 0 | 1 (6.2) | 1 (2.5) |
| Non defined | 4 (16.7) | 8 (50) | 12 (30) |
| WBC at diagnosis—median (range) [103/µL] | 5.2 (1.5–120.0) | 3.0 (1.1–309.8) | 4.5 (1.1–309.8) |
| Cytogenetics—number of results (%) | 16 (66.7) | 11 (68.7) | 27 (67.5) |
| Normal karyotype | 2 (12.5) | 2 (18.2) | 4 (14.8) |
| Complex karyotype | 3 (18.7) | 2 (18.2) | 5 (18.5)) |
| Monosomy 7 | 2 (12.5) | 2 (18.2) | 4 (14.8) |
| Monosomy Y | 0 | 3 (27.3) | 3 (11.1) |
| Trisomy 8 | 1 (6.2) | 1 (9.1) | 2 (7.4) |
| KMT2A rearrangements | 2 (12.5) | 4 (36.4) | 6 (22.2) |
| t(8;21)(q22;q22) | 2 (12.5) | 1 (9.1) | 3 (11.1) |
| inv16 | 1 (6.2) | 0 | 1 (3.7) |
| Molecular genetics—number of results (%) | 14 (87.5) | 16 (100) | 30 (75) |
| KMT2A rearrangements | 2 (14.3) | 7 (43.7) | 9 (30) |
| RUNX1::RUNX1T1 fusion | 2 (14.3) | 2 (12.5) | 4 (13.3) |
| CBFβ::MYH11 fusion | 1 (7.1) | 0 | 1 (3.3) |
| No fusion genes found | 9 (64.3) | 7 (43.7) | 16 (53.3) |
| Period | 2005–2014 | 2015–2022 |
|---|---|---|
| Number of patients | 24 | 16 |
| Standard AML therapy—number of treated patients (%) | AML-BFM 2004 Interim—23 (96) | AML-BFM 2012 Registry—7 (44) AML-BFM 2019—6 (38) AML-BFM 2019 + GO (6) |
| Other—number of treated patients (%) | IdaFLA+ FLA—(4) | IdaFLA + FLA, Venetoclax, Azacitidine—1 (6), Venetoclax, Azacitidine—1 (6) |
| Number of chemotherapy cycles before SCT—median (range) | 4 (2–4) | 2 (2–4) |
| SCT—number of patients (%) | 11 (46) | 11 (69) |
| Primary Diagnosis (N) | Non-Responders | Early Deaths | Deaths in Aplasia | Complete Remission | CCR | Deaths in Remission | Relapses | Deaths after Relapse | II CCR |
|---|---|---|---|---|---|---|---|---|---|
| N (%) | |||||||||
| ALL (13) | 1 (7.7) | 1 (7.7) | 0 | 11 (84.6) | 6 (46.1) | 0 | 5 (38.5) | 4 (30.8) | 1 (7.7) |
| Brain tumors (8) | 0 | 3 (37.5) | 1 (12.5) | 4 (50) | 2 (25) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 0 |
| NBL (4) | 1 (25) | 0 | 0 | 3 (75) | 2 (50) | 0 | 1 (25) | 0 | 1 (25) |
| RMS (4) | 0 | 1 (25) | 1 (25) | 2 (50) | 1 (25) | 0 | 1 (25) | 1 (25) | 0 |
| Osteosarcoma (3) | 0 | 0 | 0 | 3 (100) | 2 (66.7) | 1 (33.3) | 0 | 0 | 0 |
| RBL (2) | 0 | 0 | 0 | 2 (100) | 1 (50) | 0 | 1 (50) | 0 | 1 (50) |
| HLH (2) | 0 | 0 | 0 | 2 (100) | 2 (100) | 0 | 0 | 0 | 0 |
| JMML (1) | 0 | 0 | 0 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 |
| Nephroblastoma (1) | 0 | 0 | 0 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 |
| Immature teratoma (1) | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) | 0 | 0 | 0 |
| AML (1) | 0 | 0 | 0 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czogała, M.; Czogała, W.; Pawińska-Wąsikowska, K.; Książek, T.; Bukowska-Strakova, K.; Sikorska-Fic, B.; Łaguna, P.; Skalska-Sadowska, J.; Wachowiak, J.; Rodziewicz-Konarska, A.; et al. Pediatric Acute Myeloid Leukemia Post Cytotoxic Therapy—Retrospective Analysis of the Patients Treated in Poland from 2005 to 2022. Cancers 2023, 15, 734. https://doi.org/10.3390/cancers15030734
Czogała M, Czogała W, Pawińska-Wąsikowska K, Książek T, Bukowska-Strakova K, Sikorska-Fic B, Łaguna P, Skalska-Sadowska J, Wachowiak J, Rodziewicz-Konarska A, et al. Pediatric Acute Myeloid Leukemia Post Cytotoxic Therapy—Retrospective Analysis of the Patients Treated in Poland from 2005 to 2022. Cancers. 2023; 15(3):734. https://doi.org/10.3390/cancers15030734
Chicago/Turabian StyleCzogała, Małgorzata, Wojciech Czogała, Katarzyna Pawińska-Wąsikowska, Teofila Książek, Karolina Bukowska-Strakova, Barbara Sikorska-Fic, Paweł Łaguna, Jolanta Skalska-Sadowska, Jacek Wachowiak, Anna Rodziewicz-Konarska, and et al. 2023. "Pediatric Acute Myeloid Leukemia Post Cytotoxic Therapy—Retrospective Analysis of the Patients Treated in Poland from 2005 to 2022" Cancers 15, no. 3: 734. https://doi.org/10.3390/cancers15030734
APA StyleCzogała, M., Czogała, W., Pawińska-Wąsikowska, K., Książek, T., Bukowska-Strakova, K., Sikorska-Fic, B., Łaguna, P., Skalska-Sadowska, J., Wachowiak, J., Rodziewicz-Konarska, A., Moj-Hackemer, M., Kałwak, K., Muszyńska-Rosłan, K., Krawczuk-Rybak, M., Fałkowska, A., Drabko, K., Kozłowska, M., Irga-Jaworska, N., Bobeff, K., ... Skoczeń, S. (2023). Pediatric Acute Myeloid Leukemia Post Cytotoxic Therapy—Retrospective Analysis of the Patients Treated in Poland from 2005 to 2022. Cancers, 15(3), 734. https://doi.org/10.3390/cancers15030734






