FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Cancer Immunotherapy: Classification, Previous and Current Facts
2.1. Historical Overview of Cancer Immunotherapy
2.2. Immunotherapy in Lymphoma
2.2.1. Previous Footprints
2.2.2. Monoclonal Antibodies: From Rituximab to Immune Checkpoint Inhibitors
2.2.3. Adoptive Cell Therapy
2.2.4. Bispecific Antibodies: The Most Recent Addition to the Group
3. Role of PET/CT in Lymphoma Immunotherapy
3.1. Response Patterns
3.1.1. Pseudoprogression
3.1.2. Hyperprogression
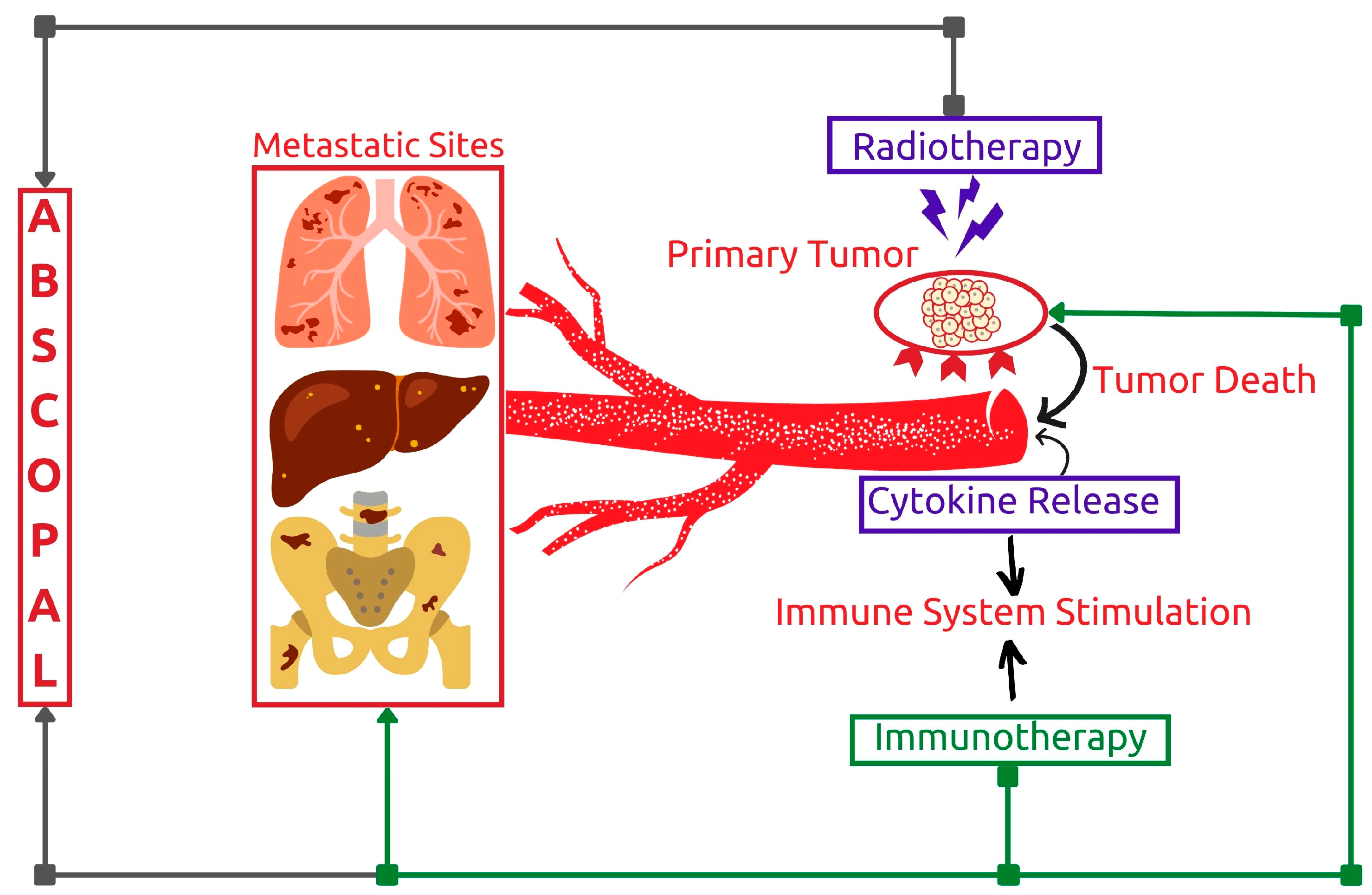
3.1.3. Potentiating Abscopal Effect
3.2. PET Response Criteria in Lymphoma
3.2.1. Lugano Classification
3.2.2. Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC)
3.2.3. Response Evaluation Criteria in Lymphoma (RECIL)
3.3. FDG PET/CT in Hodgkin’s Lymphoma (HL)
3.3.1. Immune Checkpoint Inhibitors
3.3.2. Brentuximab Vedotin (BV)
3.3.3. Chimeric Antigen Receptor Therapy (CAR-T)
3.4. FDG PET/CT in Non-Hodgkin’s Lymphoma (NHL)
3.5. FDG PET/CT in Diffuse Large B-Cell (DLBCL)
3.5.1. Rituximab
3.5.2. Immune Checkpoint Inhibitors (ICI)
3.5.3. Chimeric Antigen Receptor Therapy (CAR-T)
3.6. FDG PET/CT in Follicular Lymphoma (FL)
3.6.1. Rituximab
3.6.2. Chimeric Antigen Receptor Therapy (CAR-T)
3.6.3. Bispecific Antibodies
4. Immunotherapy-Related Adverse Effects
4.1. Monoclonal Antibodies
4.1.1. Immunotherapy-Related Inflammatory Reactions
4.1.2. Reactive Changes
4.1.3. Tumor Flare Reaction (TFR)
4.2. Chimeric Antigen Receptor Therapy (CAR-T)
4.2.1. Cytokine Release Syndrome (CRS)
4.2.2. Immune Effector Cell Associated Neurotoxicity Syndrome (ICANS)
5. Current Challenges and Future Prospects
5.1. Current Challenges
5.1.1. Limitations in Low-Income and Conflict Regions
5.1.2. Financial Burden
5.1.3. Volumetric PET Parameters: Promising Yet Overlooked
5.1.4. Lack of Harmonization in Implementing Novel Response Criteria
5.2. Future Prospects
5.2.1. FDG Alternatives
5.2.2. Radiomics and Machine Learning
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of Cancer. Eur. J. Pharmacol. 2009, 625, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.S.; Liu, S.V.; Giaccone, G. Novel Approaches in Cancer Immunotherapy. Discov. Med. 2016, 21, 267–274. [Google Scholar] [PubMed]
- Choi, B.K.; Kim, S.-H.; Kim, Y.H.; Kwon, B.S. Cancer Immunotherapy Using Tumor Antigen-Reactive T Cells. Immunotherapy 2018, 10, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Oiseth, S.J.; Aziz, M.S. Cancer Immunotherapy: A Brief Review of the History, Possibilities, and Challenges Ahead. J. Cancer Metastasis Treat. 2017, 3, 250. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J. Current Status and Future Directions of Cancer Immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Ruzicka, M.; Fabritius, M.P.; Mittlmeier, L.M.; Winkelmann, M.; Rübenthaler, J.; Brendel, M.; Subklewe, M.; von Bergwelt-Baildon, M.; Ricke, J.; et al. PET/CT Imaging for Tumour Response Assessment to Immunotherapy: Current Status and Future Directions. Eur. Radiol. Exp. 2020, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Decker, W.K.; da Silva, R.F.; Sanabria, M.H.; Angelo, L.S.; Guimarães, F.; Burt, B.M.; Kheradmand, F.; Paust, S. Cancer Immunotherapy: Historical Perspective of a Clinical Revolution and Emerging Preclinical Animal Models. Front. Immunol. 2017, 8, 829. [Google Scholar] [CrossRef]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of Treatment of 255 Patients with Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, T.D.; Janssen, A.B.; van Beusechem, V.W. Arming Oncolytic Viruses to Leverage Antitumor Immunity. Expert Opin. Biol. Ther. 2015, 15, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent Advances of Oncolytic Virus in Cancer Therapy. Hum. Vaccin. Immunother. 2020, 16, 2389–2402. [Google Scholar] [CrossRef]
- Menotti, L.; Avitabile, E. Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy. Int. J. Mol. Sci. 2020, 21, 8310. [Google Scholar] [CrossRef]
- Grillo-López, A.J.; White, C.A.; Varns, C.; Shen, D.; Wei, A.; McClure, A.; Dallaire, B.K. Overview of the Clinical Development of Rituximab: First Monoclonal Antibody Approved for the Treatment of Lymphoma. Semin. Oncol. 1999, 26, 66–73. [Google Scholar]
- Pfreundschuh, M.; Schubert, J.; Ziepert, M.; Schmits, R.; Mohren, M.; Lengfelder, E.; Reiser, M.; Nickenig, C.; Clemens, M.; Peter, N.; et al. Six versus Eight Cycles of Bi-Weekly CHOP-14 with or without Rituximab in Elderly Patients with Aggressive CD20+ B-Cell Lymphomas: A Randomised Controlled Trial (RICOVER-60). Lancet Oncol. 2008, 9, 105–116. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trümper, L.; Osterborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP Alone or with Maintenance Rituximab in Older Patients with Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; van den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Schulz, H.; Bohlius, J.; Skoetz, N.; Trelle, S.; Kober, T.; Reiser, M.; Dreyling, M.; Herold, M.; Schwarzer, G.; Hallek, M.; et al. Chemotherapy plus Rituximab versus Chemotherapy Alone for B-Cell Non-Hodgkin’s Lymphoma. Cochrane Database Syst. Rev. 2007, 2010, CD003805. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus Interference. I. The Interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Finkelstein, S.E.; Surman, D.R.; Lichtman, M.K.; Gattinoni, L.; Theoret, M.R.; Grewal, N.; Spiess, P.J.; Antony, P.A.; Palmer, D.C.; et al. IL-15 Enhances the in Vivo Antitumor Activity of Tumor-Reactive CD8+ T Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Solal-Celigny, P.; Lepage, E.; Brousse, N.; Reyes, F.; Haioun, C.; Leporrier, M.; Peuchmaur, M.; Bosly, A.; Parlier, Y.; Brice, P. Recombinant Interferon Alfa-2b Combined with a Regimen Containing Doxorubicin in Patients with Advanced Follicular Lymphoma. Groupe d’Etude Des Lymphomes de l’Adulte. N. Engl. J. Med. 1993, 329, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Fowler, N.H.; Neelapu, S.S. Targeting the Programmed Death-1/Programmed Death-Ligand 1 Axis in Lymphoma. Curr. Opin. Oncol. 2015, 27, 384–391. [Google Scholar] [CrossRef]
- Menter, T.; Bodmer-Haecki, A.; Dirnhofer, S.; Tzankov, A. Evaluation of the Diagnostic and Prognostic Value of PDL1 Expression in Hodgkin and B-Cell Lymphomas. Hum. Pathol. 2016, 54, 17–24. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Mottaghy, F.M.; Juweid, M.E. PET/CT in Hodgkin Lymphoma: An Update. Semin. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Lopci, E.; Meignan, M. Current Evidence on PET Response Assessment to Immunotherapy in Lymphomas. PET Clin. 2020, 15, 23–34. [Google Scholar] [CrossRef]
- Hutchings, M.; Radford, J.; Ansell, S.M.; Illés, Á.; Sureda, A.; Connors, J.M.; Sýkorová, A.; Shibayama, H.; Abramson, J.S.; Chua, N.S.; et al. Brentuximab Vedotin plus Doxorubicin, Vinblastine, and Dacarbazine in Patients with Advanced-stage, Classical Hodgkin Lymphoma: A Prespecified Subgroup Analysis of High-risk Patients from the ECHELON-1 Study. Hematol. Oncol. 2021, 39, 185–195. [Google Scholar] [CrossRef]
- Mohanty, R.; Chowdhury, C.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T Cell Therapy: A New Era for Cancer Treatment (Review). Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef]
- Abate-Daga, D.; Davila, M.L. CAR Models: Next-Generation CAR Modifications for Enhanced T-Cell Function. Mol. Ther. Oncolytics 2016, 3, 16014. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153. [Google Scholar] [CrossRef]
- Frigault, M.J.; Maus, M. v State of the Art in CAR T Cell Therapy for CD19+ B Cell Malignancies. J. Clin. Investig. 2020, 130, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, Y.J. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front. Immunol. 2020, 11, 176. [Google Scholar] [CrossRef]
- Ruella, M.; Kenderian, S.S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017, 31, 473–481. [Google Scholar] [CrossRef]
- Yip, A.; Webster, R.M. The Market for Chimeric Antigen Receptor T Cell Therapies. Nat. Rev. Drug. Discov. 2018, 17, 161–162. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Cahill, K.E.; Smith, S.M. Follicular Lymphoma: A Focus on Current and Emerging Therapies. Oncology 2022, 36, 97–106. [Google Scholar] [CrossRef]
- Prigent, K.; Aide, N. 18F-Fludeoxyglucose PET/Computed Tomography for Assessing Tumor Response to Immunotherapy and Detecting Immune-Related Side Effects: A Checklist for the PET Reader. PET Clin. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients with advanced melanoma treated with Pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. Clin. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef] [Green Version]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef]
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; le Tourneau, C. Patterns of Response and Progression to Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, J.; Wu, X. Pseudoprogression and Hyperprogression after Checkpoint Blockade. Int. Immunopharmacol. 2018, 58, 125–135. [Google Scholar] [CrossRef]
- Chae, Y.K.; Wang, S.; Nimeiri, H.; Kalyan, A.; Giles, F.J. Pseudoprogression in Microsatellite Instability-High Colorectal Cancer during Treatment with Combination T Cell Mediated Immunotherapy: A Case Report and Literature Review. Oncotarget 2017, 8, 57889–57897. [Google Scholar] [CrossRef]
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification Lymphoma Response Criteria in the Era of Immunomodulatory Therapy. Blood 2016, 128, 2489–2496. [Google Scholar] [CrossRef]
- Tanizaki, J.; Hayashi, H.; Kimura, M.; Tanaka, K.; Takeda, M.; Shimizu, S.; Ito, A.; Nakagawa, K. Report of Two Cases of Pseudoprogression in Patients with Non–Small Cell Lung Cancer Treated with Nivolumab—Including Histological Analysis of One Case after Tumor Regression. Lung Cancer 2016, 102, 44–48. [Google Scholar] [CrossRef]
- McGehee, E.; Patel, H.; Pearson, C.; Clements, K.; Jaso, J.M.; Chen, W.; Callan, A.; Desai, N.; Ramakrishnan Geethakumari, P. Combined Immune Checkpoint Blockade and Radiotherapy Induces Durable Remission in Relapsed Natural Killer/T-Cell Lymphoma: A Case Report and Review of the Literature. J. Med. Case Rep. 2021, 15, 221. [Google Scholar] [CrossRef]
- Kwong, Y.-L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 Blockade with Pembrolizumab Is Highly Effective in Relapsed or Refractory NK/T-Cell Lymphoma Failing l-Asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during Anti-PD-1/PD-L1 Therapy in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onesti, C.E.; Frères, P.; Jerusalem, G. Atypical Patterns of Response to Immune Checkpoint Inhibitors: Interpreting Pseudoprogression and Hyperprogression in Decision Making for Patients’ Treatment. J. Thorac. Dis. 2019, 11, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non–Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Mole, R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Stone, H.B.; Peters, L.J.; Milas, L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. JNCI J. Natl. Cancer Inst. 1979, 63, 1229–1235. [Google Scholar] [CrossRef]
- Kang, J.; Demaria, S.; Formenti, S. Current Clinical Trials Testing the Combination of Immunotherapy with Radiotherapy. J. Immunother. Cancer 2016, 4, 51. [Google Scholar] [CrossRef]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local Radiotherapy and Granulocyte-Macrophage Colony-Stimulating Factor to Generate Abscopal Responses in Patients with Metastatic Solid Tumours: A Proof-of-Principle Trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Lang, D.; Wahl, G.; Poier, N.; Graf, S.; Kiesl, D.; Lamprecht, B.; Gabriel, M. Impact of PET/CT for Assessing Response to Immunotherapy—A Clinical Perspective. J. Clin. Med. 2020, 9, 3483. [Google Scholar] [CrossRef]
- Castello, A.; Grizzi, F.; Qehajaj, D.; Rahal, D.; Lutman, F.; Lopci, E. 18F-FDG PET/CT for Response Assessment in Hodgkin Lymphoma Undergoing Immunotherapy with Checkpoint Inhibitors. Leuk. Lymphoma 2019, 60, 367–375. [Google Scholar] [CrossRef]
- Rossi, C.; Gilhodes, J.; Maerevoet, M.; Herbaux, C.; Morschhauser, F.; Brice, P.; Garciaz, S.; Borel, C.; Ysebaert, L.; Obéric, L.; et al. Efficacy of Chemotherapy or Chemo-Anti-PD-1 Combination after Failed Anti-PD-1 Therapy for Relapsed and Refractory Hodgkin Lymphoma: A Series from Lysa Centers. Am. J. Hematol. 2018, 93, 1042–1049. [Google Scholar] [CrossRef]
- Dercle, L.; Seban, R.-D.; Lazarovici, J.; Schwartz, L.H.; Houot, R.; Ammari, S.; Danu, A.; Edeline, V.; Marabelle, A.; Ribrag, V.; et al. 18F-FDG PET and CT Scans Detect New Imaging Patterns of Response and Progression in Patients with Hodgkin Lymphoma Treated by Anti–Programmed Death 1 Immune Checkpoint Inhibitor. J. Nucl. Med. 2018, 59, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group Consensus Response Evaluation Criteria in Lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Skusa, C.; Weber, M.-A.; Böttcher, S.; Thierfelder, K.M. Criteria-Based Imaging and Response Evaluation of Lymphoma 20 Years After Cheson: What Is New? RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2020, 192, 657–668. [Google Scholar] [CrossRef]
- Cheson, B.D. Rethinking Clinical Response and Outcome Assessment in a Biologic Age. Curr. Oncol. Rep. 2015, 17, 27. [Google Scholar] [CrossRef]
- Waxman, E.S.; Lee Gerber, D. Pseudoprogression and Immunotherapy Phenomena. J. Adv. Pract. Oncol. 2020, 11, 723–731. [Google Scholar] [CrossRef]
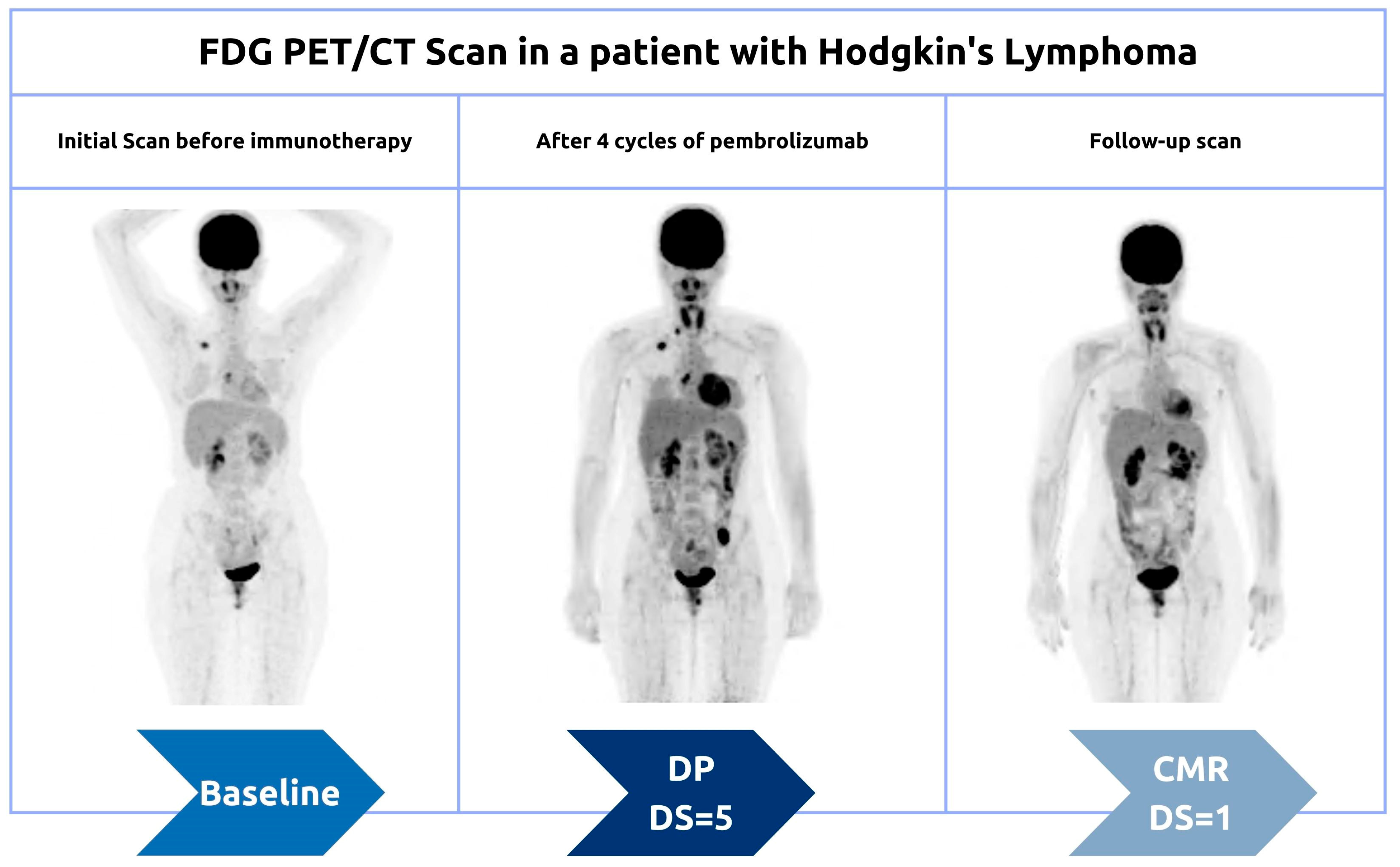
- Al-Ibraheem, A.; Anwer, F.; Juweid, M.E.; Shagera, Q.A.; Khalaf, A.N.; Obeidat, S.; Mansour, A.; Ma’koseh, M.; Halahleh, K.; Jaradat, I.; et al. Interim FDG-PET/CT for Therapy Monitoring and Prognostication in Hodgkin’s Lymphoma. Sci. Rep. 2022, 12, 17702. [Google Scholar] [CrossRef]
- Zaucha, J.M.; Małkowski, B.; Chauvie, S.; Subocz, E.; Tajer, J.; Kulikowski, W.; Fijołek-Warszewska, A.; Biggi, A.; Fallanca, F.; Kobylecka, M.; et al. The Predictive Role of Interim PET after the First Chemotherapy Cycle and Sequential Evaluation of Response to ABVD in Hodgkin’s Lymphoma Patients—The Polish Lymphoma Research Group (PLRG) Observational Study. Ann. Oncol. 2017, 28, 3051–3057. [Google Scholar] [CrossRef]
- Hutchings, M.; Kostakoglu, L.; Zaucha, J.M.; Malkowski, B.; Biggi, A.; Danielewicz, I.; Loft, A.; Specht, L.; Lamonica, D.; Czuczman, M.S.; et al. In Vivo Treatment Sensitivity Testing with Positron Emission Tomography/Computed Tomography After One Cycle of Chemotherapy for Hodgkin Lymphoma. J. Clin. Oncol. 2014, 32, 2705–2711. [Google Scholar] [CrossRef]
- Biggi, A.; Gallamini, A.; Chauvie, S.; Hutchings, M.; Kostakoglu, L.; Gregianin, M.; Meignan, M.; Malkowski, B.; Hofman, M.S.; Barrington, S.F. International Validation Study for Interim PET in ABVD-Treated, Advanced-Stage Hodgkin Lymphoma: Interpretation Criteria and Concordance Rate Among Reviewers. J. Nucl. Med. 2013, 54, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.-M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed Death-1 Blockade with Pembrolizumab in Patients with Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for Classical Hodgkin’s Lymphoma after Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multicentre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Ammari, S.; Seban, R.-D.; Schwartz, L.H.; Houot, R.; Labaied, N.; Mokrane, F.-Z.; Lazarovici, J.; Danu, A.; Marabelle, A.; et al. Kinetics and Nadir of Responses to Immune Checkpoint Blockade by Anti-PD1 in Patients with Classical Hodgkin Lymphoma. Eur. J. Cancer 2018, 91, 136–144. [Google Scholar] [CrossRef]
- Lepik, K.V.; Volkov, N.P.; Mikhailova, N.B.; Kondakova, E.V.; Tsvetkova, L.A.; Zalyalov, Y.R.; Lepik, E.E.; Fedorova, L.V.; Beinarovich, A.V.; Demchenkova, M.V.; et al. Long-Term Outcomes of Nivolumab Therapy in Patients with Relapsed/Refractory Classic Hodgkin’s Lymphoma after High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation in Real Clinical Practice. Klin. Onkogematol./Clin. Oncohematol. 2020, 13, 280–288. [Google Scholar] [CrossRef]
- Fedorova, L.; Lepik, K.; Mikhailova, N.; Kondakova, E.; Kotselyabina, P.; Shmidt, D.I.; Kozlov, A.; Zalyalov, Y.; Borzenkova, E.; Baykov, V.; et al. 903P Combination of Nivolumab with Brentuximab Vedotin in Therapy of Relapsed and Refractory Hodgkin Lymphoma. Ann. Oncol. 2020, 31, S655. [Google Scholar] [CrossRef]
- Parmar, K.; Dwarampudi, R.; Tijani, L.; Jakubski, S.; Rehman, S. Combination Therapy of Nivolumab in First Line and Relapsed/Refractory Classic Hodgkin’s Lymphoma: A Systematic Review and Meta-Analysis of Clinical Trials. Blood 2022, 140, 12031–12032. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S.; et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma. JAMA Oncol. 2020, 6, 872. [Google Scholar] [CrossRef]
- Lee, H.; Flinn, I.W.; Melear, J.; Ramchandren, R.; Friedman, J.; Burke, J.M.; Linhares, Y.; Raval, M.; Chintapatla, R.; Feldman, T.A.; et al. P1089: Brentuximab Vedotin, Nivolumab, Doxorubicin, And Dacarbazine (AN+AD) for Advanced Stage Classic Hodgkin Lymphoma: Preliminary Safety and Efficacy Results from the Phase 2 Study (SGN35 027 Part B). Hemasphere 2022, 6, 979–980. [Google Scholar] [CrossRef]
- Park, S.I.; Ansell, S.M.; Giri, S.; Svoboda, J.; Smith, S.D.; Feldman, T.; Budde, E.L.; Ness, A.J.; Choi, Y.; Bierman, P.J.; et al. Frontline PET-Directed Therapy with Brentuximab Vedotin Plus AVD Followed By Nivolumab Consolidation in Patients with Limited Stage Hodgkin Lymphoma. Blood 2022, 140, 1751–1752. [Google Scholar] [CrossRef]
- Gibb, A.; Jones, C.; Bloor, A.; Kulkarni, S.; Illidge, T.; Linton, K.; Radford, J. Brentuximab Vedotin in Refractory CD30+ Lymphomas: A Bridge to Allogeneic Transplantation in Approximately One Quarter of Patients Treated on a Named Patient Programme at a Single UK Center. Haematologica 2013, 98, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Rothe, A.; Sasse, S.; Goergen, H.; Eichenauer, D.A.; Lohri, A.; Jäger, U.; Bangard, C.; Böll, B.; von Bergwelt Baildon, M.; Theurich, S.; et al. Brentuximab Vedotin for Relapsed or Refractory CD30+ Hematologic Malignancies: The German Hodgkin Study Group Experience. Blood 2012, 120, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Viviani, S.; Anastasia, A.; Vitolo, U.; Luminari, S.; Zaja, F.; Corradini, P.; Spina, M.; Brusamolino, E.; Gianni, A.M.; et al. Brentuximab Vedotin in Relapsed/Refractory Hodgkin’s Lymphoma: The Italian Experience and Results of Its Use in Daily Clinical Practice Outside Clinical Trials. Haematologica 2013, 98, 1232–1236. [Google Scholar] [CrossRef]
- Kahraman, D.; Theurich, S.; Rothe, A.; Kuhnert, G.; Sasse, S.; Scheid, C.; Dietlein, M.; Drzezga, A.; von Bergwelt-Baildon, M.; Kobe, C. 18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Assessment of Response to Brentuximab Vedotin Treatment in Relapsed and Refractory Hodgkin Lymphoma. Leuk. Lymphoma 2014, 55, 811–816. [Google Scholar] [CrossRef]
- Kedmi, M.; Khaustov, P.; Ribakovsy, E.; Benjamini, O.; Avigdor, A. Outcomes Related to FDG-PET-CT Response in Patients with Hodgkin Lymphoma Treated with Brentuximab-Vedotin at Relapse or Consolidation. Clin. Lymphoma Myeloma Leuk. 2021, 21, e929–e937. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Chen, R.W.; Ansell, S.M.; Gallamini, A.; Connors, J.M.; Savage, K.J.; Collins, G.P.; Grigg, A.; Sureda, A.M.; Ghosh, N.; Feldman, T.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin Lymphoma (HL): Impact of Cycle 2 PET Result on Modified Progression-Free Survival (MPFS). J. Clin. Oncol. 2018, 36, 7539. [Google Scholar] [CrossRef]
- Ramchandren, R.; Advani, R.H.; Ansell, S.M.; Bartlett, N.L.; Chen, R.W.; Feldman, T.; Forero-Torres, A.; Friedberg, J.W.; Gopal, A.K.; Gordon, L.I.; et al. Brentuximab Vedotin (BV) plus Chemotherapy in Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma (HL): North American Results. J. Clin. Oncol. 2018, 36, 7541. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Kreissl, S.; Sökler, M.; Hellmuth, J.C.; Meissner, J.; Mathas, S.; Topp, M.S.; Behringer, K.; Klapper, W.; et al. Incorporation of Brentuximab Vedotin into First-Line Treatment of Advanced Classical Hodgkin’s Lymphoma: Final Analysis of a Phase 2 Randomised Trial by the German Hodgkin Study Group. Lancet Oncol. 2017, 18, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Gavane, S.C.; Ito, K.; Moskowitz, C.H.; Moskowitz, A.J.; Schöder, H. Metabolic Tumor Volume to Predict Event Free Survival in Patients with Relapsed/Refractory HL Treated with Brentuximab Vedotin-Based Salvage Therapy. J. Clin. Oncol. 2016, 34, 11566. [Google Scholar] [CrossRef]
- Abramson, J.S.; Arnason, J.E.; LaCasce, A.S.; Redd, R.; Barnes, J.A.; Sokol, L.; Joyce, R.; Avigan, D.; Neuberg, D.; Takvorian, R.W.; et al. Brentuximab Vedotin, Doxorubicin, Vinblastine, and Dacarbazine for Nonbulky Limited-Stage Classical Hodgkin Lymphoma. Blood 2019, 134, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Shea, T.C.; Olajide, O.; Reddy, N.M.; Budde, L.E.; Ghosh, N.; Deal, A.M.; Noe, J.F.; Ansell, S.M. ABVD Followed by BV Consolidation in Risk-Stratified Patients with Limited-Stage Hodgkin Lymphoma. Blood Adv. 2020, 4, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Linguanti, F.; Abenavoli, E.M.; Berti, V.; Lopci, E. Metabolic Imaging in B-Cell Lymphomas during CAR-T Cell Therapy. Cancers 2022, 14, 4700. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Bar, M.; Kansagra, A.J.; Chong, E.A.; Hashmi, S.K.; Neelapu, S.S.; Byrne, M.; Jacoby, E.; Lazaryan, A.; Jacobson, C.A.; et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2019, 25, 2305–2321. [Google Scholar] [CrossRef]
- Ruff, A.; Ballard, H.J.; Pantel, A.R.; Namoglu, E.C.; Hughes, M.E.; Nasta, S.D.; Chong, E.A.; Bagg, A.; Ruella, M.; Farwell, M.D.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Following Chimeric Antigen Receptor T-Cell Therapy in Large B-Cell Lymphoma. Mol. Imaging Biol. 2021, 23, 818–826. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Li, J.; Liu, Z.; Mi, M.; Zhu, F.; Wu, G.; Lan, X.; Zhang, L. Interim PET/CT Based on Visual and Semiquantitative Analysis Predicts Survival in Patients with Diffuse Large B-cell Lymphoma. Cancer Med. 2019, 8, 5012–5022. [Google Scholar] [CrossRef]
- Meignan, M.; Gallamini, A.; Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on Interim-PET Scan in Lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260. [Google Scholar] [CrossRef]
- Schmitz, C.; Hüttmann, A.; Müller, S.P.; Hanoun, M.; Boellaard, R.; Brinkmann, M.; Jöckel, K.-H.; Dührsen, U.; Rekowski, J. Dynamic Risk Assessment Based on Positron Emission Tomography Scanning in Diffuse Large B-Cell Lymphoma: Post-Hoc Analysis from the PETAL Trial. Eur. J. Cancer 2020, 124, 25–36. [Google Scholar] [CrossRef]
- Wang, X. PET/CT: Appropriate Application in Lymphoma. Chin. Clin. Oncol. 2015, 4, 4. [Google Scholar] [PubMed]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and Immunological Responses after CD30-Specific Chimeric Antigen Receptor-Redirected Lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, T.J.; Zhao, B.; Oldan, J.; Hucks, G.; Khandani, A.; Dittus, C.; Smith, J.; Morrison, J.K.; Cheng, C.J.; Ivanova, A.; et al. Pretherapy Metabolic Tumor Volume Is Associated with Response to CD30 CAR T Cells in Hodgkin Lymphoma. Blood Adv. 2022, 6, 1255–1263. [Google Scholar] [CrossRef]
- “Re-Priming” RT After Incomplete Response to CAR-T in R/R NHL—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04601831?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2 (accessed on 11 December 2022).
- Radiomics and Metabolomics in the Follow-up of CAR T-Cells for Refractory or Relapsed Non-Hodgkin’s Lymphoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05422521?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2 (accessed on 11 December 2022).
- 18F-F-AraG PET Imaging to Evaluate Immunological Response to CAR T Cell Therapy in Lymphoma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05096234?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2&rank=2 (accessed on 11 December 2022).
- PD-L1 PET-Imaging During CAR T-Cell Therapy—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05404048?term=CAR-T%2C+FDG+PET%2FCT&cond=Lymphoma&draw=2&rank=1 (accessed on 11 December 2022).
- Weiler-Sagie, M.; Bushelev, O.; Epelbaum, R.; Dann, E.J.; Haim, N.; Avivi, I.; Ben-Barak, A.; Ben-Arie, Y.; Bar-Shalom, R.; Israel, O. (18)F-FDG Avidity in Lymphoma Readdressed: A Study of 766 Patients. J. Nucl. Med. 2010, 51, 25–30. [Google Scholar] [CrossRef]
- Haioun, C.; Itti, E.; Rahmouni, A.; Brice, P.; Rain, J.-D.; Belhadj, K.; Gaulard, P.; Garderet, L.; Lepage, E.; Reyes, F.; et al. [18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) in Aggressive Lymphoma: An Early Prognostic Tool for Predicting Patient Outcome. Blood 2005, 106, 1376–1381. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Guo, C.; Li, S.; Hong, H.; Li, X.; Zhang, M.; Xia, Z.; Lin, T. Predictive Value of Interim 18F-FDG PET-CT Scans on Diffuse Large B-Cell Lymphoma Treated with R-CHOP: A Prospective Study. Blood 2015, 126, 1458. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, J.; Qiao, W.; Wang, T. Predictive Value of Interim PET/CT in DLBCL Treated with R-CHOP: Meta-Analysis. Biomed. Res. Int. 2015, 2015, 648572. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Martelli, M.; Sehn, L.H.; Belada, D.; Carella, A.-M.; Chua, N.; Gonzalez-Barca, E.; Hong, X.; Pinto, A.; Shi, Y.; et al. End-of-Treatment PET/CT Predicts PFS and OS in DLBCL after First-Line Treatment: Results from GOYA. Blood Adv. 2021, 5, 1283–1290. [Google Scholar] [CrossRef]
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A Randomized, Open-Label, Phase III Study of Obinutuzumab or Rituximab plus CHOP in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma: Final Analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, Y.; Guo, L.; Zhao, H.; Shi, Y.; Li, S.; Wang, A.; Zhang, X.; Shi, J.; Zhu, J.; et al. Predictive Value of Baseline 18F-FDG PET/CT and Interim Treatment Response for the Prognosis of Patients with Diffuse Large B-cell Lymphoma Receiving R-CHOP Chemotherapy. Oncol. Lett. 2020, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Cottereau, A.-S.; Lanic, H.; Mareschal, S.; Meignan, M.; Vera, P.; Tilly, H.; Jardin, F.; Becker, S. Molecular Profile and FDG-PET/CT Total Metabolic Tumor Volume Improve Risk Classification at Diagnosis for Patients with Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2016, 22, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Islam, P.; Goldstein, J.; Flowers, C.R. PET-Derived Tumor Metrics Predict DLBCL Response and Progression-Free Survival. Leuk. Lymphoma 2019, 60, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef]
- Smith, S.D.; Till, B.G.; Shadman, M.S.; Lynch, R.C.; Cowan, A.J.; Wu, Q.V.; Voutsinas, J.; Rasmussen, H.A.; Blue, K.; Ujjani, C.S.; et al. Pembrolizumab with R-CHOP in Previously Untreated Diffuse Large B-cell Lymphoma: Potential for Biomarker Driven Therapy. Br. J. Haematol. 2020, 189, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Burke, J.M.; Cheson, B.D.; Diefenbach, C.; Ferrari, S.; Hahn, U.H.; Hawkes, E.A.; Khan, C.; Lossos, I.S.; Musuraca, G.; et al. Safety and Efficacy of Atezolizumab in Combination with Rituximab Plus CHOP in Previously Untreated Patients with Diffuse Large B-Cell Lymphoma (DLBCL): Updated Analysis of a Phase I/II Study. Blood 2019, 134, 2874. [Google Scholar] [CrossRef]
- Shah, N.N.; Nagle, S.J.; Torigian, D.A.; Farwell, M.D.; Hwang, W.-T.; Frey, N.; Nasta, S.D.; Landsburg, D.; Mato, A.; June, C.H.; et al. Early Positron Emission Tomography/Computed Tomography as a Predictor of Response after CTL019 Chimeric Antigen Receptor -T-Cell Therapy in B-Cell Non-Hodgkin Lymphomas. Cytotherapy 2018, 20, 1415–1418. [Google Scholar] [CrossRef]
- Cohen, D.; Luttwak, E.; Beyar-Katz, O.; Hazut Krauthammer, S.; Bar-On, Y.; Amit, O.; Gold, R.; Perry, C.; Avivi, I.; Ram, R.; et al. [18F]FDG PET-CT in Patients with DLBCL Treated with CAR-T Cell Therapy: A Practical Approach of Reporting Pre- and Post-Treatment Studies. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 953–962. [Google Scholar] [CrossRef]
- Galtier, J.; Vercellino, L.; Chartier, L.; Olivier, P.; Tabouret-Viaud, C.; Mesguich, C.; di Blasi, R.; Durand, A.; Raffy, L.; Gros, F.-X.; et al. PET-Imaging Assessment for Guiding Strategy in Patients with Relapsed/Refractory Large B-Cell Lymphoma Receiving CAR T-Cells. Haematologica 2022, 108, 171–180. [Google Scholar] [CrossRef]
- Kuhnl, A.; Roddie, C.; Kirkwood, A.A.; Menne, T.; Cuadrado, M.; Marzolini, M.A.V.; Osborne, W.; Sanderson, R.; O’Reilly, M.; Townsend, W.; et al. Early FDG-PET Response Predicts CAR-T Failure in Large B-Cell Lymphoma. Blood Adv. 2022, 6, 321–326. [Google Scholar] [CrossRef]
- Breen, W.G.; Hathcock, M.A.; Young, J.R.; Kowalchuk, R.O.; Bansal, R.; Khurana, A.; Bennani, N.N.; Paludo, J.; Villasboas Bisneto, J.C.; Wang, Y.; et al. Metabolic Characteristics and Prognostic Differentiation of Aggressive Lymphoma Using One-Month Post-CAR-T FDG PET/CT. J. Hematol. Oncol. 2022, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- al Zaki, A.; Feng, L.; Watson, G.; Ahmed, S.A.; Mistry, H.; Nastoupil, L.J.; Hawkins, M.; Nair, R.; Iyer, S.P.; Lee, H.J.; et al. Day 30 SUVmax Predicts Progression in Patients with Lymphoma Achieving PR/SD after CAR T-Cell Therapy. Blood Adv. 2022, 6, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Yang, S.; Wei, G.; Zhao, X.; Wu, W.; Zhang, Y.; Zhang, Y.; Chen, D.; Wu, Z.; et al. Role of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Predicting the Adverse Effects of Chimeric Antigen Receptor T Cell Therapy in Patients with Non-Hodgkin Lymphoma. Biol. Blood Marrow Transplant. 2019, 25, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Carlier, T.; Tessoulin, B.; Gastinne, T.; Kraeber-Bodere, F.; le Gouill, S.; Bodet-Milin, C. Prognostic Value of FDG-PET/CT Response for Patient Selection before Chimeric Antigen Receptor-T-cells Therapy in Non-Hodgkin Lymphoma. Hematol. Oncol. 2022, 40, 796–800. [Google Scholar] [CrossRef]
- Armitage, J.O.; Weisenburger, D.D. New Approach to Classifying Non-Hodgkin’s Lymphomas: Clinical Features of the Major Histologic Subtypes. Non-Hodgkin’s Lymphoma Classification Project. J. Clin. Oncol. 1998, 16, 2780–2795. [Google Scholar] [CrossRef]
- Dillman, R.O. Radioimmunotherapy of B-Cell Lymphoma with Radiolabelled Anti-CD20 Monoclonal Antibodies. Clin. Exp. Med. 2006, 6, 1–12. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Musuraca, G.; Alinari, L.; Fanti, S.; Tani, M.; Stefoni, V.; Marchi, E.; Fina, M.; Pellegrini, C.; Castellucci, P.; et al. Predictive Role of Positron Emission Tomography in the Outcome of Patients with Follicular Lymphoma. Clin. Lymphoma Myeloma 2007, 7, 291–295. [Google Scholar] [CrossRef]
- Trotman, J.; Pettitt, A.R. Is It Time for PET-Guided Therapy in Follicular Lymphoma? Blood 2022, 139, 1631–1641. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G. Imaging Follicular Lymphoma Using Positron Emission Tomography with [18F]Fluorodeoxyglucose: To What Purpose? J. Clin. Oncol. 2012, 30, 4285–4287. [Google Scholar] [CrossRef]
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis from the National LymphoCare Study. J. Clin. Oncol. 2015, 33, 2516–2522. [Google Scholar] [CrossRef]
- Trotman, J.; Fournier, M.; Lamy, T.; Seymour, J.F.; Sonet, A.; Janikova, A.; Shpilberg, O.; Gyan, E.; Tilly, H.; Estell, J.; et al. Positron Emission Tomography–Computed Tomography (PET-CT) After Induction Therapy Is Highly Predictive of Patient Outcome in Follicular Lymphoma: Analysis of PET-CT in a Subset of PRIMA Trial Participants. J. Clin. Oncol. 2011, 29, 3194–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuis, J.; Berriolo-Riedinger, A.; Julian, A.; Brice, P.; Tychyj-Pinel, C.; Tilly, H.; Mounier, N.; Gallamini, A.; Feugier, P.; Soubeyran, P.; et al. Impact of [18F]Fluorodeoxyglucose Positron Emission Tomography Response Evaluation in Patients with High–Tumor Burden Follicular Lymphoma Treated with Immunochemotherapy: A Prospective Study from the Groupe d’Etudes Des Lymphomes de l’Adulte and GOELAMS. J. Clin. Oncol. 2012, 30, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Luminari, S.; Biasoli, I.; Versari, A.; Rattotti, S.; Bottelli, C.; Rusconi, C.; Merli, F.; Spina, M.; Ferreri, A.J.M.; Zinzani, P.L.; et al. The Prognostic Role of Post-Induction FDG-PET in Patients with Follicular Lymphoma: A Subset Analysis from the FOLL05 Trial of the Fondazione Italiana Linfomi (FIL). Ann. Oncol. 2014, 25, 442–447. [Google Scholar] [CrossRef]
- Trotman, J.; Luminari, S.; Boussetta, S.; Versari, A.; Dupuis, J.; Tychyj, C.; Marcheselli, L.; Berriolo-Riedinger, A.; Franceschetto, A.; Julian, A.; et al. Prognostic Value of PET-CT after First-Line Therapy in Patients with Follicular Lymphoma: A Pooled Analysis of Central Scan Review in Three Multicentre Studies. Lancet Haematol. 2014, 1, e17–e27. [Google Scholar] [CrossRef] [PubMed]
- Trotman, J.; Barrington, S.F.; Belada, D.; Meignan, M.; MacEwan, R.; Owen, C.; Ptáčník, V.; Rosta, A.; Fingerle-Rowson, G.R.; Zhu, J.; et al. Prognostic Value of End-of-Induction PET Response after First-Line Immunochemotherapy for Follicular Lymphoma (GALLIUM): Secondary Analysis of a Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1530–1542. [Google Scholar] [CrossRef]
- Luminari, S.; Manni, M.; Galimberti, S.; Versari, A.; Tucci, A.; Boccomini, C.; Farina, L.; Olivieri, J.; Marcheselli, L.; Guerra, L.; et al. Response-Adapted Postinduction Strategy in Patients with Advanced-Stage Follicular Lymphoma: The FOLL12 Study. J. Clin. Oncol. 2022, 40, 729–739. [Google Scholar] [CrossRef]
- Bouchkouj, N.; Zimmerman, M.; Kasamon, Y.L.; Wang, C.; Dai, T.; Xu, Z.; Wang, X.; Theoret, M.; Purohit-Sheth, T.; George, B. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Follicular Lymphoma. Oncologist 2022, 27, 587–594. [Google Scholar] [CrossRef]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Shi, Y.; Su, H.; Song, Y.; Jiang, W.; Sun, X.; Qian, W.; Zhang, W.; Gao, Y.; Jin, Z.; Zhou, J.; et al. Safety and Activity of Sintilimab in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma (ORIENT-1): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2019, 6, e12–e19. [Google Scholar] [CrossRef]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef]
- Petersen, H.; Holdgaard, P.C.; Madsen, P.H.; Knudsen, L.M.; Gad, D.; Gravergaard, A.E.; Rohde, M.; Godballe, C.; Engelmann, B.E.; Bech, K.; et al. FDG PET/CT in Cancer: Comparison of Actual Use with Literature-Based Recommendations. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18F-FDG PET/CT Longitudinal Studies in Patients with Advanced Metastatic Melanoma for Response Evaluation of Combination Treatment with Vemurafenib and Ipilimumab. Melanoma Res. 2019, 29, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Maggialetti, N.; Masi, T.; Nappi, A.G.; Santo, G.; Niccoli Asabella, A.; Rubini, G. Early Evaluation of Immunotherapy Response in Lymphoma Patients by 18F-FDG PET/CT: A Literature Overview. J. Pers. Med. 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Hicks, R.J.; le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for Assessing Tumour Response to Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef]
- Pektor, S.; Hilscher, L.; Walzer, K.C.; Miederer, I.; Bausbacher, N.; Loquai, C.; Schreckenberger, M.; Sahin, U.; Diken, M.; Miederer, M. In Vivo Imaging of the Immune Response upon Systemic RNA Cancer Vaccination by FDG-PET. EJNMMI Res. 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Avivi, I.; Zilberlicht, A.; Dann, E.J.; Leiba, R.; Faibish, T.; Rowe, J.M.; Bar-Shalom, R. Strikingly High False Positivity of Surveillance FDG-PET/CT Scanning among Patients with Diffuse Large Cell Lymphoma in the Rituximab Era. Am. J. Hematol. 2013, 88, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Gandy, N.; Arshad, M.A.; Wallitt, K.L.; Dubash, S.; Khan, S.; Barwick, T.D. Immunotherapy-Related Adverse Effects on 18F-FDG PET/CT Imaging. Br. J. Radiol. 2020, 93, 20190832. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Nguyen, V.A.; Plaickner, J.; Jaschke, W. Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Radiol. Rep. 2017, 5, 59. [Google Scholar] [CrossRef]
- Witzig, T.E.; Vose, J.M.; Kaplan, H.P.; Wolf, J.L.; Pietronigro, D.; Takeshita, K.; Ervin-Haynes, A.; Zeldis, J.B.; Wiernik, P.H. Early Results from a Phase II Study of Lenalidomide Monotherapy in Relapsed/Refractory Indolent Non-Hodgkin’s Lymphoma. Blood 2006, 108, 2482. [Google Scholar] [CrossRef]
- Witzig, T.E.; Vose, J.M.; Zinzani, P.L.; Reeder, C.B.; Buckstein, R.; Polikoff, J.A.; Bouabdallah, R.; Haioun, C.; Tilly, H.; Guo, P.; et al. An International Phase II Trial of Single-Agent Lenalidomide for Relapsed or Refractory Aggressive B-Cell Non-Hodgkin’s Lymphoma. Ann. Oncol. 2011, 22, 1622–1627. [Google Scholar] [CrossRef]
- Corazzelli, G.; de Filippi, R.; Capobianco, G.; Frigeri, F.; de Rosa, V.; Iaccarino, G.; Russo, F.; Arcamone, M.; Becchimanzi, C.; Crisci, S.; et al. Tumor Flare Reactions and Response to Lenalidomide in Patients with Refractory Classic Hodgkin Lymphoma. Am. J. Hematol. 2009, 85, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Skoura, E.; Ardeshna, K.; Halsey, R.; Wan, S.; Kayani, I. False-Positive 18F-FDG PET/CT Imaging. Clin. Nucl. Med. 2016, 41, e171–e172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Y.; Yu, F.; Huang, W.; Wu, W.; He, J.; Cai, Z.; He, D. Tumor Flare Reaction in a Classic Hodgkin Lymphoma Patient Treated with Brentuximab Vedotin and Tislelizumab: A Case Report. Front. Immunol. 2022, 12, 756583. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Vercellino, L.; di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive Factors of Early Progression after CAR T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Adv. 2020, 4, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.A.; Mhaskar, R.S.; Lu, H.; Mousa, M.S.; Krivenko, G.S.; Lazaryan, A.; Bachmeier, C.A.; Chavez, J.C.; Nishihori, T.; Davila, M.L.; et al. High Metabolic Tumor Volume Is Associated with Decreased Efficacy of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 3268–3276. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef]
- Rubin, D.B.; Danish, H.H.; Ali, A.B.; Li, K.; LaRose, S.; Monk, A.D.; Cote, D.J.; Spendley, L.; Kim, A.H.; Robertson, M.S.; et al. Neurological Toxicities Associated with Chimeric Antigen Receptor T-Cell Therapy. Brain 2019, 142, 1334–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paccagnella, A.; Farolfi, A.; Casadei, B.; Garibotto, V.; Zinzani, P.; Fanti, S. 2-[18F]FDG-PET/CT for Early Response and Brain Metabolic Pattern Assessment after CAR-T Cell Therapy in a Diffuse Large B Cell Lymphoma Patient with ICANS. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1090–1091. [Google Scholar] [CrossRef] [PubMed]
- Vernier, V.; Ursu, R.; Belin, C.; Maillet, D.; Thieblemont, C.; Carpentier, A.F. Hypometabolism on Brain FDG-PET as a Marker for Neurotoxicity after CAR T-Cell Therapy: A Case Report. Rev. Neurol. 2022, 178, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibraheem, A.; Abdlkadir, A.; Mohamedkhair, A.; Mikhail-Lette, M.; Al-Qudah, M.; Paez, D.; Mansour, A. Cancer Diagnosis in Areas of Conflict. Front. Oncol. 2022, 12, 1087476. [Google Scholar] [CrossRef]
- El Sayed, R.; Abdul-Sater, Z.; Mukherji, D. Cancer Care During War and Conflict. In Cancer in the Arab World; Springer: Singapore, 2022; pp. 461–476. [Google Scholar]
- Gajra, A.; Zalenski, A.; Sannareddy, A.; Jeune-Smith, Y.; Kapinos, K.; Kansagra, A. Barriers to Chimeric Antigen Receptor T-Cell (CAR-T) Therapies in Clinical Practice. Pharmaceut. Med. 2022, 36, 163–171. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Abdlkadir, A.; Albalooshi, B.; Muhsen, H.; Haider, M.; Omar, Y.; Usmani, S.; Al-kandri, F. Theranostics in the Arab World; Achievements & Challenges. Jordan Med. J. 2022, 56, 188–205. [Google Scholar]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef]
- Hirata, K.; Tamaki, N. Quantitative FDG PET Assessment for Oncology Therapy. Cancers 2021, 13, 869. [Google Scholar] [CrossRef]
- Sprinz, C.; Altmayer, S.; Zanon, M.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of Blood Glucose Level on 18F-FDG Uptake for PET/CT in Normal Organs: A Systematic Review. PLoS ONE 2018, 13, e0193140. [Google Scholar] [CrossRef]
- Zasadny, K.R.; Wahl, R.L. Standardized Uptake Values of Normal Tissues at PET with 2-[Fluorine-18]-Fluoro-2-Deoxy-D-Glucose: Variations with Body Weight and a Method for Correction. Radiology 1993, 189, 847–850. [Google Scholar] [CrossRef]
- Kitao, T.; Shiga, T.; Hirata, K.; Sekizawa, M.; Takei, T.; Yamashiro, K.; Tamaki, N. Volume-Based Parameters on FDG PET May Predict the Proliferative Potential of Soft-Tissue Sarcomas. Ann. Nucl. Med. 2019, 33, 22–31. [Google Scholar] [CrossRef]
- Pak, K.; Cheon, G.J.; Nam, H.-Y.; Kim, S.-J.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitao, T.; Hirata, K.; Shima, K.; Hayashi, T.; Sekizawa, M.; Takei, T.; Ichimura, W.; Harada, M.; Kondo, K.; Tamaki, N. Reproducibility and Uptake Time Dependency of Volume-Based Parameters on FDG-PET for Lung Cancer. BMC Cancer 2016, 16, 576. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Penney, B.C.; Wroblewski, K.; Zhang, H.; Simon, C.A.; Kampalath, R.; Shih, M.-C.; Shimada, N.; Chen, S.; Salgia, R.; et al. Prognostic Value of Metabolic Tumor Burden on 18F-FDG PET in Nonsurgical Patients with Non-Small Cell Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 27–38. [Google Scholar] [CrossRef]
- Im, H.-J.; Pak, K.; Cheon, G.J.; Kang, K.W.; Kim, S.-J.; Kim, I.-J.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic Value of Volumetric Parameters of 18F-FDG PET in Non-Small-Cell Lung Cancer: A Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 241–251. [Google Scholar] [CrossRef]
- Hirata, K.; Kobayashi, K.; Wong, K.-P.; Manabe, O.; Surmak, A.; Tamaki, N.; Huang, S.-C. A Semi-Automated Technique Determining the Liver Standardized Uptake Value Reference for Tumor Delineation in FDG PET-CT. PLoS ONE 2014, 9, e105682. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V. Toward Common Response Evaluation Criteria for Solid Tumors and Lymphomas: RECIL and RECIST? Ann. Oncol. 2017, 28, 1409–1411. [Google Scholar] [CrossRef]
- al Tabaa, Y.; Bailly, C.; Kanoun, S. FDG-PET/CT in Lymphoma: Where Do We Go Now? Cancers 2021, 13, 5222. [Google Scholar] [CrossRef]
- Luo, Y.; Cao, X.; Pan, Q.; Li, J.; Feng, J.; Li, F. 68Ga-Pentixafor PET/CT for Imaging of Chemokine Receptor 4 Expression in Waldenström Macroglobulinemia/Lymphoplasmacytic Lymphoma: Comparison to 18F-FDG PET/CT. J. Nucl. Med. 2019, 60, 1724–1729. [Google Scholar] [CrossRef]
- Pan, Q.; Luo, Y.; Zhang, Y.; Chang, L.; Li, J.; Cao, X.; Li, J.; Li, F. Preliminary Evidence of Imaging of Chemokine Receptor-4-Targeted PET/CT with [68Ga]Pentixafor in Non-Hodgkin Lymphoma: Comparison to [18F]FDG. EJNMMI Res. 2020, 10, 89. [Google Scholar] [CrossRef]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-Art of FAPI-PET Imaging: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, M.; Wang, S.; Wang, G.; Lai, Y.; Shi, Y.; Zhang, Y.; Yang, Z.; Wang, X. Detecting Fibroblast Activation Proteins in Lymphoma Using 68Ga-FAPI PET/CT. J. Nucl. Med. 2022, 63, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Chantepie, S.; Hovhannisyan, N.; Guillouet, S.; Pelage, J.-P.; Ibazizene, M.; Bodet-Milin, C.; Carlier, T.; Gac, A.-C.; Réboursière, E.; Vilque, J.-P.; et al. 18F-Fludarabine PET for Lymphoma Imaging: First-in-Humans Study on DLBCL and CLL Patients. J. Nucl. Med. 2018, 59, 1380–1385. [Google Scholar] [CrossRef]
- Hovhannisyan, N.; Dhilly, M.; Guillouet, S.; Leporrier, M.; Barré, L. Comparative Analysis between [18F]Fludarabine-PET and [18F]FDG-PET in a Murine Model of Inflammation. Mol. Pharm. 2016, 13, 2136–2139. [Google Scholar] [CrossRef]
- Barré, L.; Hovhannisyan, N.; Bodet-Milin, C.; Kraeber-Bodéré, F.; Damaj, G. [18F]-Fludarabine for Hematological Malignancies. Front. Med. 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, N.; Guillouet, S.; Fillesoye, F.; Dhilly, M.; Patin, D.; Galateau, F.; Leporrier, M.; Barré, L. Evaluation of the Specificity of [18F]Fludarabine PET/CT in a Xenograft Model of Follicular Lymphoma: Comparison with [18F]FDG and Impact of Rituximab Therapy. EJNMMI Res. 2015, 5, 23. [Google Scholar] [CrossRef]
- Frood, R.; Clark, M.; Burton, C.; Tsoumpas, C.; Frangi, A.F.; Gleeson, F.; Patel, C.; Scarsbrook, A. Utility of Pre-Treatment FDG PET/CT–Derived Machine Learning Models for Outcome Prediction in Classical Hodgkin Lymphoma. Eur. Radiol. 2022, 32, 7237–7247. [Google Scholar] [CrossRef]
- Sollini, M.; Kirienko, M.; Cavinato, L.; Ricci, F.; Biroli, M.; Ieva, F.; Calderoni, L.; Tabacchi, E.; Nanni, C.; Zinzani, P.L.; et al. Methodological Framework for Radiomics Applications in Hodgkin’s Lymphoma. Eur. J. Hybrid. Imaging 2020, 4, 9. [Google Scholar] [CrossRef]






| Drug Name | Class | Main Action | Treatment Protocol | Approved for |
|---|---|---|---|---|
| Rituximab | mAb 1 | CD-20 Antibody | With chemotherapy | First line for NHL 2 |
| Brentuximab Vedotin | mAb 1 | CD-30 Antibody | With chemotherapy | Advanced HL 3 |
| Nivolumab | ICI 4 | PD-1 Blockade | Standalone | cHL 5 |
| Pembrolizumab | ICI 4 | PD-1 Blockade | Standalone | Refractory cHL 5 |
| Tisagenlecleuce | CAR-T 6 | T-lymphocyte-mediated CD-19 expression | Standalone | Adult R/R DLBCL 7 |
| Lisocabtagenel maraleuecel | CAR-T 6 | T-lymphocyte-mediated CD-19 expression | Standalone | R/R large B-cell lymphoma |
| Mosunetuzumab | BiTes 7 | Follicular Lymphoma | Standalone | R/R Follicular Lymphoma |
| Deauville 5-Point Scale (5PS) | |
| DS *1 | No uptake |
| DS2 | Uptake ≤ mediastinum |
| DS3 | Uptake > mediastinum but ≤ liver |
| DS4 | Uptake moderately higher than liver |
| DS5 | Uptake markedly higher than liver and/or new lesions + |
| Category | Subtype of Lymphoma | FDG Avidity | Degree of FDG Avidity |
|---|---|---|---|
| HL 1 | Classical | Avid | High |
| Mixed cellularity | Avid | Moderate to high | |
| Lymphocyte depletion | Avid | Moderate to high | |
| Lymphocyte predominance | Avid | Moderate | |
| Aggressive NHL 2 | Diffuse large B-cell | Avid | High |
| Burkitt | Avid | High | |
| Anaplastic Large cell | Avid | High | |
| Mantle Cell | Avid | Moderate | |
| Indolent NHL 2 | Follicular | Variable | Low-high |
| Lymphoplasmacytic | Variable | Low-high | |
| Marginal zone | Variable | None-high | |
| Small lymphocytic | Variable | None-high | |
| Cutaneous Anaplastic | Variable | None-moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ibraheem, A.; Abdlkadir, A.S.; Juweid, M.E.; Al-Rabi, K.; Ma’koseh, M.; Abdel-Razeq, H.; Mansour, A. FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects. Cancers 2023, 15, 1063. https://doi.org/10.3390/cancers15041063
Al-Ibraheem A, Abdlkadir AS, Juweid ME, Al-Rabi K, Ma’koseh M, Abdel-Razeq H, Mansour A. FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects. Cancers. 2023; 15(4):1063. https://doi.org/10.3390/cancers15041063
Chicago/Turabian StyleAl-Ibraheem, Akram, Ahmed Saad Abdlkadir, Malik E. Juweid, Kamal Al-Rabi, Mohammad Ma’koseh, Hikmat Abdel-Razeq, and Asem Mansour. 2023. "FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects" Cancers 15, no. 4: 1063. https://doi.org/10.3390/cancers15041063
APA StyleAl-Ibraheem, A., Abdlkadir, A. S., Juweid, M. E., Al-Rabi, K., Ma’koseh, M., Abdel-Razeq, H., & Mansour, A. (2023). FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects. Cancers, 15(4), 1063. https://doi.org/10.3390/cancers15041063