Multi-Morbidity and Risk of Breast Cancer among Women in the UK Biobank Cohort
Abstract
Simple Summary
Abstract
1. Introduction
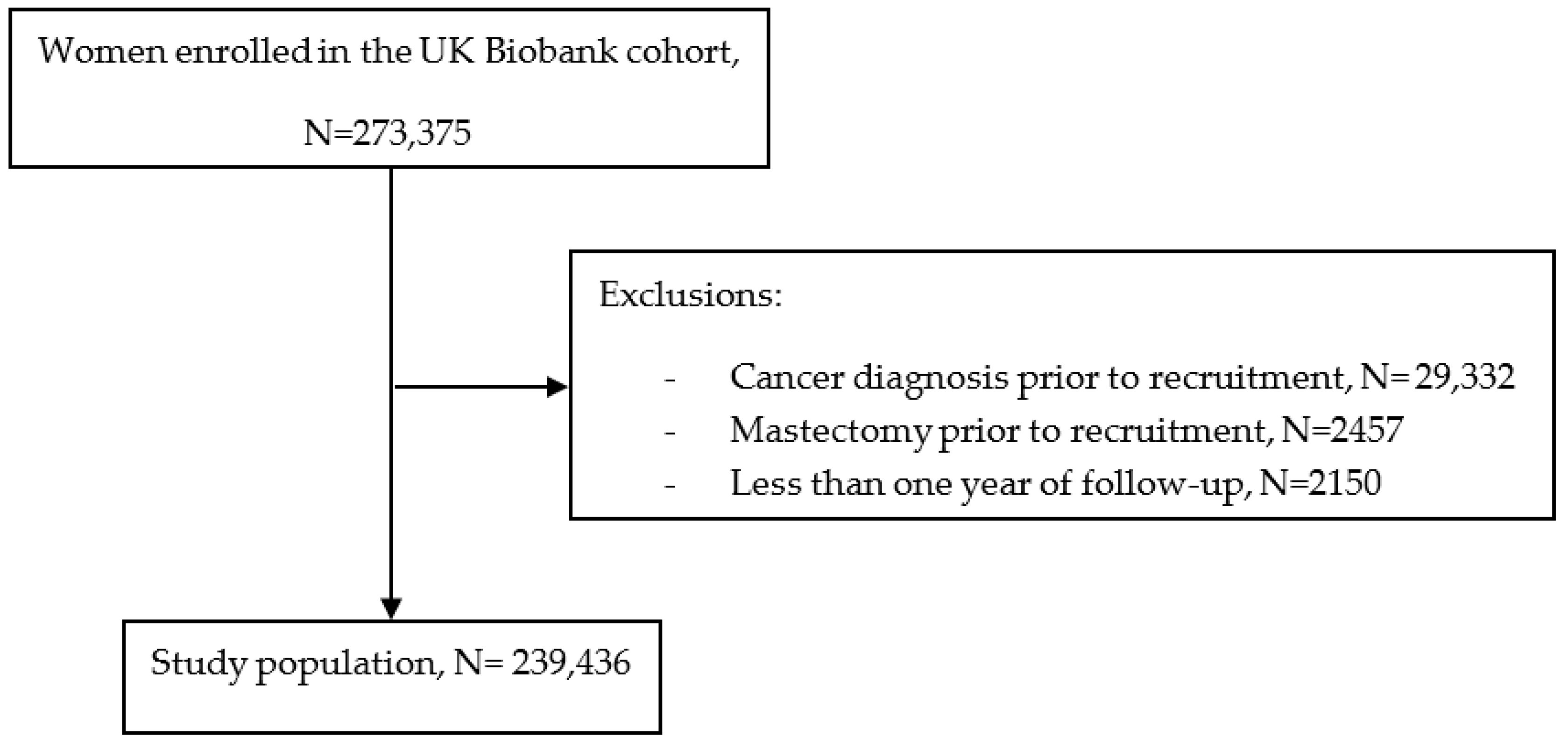
2. Materials and Methods
2.1. Data Source and Study Design
2.2. Study Population
2.3. Baseline Morbidity Identification
2.4. Breast Cancer Ascertainment
2.5. Baseline Confounding Factors
2.6. Statistical Analysis
2.6.1. Multiple Correspondence Analysis (MCA) and Cluster Analysis (See Appendix B)
2.6.2. Association among the Number of Morbidities, Morbidity Patterns, and Breast Cancer Risk
3. Results
3.1. Description of Morbidity Patterns
3.1.1. Pattern 1: No-Predominant Morbidity [n = 159,083 (66.4%), 3534 Breast Cancer Cases (2.0% of Cases)]
3.1.2. Pattern 2: Psychiatric Morbidities [n = 16,627 (7.0%), 381 Breast Cancer Cases (2.0% of Cases)]
3.1.3. Pattern 3: Respiratory/Immunological Morbidities [n = 27,920 (11.7%), 611 Breast Cancer Cases (2.0% of cases)]
3.1.4. Pattern 4: Cardiovascular/Metabolic Morbidities [n = 11,041 (4.6%), 246 Breast Cancer Cases (2.0% of cases)]
3.1.5. Pattern 5: Unspecific Morbidities [n = 24,765 (10.3%), 554 Breast Cancer Cases (2.0%)]
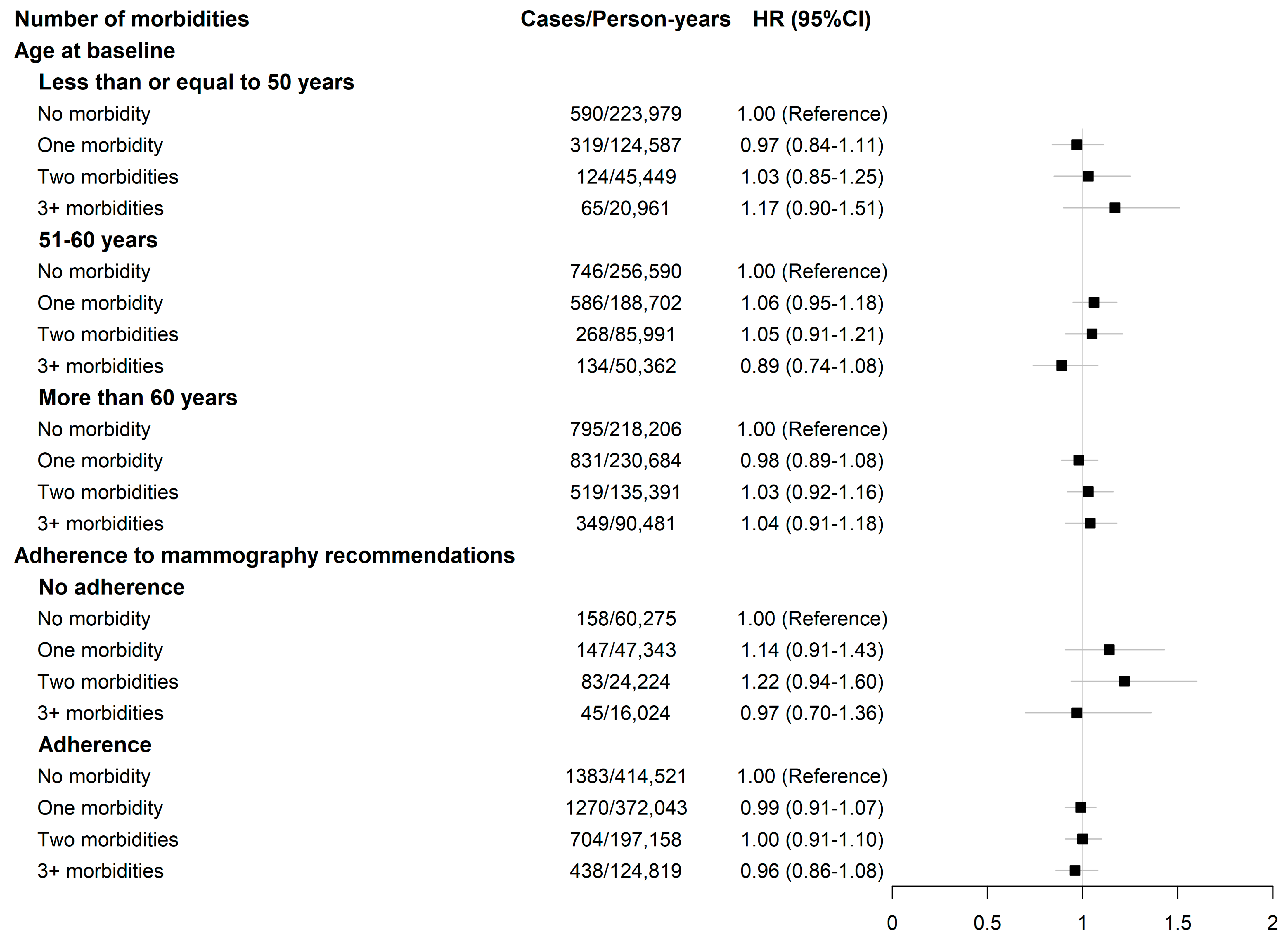
3.2. Breast Cancer Risk According to the Number of Morbidities and Morbidity Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Morbidity ^ | Conditions Included |
|---|---|
| 1. Painful conditions * | Back pain |
| Joint pain | |
| Headaches (not migraine) | |
| Sciatica | |
| Plantar fasciitis | |
| Carpal tunnel syndrome | |
| Fibromyalgia | |
| Arthritis | |
| Shingles | |
| Disc problem | |
| Prolapsed disc/slipped disc | |
| Spine arthritis/spondylitis | |
| Ankylosing spondylitis | |
| Back problem | |
| Osteoarthritis | |
| Gout | |
| Cervical spondylosis | |
| Trigeminal neuralgia | |
| Disc degeneration | |
| Trapped nerve/compressed nerve | |
| 2. Hypertension | Hypertension |
| Essential hypertension | |
| 3. Depression * | Depression |
| Postnatal depression | |
| 4. Asthma | Asthma |
| 5. Coronary heart disease | Heart attack/MI |
| Angina | |
| 6. Treated dyspepsia | Gastro-esophageal reflux (GORD)/gastric reflux |
| Esophagitis/Barrett’s esophagus | |
| Gastric stomach ulcers | |
| Gastric erosions/gastritis | |
| Duodenal ulcer | |
| Dyspepsia/indigestion | |
| Hiatus hernia | |
| Helicobacter pylori | |
| 7. Diabetes | Diabetic nephropathy |
| Diabetic neuropathy/ulcers | |
| Diabetes | |
| Type 1 diabetes | |
| Type 2 diabetes | |
| Diabetic eye disease | |
| 8. Thyroid disorders | Thyroid problem (not cancer) |
| Hyperthyroidism/thyrotoxicosis | |
| Hypothyroidism/myxedema | |
| Graves’ disease | |
| Thyroid goitre | |
| Thyroiditis | |
| 9. Rheumatoid arthritis, other inflammatory polyarthropathies, systemic connective tissue disorders and systemic autoimmune disorders | Myositis/myopathy |
| Systemic lupus erythematosus | |
| Connective tissue disorder | |
| Sjogren’s syndrome/sicca syndrome | |
| Dermatopolymyositis | |
| Scleroderma/systemic sclerosis | |
| Rheumatoid arthritis | |
| Psoriatic arthropathy | |
| Dermatomyositis | |
| Polymyositis | |
| Polymyalgia rheumatica | |
| Malabsorption/celiac disease | |
| 10. Chronic obstructive pulmonary disease (COPD) | COPD/chronic obstructive airways disease |
| Emphysema/chronic bronchitis | |
| Emphysema | |
| 11. Anxiety, other neurotic, stress-related, and somatoform disorders * | Anxiety/panic attacks |
| Nervous breakdown | |
| Post-traumatic stress disorder | |
| Obsessive compulsive disorder | |
| Stress | |
| Insomnia | |
| Psychological/psychiatric problem | |
| 12. Irritable bowel syndrome | Irritable bowel syndrome |
| 13. Alcohol problems * | Alcohol dependency |
| Alcoholic liver disease/alcoholic cirrhosis | |
| 14. Other psychoactive substance abuse * | Opioid dependency |
| Other substance abuse/dependency | |
| 15. Treated constipation | Constipation |
| 16. Stroke and transient ischemic attack (TIA) | Stroke |
| TIA | |
| Subarachnoid hemorrhage | |
| Brain hemorrhage | |
| Ischemic stroke | |
| 17. Chronic kidney disease | Polycystic kidney |
| Diabetic nephropathy | |
| Renal/kidney failure | |
| Renal failure requiring dialysis | |
| Renal failure not requiring dialysis | |
| Kidney nephropathy | |
| Immunoglobulin A (IgA) nephropathy | |
| 18. Diverticular disease of intestine | Diverticular disease/diverticulitis |
| 19. Atrial fibrillation | Atrial fibrillation |
| 20. Peripheral vascular disease | Peripheral vascular disease |
| Leg claudication/intermittent claudication | |
| 21. Heart failure | Cardiomyopathy |
| Hypertrophic cardiomyopathy | |
| Heart failure/pulmonary edema | |
| 22. Prostate disorders | Prostate problem (not cancer) |
| Enlarged prostate | |
| Benign prostatic hypertrophy | |
| 23. Glaucoma | Glaucoma |
| 24. Epilepsy | Epilepsy |
| 25. Dementia | Dementia/Alzheimer/cognitive impairment |
| 26. Schizophrenia (and related non-organic psychosis) and bipolar disorder * | Schizophrenia |
| Mania/bipolar disorder/manic depression | |
| 27. Psoriasis or eczema | Eczema/dermatitis |
| Psoriasis | |
| 28. Inflammatory bowel disease | Inflammatory bowel disease |
| Crohn’s disease | |
| Ulcerative colitis | |
| 29. Migraine | Migraine |
| 30. Chronic sinusitis | Chronic sinusitis |
| 31. Anorexia or bulimia * | Anorexia, bulimia/other eating disorder |
| 32. Bronchiectasis | Bronchiectasis |
| 33. Parkinson’s disease | Parkinson’s disease |
| 34. Multiple sclerosis | Multiple sclerosis |
| 35. Viral Hepatitis | Infective/viral hepatitis |
| Hepatitis B | |
| Hepatitis C | |
| Hepatitis D | |
| Hepatitis E | |
| 36. Chronic liver disease | Esophageal varices |
| Non infective hepatitis | |
| Liver failure/cirrhosis | |
| Primary biliary cirrhosis | |
| 37. Osteoporosis ~ | Osteoporosis |
| 38. Chronic fatigue syndrome ~ | Chronic fatigue syndrome |
| 39. Endometriosis ~ | Endometriosis |
| 40. Meniere disease ~ | Meniere disease |
| 41. Pernicious Anemia ~ | Pernicious anemia |
| 42. Polycystic ovaries ~ | Polycystic ovaries |
| 43. Cancer | Lifetime diagnosis |
| Risk Factors | Coding | Information Source | Testing for Confounding Effect | Testing for Modification Effect |
|---|---|---|---|---|
| Socio-demographic and economic characteristics | ||||
| Age at baseline | Continuous | SR-Q | Yes | Yes |
| Occupation | Administrative and Secretarial Occupations Associate Professional and Technical Occupations Elementary Occupations Managers and Senior Officials Personal Service Occupations Process, Plant, and Machine Operatives Professional Occupations Sales and Customer Service Occupations Skilled Trades Occupations Unknown | SR-Q | Yes | No |
| Race | Asian Black and Caribbean White Other/Unknown | SR-Q | Yes | No |
| Townsend score | Continuous | UK data service | Yes | Yes |
| Hormone-related factors | ||||
| Age at menarche | Continuous | SR-Q | Yes | No |
| Age at menopause | Still had periods Had menopause before the age of 45 years Had menopause between the age of 45 and 54 Had menopause after the age of 55 | SR-Q | Yes | Yes |
| Menopausal hormone therapy use | Never Ever, less than 5-year duration Ever, 5 years and longer Ever, unknown duration | Reporting menopause (periods stopped) (SR-Q)OR Reporting use of menopausal hormone therapy (SR-Q)OR Undergoing a bilateral oophorectomy (SR-I)OR ≥51 years of age at baseline | Yes | No |
| Oral contraception use | Never Ever, less than 10-year duration; Ever, at least 10-year duration; Ever, unknown duration; Unknown status | SR-Q | Yes | No |
| Parity and age at first birth | No live birth At least one birth before age 30 At least one birth after age 30 | SR-Q | Yes | No |
| Health and health care-related factors | ||||
| BMI | Continuous | PM | Yes | Yes |
| Level of physical activity | Low Moderate High | SR-Q | Yes | Yes |
| Alcohol consumption | Never Twice a week or less Three times a week or more Unknown status | SR-Q | Yes | No |
| Adherence to mammography guidelines | Never Ever, last use since more than 3 years ago Ever, in the last 3 years Ever, unknown time of last use | SR-Q | Yes | Yes |
Appendix B
Appendix B.1. Multiple Correspondence Analysis (MCA)
Appendix B.2. Cluster Analysis
- Agglomerative hierarchical clustering (AHC)
- Ward’s method for cluster analysis
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Spiegelman, D.; Smith-Warner, S.A.; Wang, M.; Pazaris, M.; Willett, W.C.; Eliassen, A.H.; Hunter, D.J. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am. J. Epidemiol. 2016, 184, 884–893. [Google Scholar] [CrossRef]
- van Gemert, W.A.; Lanting, C.I.; Goldbohm, R.A.; van den Brandt, P.A.; Grooters, H.G.; Kampman, E.; Kiemeney, L.a.L.M.; van Leeuwen, F.E.; Monninkhof, E.M.; de Vries, E.; et al. The proportion of postmenopausal breast cancer cases in the Netherlands attributable to lifestyle-related risk factors. Breast Cancer Res. Treat. 2015, 152, 155–162. [Google Scholar] [CrossRef]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Xu, X.; Mishra, G.D.; Jones, M. Evidence on multimorbidity from definition to intervention: An overview of systematic reviews. Ageing Res. Rev. 2017, 37, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Cassell, A.; Edwards, D.; Harshfield, A.; Rhodes, K.; Brimicombe, J.; Payne, R.; Griffin, S. The epidemiology of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2018, 68, e245–e251. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Boniol, M.; Koechlin, A.; Robertson, C.; Valentini, F.; Coppens, K.; Fairley, L.-L.; Boniol, M.; Zheng, T.; Zhang, Y.; et al. Diabetes and breast cancer risk: A meta-analysis. Br. J. Cancer 2012, 107, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-V.-T.; Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N. Thyroid dysfunction and cancer incidence: A systematic review and meta-analysis. Endocr. Relat. Cancer 2020, 27, 245–259. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Chen, H.-P.; Hung, C.-M.; Lee, P.-H.; Lin, C.-L.; Kao, C.-H. Hospitalization for Inflammatory Bowel Disease is Associated with Increased Risk of Breast Cancer: A Nationwide Cohort Study of an Asian Population. Ann. Surg. Oncol. 2015, 22, 1996–2002. [Google Scholar] [CrossRef]
- Schairer, C.; Pfeiffer, R.M.; Gadalla, S.M. Autoimmune diseases and breast cancer risk by tumor hormone-receptor status among elderly women. Int. J. Cancer 2018, 142, 1202–1208. [Google Scholar] [CrossRef]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Xue, F.; Michels, K.B. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am. J. Clin. Nutr. 2007, 86, s823–s835. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Poblador-Plou, B.; Gimeno-Miguel, A.; Calderón-Larrañaga, A.; Poncel-Falcó, A.; Gimeno-Feliú, L.A.; González-Rubio, F.; Laguna-Berna, C.; Marta-Moreno, J.; Clerencia-Sierra, M.; et al. Cohort Profile: The Epidemiology of Chronic Diseases and Multimorbidity. The EpiChron Cohort Study. Int. J. Epidemiol. 2018, 47, 382–384f. [Google Scholar] [CrossRef]
- Britt, H.C.; Harrison, C.M.; Miller, G.C.; Knox, S.A. Prevalence and patterns of multimorbidity in Australia. Med. J. Aust. 2008, 189, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Roso-Llorach, A.; Fernández, S.; Guisado-Clavero, M.; Violán, C.; Onder, G.; Fratiglioni, L.; Calderón-Larrañaga, A.; Marengoni, A. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat. Commun. 2020, 11, 3223. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration Association of Cardiometabolic Multimorbidity With Mortality. JAMA 2015, 314, 52–60. [CrossRef] [PubMed]
- Yasmeen, S.; Hubbard, R.A.; Romano, P.S.; Zhu, W.; Geller, B.M.; Onega, T.; Yankaskas, B.C.; Miglioretti, D.L.; Kerlikowske, K. Risk of Advanced-Stage Breast Cancer Among Older Women with Comorbidities. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2012, 21, 1510–1519. [Google Scholar] [CrossRef]
- Jensen, L.F.; Pedersen, A.F.; Andersen, B.; Vestergaard, M.; Vedsted, P. Non-participation in breast cancer screening for women with chronic diseases and multimorbidity: A population-based cohort study. BMC Cancer 2015, 15, 798. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.F.; Koroukian, S.M.; Schiltz, N.K.; Smyth, K.A.; Cooper, G.S.; Owusu, C.; Stange, K.C.; Berger, N.A. Complex Multimorbidity and Breast Cancer Screening Among Midlife and Older Women: The Role of Perceived Need. Gerontologist 2019, 59, S77–S87. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Nicholl, B.I.; Mackay, D.; Cullen, B.; Martin, D.J.; Ul-Haq, Z.; Mair, F.S.; Evans, J.; McIntosh, A.M.; Gallagher, J.; Roberts, B.; et al. Chronic multisite pain in major depression and bipolar disorder: Cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry 2014, 14, 350. [Google Scholar] [CrossRef]
- Anderson, K.N.; Schwab, R.B.; Martinez, M.E. Reproductive Risk Factors and Breast Cancer Subtypes: A Review of the Literature. Breast Cancer Res. Treat. 2014, 144, 1–10. [Google Scholar] [CrossRef]
- McPherson, K.; Steel, C.M.; Dixon, J.M. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 2000, 321, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Arvizu, M.; Rich-Edwards, J.W.; Stuart, J.J.; Manson, J.E.; Missmer, S.A.; Pan, A.; Chavarro, J.E. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: Prospective cohort study. BMJ 2020, 371, m3464. [Google Scholar] [CrossRef] [PubMed]
- Violán, C.; Roso-Llorach, A.; Foguet-Boreu, Q.; Guisado-Clavero, M.; Pons-Vigués, M.; Pujol-Ribera, E.; Valderas, J.M. Multimorbidity patterns with K-means nonhierarchical cluster analysis. BMC Fam. Pract. 2018, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Sourial, N.; Wolfson, C.; Zhu, B.; Quail, J.; Fletcher, J.; Karunananthan, S.; Bandeen-Roche, K.; Béland, F.; Bergman, H. Correspondence analysis is a useful tool to uncover the relationships among categorical variables. J. Clin. Epidemiol. 2010, 63, 638–646. [Google Scholar] [CrossRef]
- García-Gil, M.; Blanch, J.; Comas-Cufí, M.; Daunis-i-Estadella, J.; Bolíbar, B.; Martí, R.; Ponjoan, A.; Alves-Cabratosa, L.; Ramos, R. Patterns of statin use and cholesterol goal attainment in a high-risk cardiovascular population: A retrospective study of primary care electronic medical records. J. Clin. Lipidol. 2016, 10, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dimensionality Assessment of Ordered Polytomous Items with Parallel Analysis. PsycNET. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Fa0023353 (accessed on 21 July 2021).
- Xu, R.; Wunsch, D. Survey of Clustering Algorithms. IEEE Trans. Neural Netw. 2005, 16, 645–678. [Google Scholar] [CrossRef]
- Petushkova, N.A.; Pyatnitskiy, M.A.; Rudenko, V.A.; Larina, O.V.; Trifonova, O.P.; Kisrieva, J.S.; Samenkova, N.F.; Kuznetsova, G.P.; Karuzina, I.I.; Lisitsa, A.V. Applying of Hierarchical Clustering to Analysis of Protein Patterns in the Human Cancer-Associated Liver. PLoS ONE 2014, 9, e103950. [Google Scholar] [CrossRef]
- Muntaner, C.; Chung, H.; Benach, J.; Ng, E. Hierarchical cluster analysis of labour market regulations and population health: A taxonomy of low- and middle-income countries. BMC Public Health 2012, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Kimes, P.K.; Liu, Y.; Hayes, D.N.; Marron, J.S. Statistical Significance for Hierarchical Clustering. Biometrics 2017, 73, 811–821. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. La Librairie NbClust pour L’estimation du Nombre Optimal de Classes dans un Jeu de Données. In Proceedings of the XXIème Rencontre de la Société Francophone de Classification, Rabat, Morocco, 10–12 September 2014. [Google Scholar]
- Estimating the Number of Clusters in a Data Set via the Gap Statistic | Request PDF. Available online: https://www.researchgate.net/publication/4772044_Estimating_the_Number_of_Clusters_in_a_Data_Set_Via_the_Gap_Statistic (accessed on 24 July 2021).
- Schäfer, I.; Kaduszkiewicz, H.; Wagner, H.-O.; Schön, G.; Scherer, M.; van den Bussche, H. Reducing complexity: A visualisation of multimorbidity by combining disease clusters and triads. BMC Public Health 2014, 14, 1285. [Google Scholar] [CrossRef]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef] [PubMed]
- Higgs, N.T. Practical and Innovative Uses of Correspondence Analysis. Statistician 1991, 40, 183. [Google Scholar] [CrossRef]
- Schäfer, I.; von Leitner, E.-C.; Schön, G.; Koller, D.; Hansen, H.; Kolonko, T.; Kaduszkiewicz, H.; Wegscheider, K.; Glaeske, G.; van den Bussche, H. Multimorbidity Patterns in the Elderly: A New Approach of Disease Clustering Identifies Complex Interrelations between Chronic Conditions. PLoS ONE 2010, 5, e15941. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Poblador-Plou, B.; Calderón-Larrañaga, A.; Gimeno-Feliu, L.A.; González-Rubio, F.; Poncel-Falcó, A.; Sicras-Mainar, A.; Alcalá-Nalvaiz, J.T. Multimorbidity Patterns in Primary Care: Interactions among Chronic Diseases Using Factor Analysis. PLoS ONE 2012, 7, e32190. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The State of Our Understanding of the Pathophysiology and Optimal Treatment of Depression: Glass Half Full or Half Empty? Am. J. Psychiatry 2020, 177, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Generalized Anxiety Disorder | NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMcp1502514 (accessed on 19 November 2022).
- Lotfaliany, M.; Bowe, S.J.; Kowal, P.; Orellana, L.; Berk, M.; Mohebbi, M. Depression and chronic diseases: Co-occurrence and communality of risk factors. J. Affect. Disord. 2018, 241, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Momen, N.C.; Plana-Ripoll, O.; Agerbo, E.; Benros, M.E.; Børglum, A.D.; Christensen, M.K.; Dalsgaard, S.; Degenhardt, L.; de Jonge, P.; Debost, J.-C.P.G.; et al. Association between Mental Disorders and Subsequent Medical Conditions. N. Engl. J. Med. 2020, 382, 1721–1731. [Google Scholar] [CrossRef]
- Ording, A.G.; Garne, J.P.; Nyström, P.M.W.; Cronin-Fenton, D.; Tarp, M.; Sørensen, H.T.; Lash, T.L. Hospital Recorded Morbidity and Breast Cancer Incidence: A Nationwide Population-Based Case-Control Study. PLoS ONE 2012, 7, e47329. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Gierach, G.L.; Carter, B.D.; Luo, J.; Milne, R.L.; Weiderpass, E.; Giles, G.G.; Tamimi, R.M.; Eliassen, A.H.; Rosner, B.; et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res. 2018, 78, 6011–6021. [Google Scholar] [CrossRef]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of Breast Cancer Risk Factors With Tumor Subtypes: A Pooled Analysis From the Breast Cancer Association Consortium Studies. JNCI J. Natl. Cancer Inst. 2011, 103, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Andaya, A.A.; Enewold, L.; Horner, M.-J.; Jatoi, I.; Shriver, C.D.; Zhu, K. Socioeconomic disparities and breast cancer hormone receptor status. Cancer Causes Control CCC 2012, 23, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.E.; Zirpoli, G.R.; Cozier, Y.C.; Rosenberg, L.; Petrick, J.L.; Bertrand, K.A.; Palmer, J.R. Neighborhood disadvantage and individual-level life stressors in relation to breast cancer incidence in US Black women. Breast Cancer Res. BCR 2021, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, M.J.; Jones, M.E.; Wright, L.B.; Griffin, J.; McFadden, E.; Ashworth, A.; Swerdlow, A.J. Psychological stress, adverse life events and breast cancer incidence: A cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. BCR 2016, 18, 72. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
- Batty, G.D.; Gale, C.R.; Kivimäki, M.; Deary, I.J.; Bell, S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: Prospective cohort study and individual participant meta-analysis. BMJ 2020, 368, m131. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Clustering Method: Clustering Criterion and Agglomerative Algorithm. arXiv 2011, arXiv:1111.6285. [Google Scholar]
- Zhang, Z.; Murtagh, F.; Van Poucke, S.; Lin, S.; Lan, P. Hierarchical cluster analysis in clinical research with heterogeneous study population: Highlighting its visualization with R. Ann. Transl. Med. 2017, 5, 75. [Google Scholar] [CrossRef]


| Characteristics | Overall Study Population N = 239,436 | Pattern 1: No-Predominant Morbidity N = 159,083 | Pattern 2: Psychiatric Morbidities N = 16,627 | Pattern 3: Respiratory/Immunological Morbidities N = 27,920 | Pattern 4: Cardiovascular/Metabolic Morbidities N = 11,041 | Pattern 5: Unspecific Morbidities N = 24,765 | p-Value * |
| Year of follow-up, median (IQR) | 7.1 (6.4, 7.8) | 7.1 (6.4, 7.8) | 7.0 (6.3, 7.8) | 7.1 (6.4, 7.8) | 7.0 (6.3, 7.8) | 7.1 (6.4, 7.8) | <0.001 |
| Breast cancer cases, n (%) | 5326 (2) | 3534 (2) | 381 (2) | 611 (2) | 246 (2) | 554 (2) | 0.97 |
| Number of comorbid conditions, n (%) | <0.001 | ||||||
| None | 99,614 (41.6) | 99,614 (62.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| One | 77,994 (32.6) | 48,489 (30.5) | 6260 (37.6) | 14,283 (51.2) | 379 (3.4) | 8583 (34.7) | |
| Two | 38,424 (16.0) | 10,145 (6.4) | 5974 (35.9) | 9156 (32.8) | 4717 (42.7) | 8432 (34.0) | |
| Three and more | 23,404 (9.8) | 835 (0.5) | 4393 (26.4) | 4481 (16.0) | 5945 (53.8) | 7750 (31.3) | |
| Morbidity, n (%) | |||||||
| Stroke and transient ischemic attack (TIA) | 3149 (1.3) | 833 (0.5) | 90 (0.5) | 181 (0.6) | 1761 (15.9) | 284 (1.1) | <0.001 |
| Diabetes | 8122 (3.4) | 1429 (0.9) | 63 (0.4) | 282 (1.0) | 5924 (53.7) | 424 (1.7) | <0.001 |
| Coronary heart disease | 5566 (2.3) | 796 (0.5) | 53 (0.3) | 329 (1.2) | 3978 (36.0) | 410 (1.7) | <0.001 |
| Migraine | 9947 (4.2) | 247 (0.2) | 940 (5.7) | 686 (2.5) | 102 (0.9) | 7972 (32.2) | <0.001 |
| Diverticular disease of intestine | 3048 (1.3) | 0 (0.0) | 10 (0.1) | 109 (0.4) | 247 (2.2) | 2682 (10.8) | <0.001 |
| Irritable bowel syndrome | 7622 (3.2) | 32 (0.0) | 642 (3.9) | 195 (0.7) | 276 (2.5) | 6477 (26.2) | <0.001 |
| Rheumatoid arthritis | 6778 (2.8) | 0 (0.0) | 81 (0.5) | 201 (0.7) | 4 (0.0) | 6492 (26.2) | <0.001 |
| Treated dyspepsia | 17,733 (7.4) | 6427 (4.0) | 1704 (10.2) | 2053 (7.4) | 1807 (16.4) | 5742 (23.2) | <0.001 |
| Psoriasis or eczema | 8344 (3.5) | 0 (0.0) | 773 (4.6) | 5823 (20.9) | 190 (1.7) | 1558 (6.3) | <0.001 |
| Chronic obstructive respiratory disease (COPD) | 3355 (1.4) | 0 (0.0) | 7 (0.0) | 3333 (11.9) | 2 (0.0) | 13 (0.1) | <0.001 |
| Asthma | 29,541 (12.3) | 0 (0.0) | 2311 (13.9) | 21,708 (77.8) | 2473 (22.4) | 3049 (12.3) | <0.001 |
| Anxiety | 4964 (2.1) | 0 (0.0) | 4460 (26.8) | 113 (0.4) | 216 (2.0) | 175 (0.7) | <0.001 |
| Depression | 16,368 (6.8) | 0 (0.0) | 13,362 (80.4) | 424 (1.5) | 1157 (10.5) | 1425 (5.8) | <0.001 |
| Thyroid disorders | 22,718 (9.5) | 13,277 (8.3) | 1776 (10.7) | 2213 (7.9) | 2806 (25.4) | 2646 (10.7) | <0.001 |
| Hypertension | 55,223 (23.1) | 31,013 (19.5) | 3647 (21.9) | 6112 (21.9) | 8505 (77.0) | 5946 (24.0) | <0.001 |
| Pain conditions | 41,258 (17.2) | 21,363 (13.4) | 3665 (22.0) | 4767 (17.1) | 3132 (28.4) | 8331 (33.6) | <0.001 |
| Age at baseline, median (IQR) | 57.7 (50.2, 63.2) | 57.4 (49.9, 63.0) | 55.7 (48.7, 61.7) | 56.7 (49.1, 62.8) | 62.6 (57.2, 66.4) | 59.2 (51.9, 64.0) | <0.001 |
| Family history of breast cancer, n (%) | 25,330 (10.6) | 16,858 (10.6) | 1765 (10.6) | 2885 (10.3) | 1102 (10.0) | 2720 (11.0) | 0.035 |
| BMI, n (%) | <0.001 | ||||||
| <18.5 | 1803 (0.8) | 1215 (0.8) | 115 (0.7) | 225 (0.8) | 23 (0.2) | 225 (0.9) | |
| 18.5–25 | 92,857 (38.8) | 66,570 (41.8) | 5644 (33.9) | 10,139 (36.3) | 1547 (14.0) | 8957 (36.2) | |
| 25–30 | 87,381 (36.5) | 58,431 (36.7) | 6067 (36.5) | 10,161 (36.4) | 3581 (32.4) | 9141 (36.9) | |
| >30 | 56,150 (23.5) | 31,992 (20.1) | 4725 (28.4) | 7282 (26.1) | 5799 (52.5) | 6352 (25.6) | |
| Unknown | 1245 (0.5) | 875 (0.6) | 76 (0.5) | 113 (0.4) | 91 (0.8) | 90 (0.4) | |
| Adherence to breast cancer screening, n (%) | <0.001 | ||||||
| <50 years of age | 58,722 (24.5) | 40,371 (25.4) | 4902 (29.5) | 7745 (27.7) | 873 (7.9) | 4831 (19.5) | |
| >50 years of age, >3 years ago | 7929 (3.3) | 5072 (3.2) | 554 (3.3) | 889 (3.2) | 545 (4.9) | 869 (3.5) | |
| >50 years of age, in the last 3 years | 159,407 (66.6) | 104,789 (65.9) | 10,158 (61.1) | 17,761 (63.6) | 8937 (80.9) | 17,762 (71.7) | |
| >50 years of age, never | 8013 (3.3) | 5384 (3.4) | 631 (3.8) | 943 (3.4) | 348 (3.2) | 707 (2.9) | |
| >50 years of age, unknown | 5365 (2.2) | 3467 (2.2) | 382 (2.3) | 582 (2.1) | 338 (3.1) | 596 (2.4) | |
| Age at menarche, median (IQR) | 13.0 (12.0, 14.0) | 13 (12.0, 14.0) | 13 (12.0, 14.0) | 13.0 (12.0, 14.0) | 13 (12.0, 14.0) | 13 (12.0, 14.0) | <0.001 |
| Age at menopause µ, median (IQR) | 50.0 (47.0, 52.0) | 50.0 (47.0, 52.0) | 50.0 (45.5, 52.0) | 50.0 (46.0, 52.0) | 50.0 (45.0, 52.0) | 50.0 (46.0, 52.0) | <0.001 |
| Menopause status at baseline, n (%) | <0.001 | ||||||
| Still had periods | 63,488 (26.5) | 44,275 (27.8) | 4979 (29.9) | 8152 (29.2) | 951 (8.6) | 5131 (20.7) | |
| Had menopause before the age of 45 | 25,659 (10.7) | 14,768 (9.3) | 2095 (12.6) | 3356 (12.0) | 2024 (18.3) | 3416 (13.8) | |
| Had menopause between the age of 45 and 54 | 129,114 (53.9) | 85,911 (54.0) | 8332 (50.1) | 14,084 (50.4) | 6796 (61.6) | 13,991 (56.5) | |
| Had menopause after the age of 54 | 21,175 (8.8) | 14,129 (8.9) | 1221 (7.3) | 2328 (8.3) | 1270 (11.5) | 2227 (9.1) | |
| Menopausal hormone therapy use µ, n (%) | <0.001 | ||||||
| Never | 85,613 (48.7) | 59,734 (52.0) | 4572 (39.3) | 8935 (45.2) | 4485 (44.4) | 7887 (40.2) | |
| Ever, less than 5 years duration | 31,000 (17.6) | 19,322 (16.8) | 2553 (21.9) | 3683 (18.6) | 1620 (16.1) | 3822 (19.5) | |
| Ever, 5 years and longer duration | 47,233 (26.8) | 28,799 (25.1) | 3496 (30.0) | 5759 (29.1) | 2898 (28.7) | 6281 (32.0) | |
| Ever, unknown duration | 11,229 (6.4) | 6386 (5.6) | 975 (8.4) | 1314 (6.6) | 1004 (9.9) | 1550 (7.9) | |
| Unknown status | 874 (0.5) | 567 (0.5) | 52 (0.4) | 77 (0.4) | 84 (0.8) | 94 (0.5) | |
| Oral contraception use, n (%) | <0.001 | ||||||
| Never | 44,767 (18.7) | 29,175 (18.3) | 2795 (16.8) | 4818 (17.3) | 3147 (28.5) | 4832 (19.5) | |
| Ever, less than 10 years duration | 87,270 (36.4) | 57,671 (36.3) | 5929 (35.7) | 10,134 (36.3) | 4074 (36.9) | 9462 (38.2) | |
| Ever, 10 years and longer duration | 84,462 (35.3) | 57,626 (36.2) | 6117 (36.8) | 10,315 (36.9) | 2505 (22.7) | 7899 (31.9) | |
| Ever, unknown duration | 22,542 (9.4) | 14,354 (9.0) | 1758 (10.6) | 2628 (9.4) | 1270 (11.5) | 2532 (10.2) | |
| Unknown status | 395 (0.2) | 257 (0.2) | 28 (0.2) | 25 (0.1) | 45 (0.4) | 40 (0.2) | |
| Parity and age at first birth, n (%) | <0.001 | ||||||
| None of live birth | 44,601 (18.6) | 29,572 (18.6) | 3575 (21.5) | 5497 (19.7) | 1614 (14.6) | 4343 (17.5) | |
| At least one birth before 30 | 150,386 (62.8) | 98,115 (61.7) | 10,088 (60.7) | 17,341 (62.1) | 8183 (74.1) | 16,659 (67.3) | |
| At least one birth after age 30 | 43,302 (18.1) | 30,569 (19.2) | 2910 (17.5) | 5003 (17.9) | 1154 (10.5) | 3666 (14.8) | |
| Unknown | 1147 (0.5) | 827 (0.5) | 54 (0.3) | 79 (0.3) | 90 (0.8) | 97 (0.4) | |
| Levels of physical activities, n (%) | <0.001 | ||||||
| Low | 76,618 (32.0) | 47,554 (29.9) | 5964 (35.9) | 9211 (33.0) | 4867 (44.1) | 9022 (36.4) | |
| Moderate | 85,403 (35.7) | 57,868 (36.4) | 5758 (34.6) | 9893 (35.4) | 3341 (30.3) | 8543 (34.5) | |
| High | 77,415 (32.3) | 53,661 (33.7) | 4905 (29.5) | 8816 (31.6) | 2833 (25.7) | 7200 (29.1) | |
| Alcohol consumption, n (%) | <0.001 | ||||||
| Never | 22,751 (9.5) | 12,842 (8.1) | 1952 (11.7) | 2650 (9.5) | 2201 (19.9) | 3106 (12.5) | |
| Once or twice a week or less | 128,606 (53.7) | 84,178 (52.9) | 8816 (53.0) | 14,979 (53.6) | 6553 (59.4) | 14,080 (56.9) | |
| Three times a week or more | 87,417 (36.5) | 61,568 (38.7) | 5819 (35.0) | 10,247 (36.7) | 2255 (20.4) | 7528 (30.4) | |
| Unknown | 662 (0.3) | 495 (0.3) | 40 (0.2) | 44 (0.2) | 32 (0.3) | 51 (0.2) | |
| Ethnicity, n (%) | <0.001 | ||||||
| White | 224,792 (93.9) | 149,010 (93.7) | 15,960 (96.0) | 26,260 (94.1) | 9802 (88.8) | 23,760 (95.9) | |
| Asia | 5200 (2.2) | 3615 (2.3) | 192 (1.2) | 558 (2.0) | 508 (4.6) | 327 (1.3) | |
| Black and Caribbean | 4286 (1.8) | 2975 (1.9) | 146 (0.9) | 491 (1.8) | 427 (3.9) | 247 (1.0) | |
| Other/unknown | 5158 (2.2) | 3483 (2.2) | 329 (2.0) | 611 (2.2) | 304 (2.8) | 431 (1.7) | |
| Region, n (%) | <0.001 | ||||||
| England | 212,190 (88.6) | 140,684 (88.4) | 15,006 (90.3) | 24,840 (89.0) | 9744 (88.3) | 21,916 (88.5) | |
| Scotland | 17,382 (7.3) | 11,914 (7.5) | 1022 (6.1) | 1786 (6.4) | 837 (7.6) | 1823 (7.4) | |
| Wales | 9864 (4.1) | 6485 (4.1) | 599 (3.6) | 1294 (4.6) | 460 (4.2) | 1026 (4.1) | |
| Socioeconomic status based on Townsend Score, n (%) | <0.001 | ||||||
| Interquartile 1 | 59,168 (24.7) | 40,773 (25.6) | 3653 (22.0) | 6715 (24.1) | 1904 (17.2) | 6123 (24.7) | |
| Interquartile 2 | 58,909 (24.6) | 40,010 (25.2) | 3918 (23.6) | 6477 (23.2) | 2333 (21.1) | 6171 (24.9) | |
| Interquartile 3 | 59,853 (25.0) | 39,856 (25.1) | 4195 (25.2) | 6949 (24.9) | 2708 (24.5) | 6145 (24.8) | |
| Interquartile 4 | 61,506 (25.7) | 38,444 (24.2) | 4861 (29.2) | 7779 (27.9) | 4096 (37.1) | 6326 (25.5) |
| Pre-Existing Disease at Baseline | Number of Breast Cancer Cases/Person Years | Age-Adjusted Model HR (95%CI) | Multivariable Model HR (95%CI) |
|---|---|---|---|
| Hypertension | |||
| No | 3979/1,287,967 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 1347/383,417 | 1.06 (0.99–1.13) | 1.03 (0.97–1.11) |
| Pain condition | |||
| No | 4336/1,386,565 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 990/284,820 | 1.06 (0.98–1.13) | 1.04 (0.97–1.12) |
| Asthma | |||
| No | 4692/1,465,134 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 634/206,250 | 0.97 (0.89–1.05) | 0.96 (0.88–1.04) |
| Thyroid disorders | |||
| No | 4836/1,513,768 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 490/157,617 | 0.94 (0.85–1.03) | 0.93 (0.85–1.02) |
| Treated dyspepsia | |||
| No | 4898/1,548,551 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 428/122,834 | 1.04 (0.95–1.15) | 1.04 (0.94–1.15) |
| Depression | |||
| No | 4927/1,557,562 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 399/113,821 | 1.13 (1.02–1.26) | 1.12 (1.01–1.24) |
| Migraine | |||
| No | 5099/1,601,276 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 227/70,109 | 1.04 (0.91–1.18) | 1.05 (0.91–1.19) |
| Psoriasis | |||
| No | 5131/1,612,546 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 195/58,839 | 1.06 (0.92–1.22) | 1.04 (0.90–1.2) |
| Diabetes | |||
| No | 5138/1,616,001 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 188/55,384 | 1.02 (0.88–1.18) | 0.99 (0.85–1.15) |
| Irritable bowel syndrome | |||
| No | 5157/1,617,608 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 169/53,776 | 0.98 (0.84–1.15) | 0.99 (0.85–1.15) |
| Rheumatoid arthritis | |||
| No | 5181/1,624,015 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 145/473,698 | 0.92 (0.78–1.09) | 0.92 (0.78–1.09) |
| Coronary heart disease | |||
| No | 5227/1,632,796 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 99/38,589 | 0.72 (0.59–0.88) | 0.73 (0.60–0.89) |
| Anxiety | |||
| No | 5221/1,637,202 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 105/34,183 | 0.97 (0.80–1.18) | 0.96 (0.79–1.17) |
| COPD | |||
| No | 5245/1,648,455 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 81/22,930 | 1.05 (0.84–1.30) | 1.07 (0.86–1.33) |
| Stroke | |||
| No | 5260/1,649,817 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 66/21,568 | 0.89 (0.70–1.14) | 0.91 (0.71–1.16) |
| Diverticular disease of intestine | |||
| No | 5258/1,650,114 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 68/21,271 | 0.92 (0.72–1.17) | 0.9 (0.71–1.15) |
| Study Population (n = 239,436) | Postmenopausal Women Only (n = 175,949) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Breast Cancer Cases/Person- Years | Age-Adjusted Models HR (95%CI) | Fully Adjusted Models HR (95%CI) | Breast Cancer Cases/Person Years | Age-Adjusted Models HR (95%CI) | Fully Adjusted Models HR (95%CI) |
| Number of morbidities | ||||||
| No morbidity | 2131/69,8776 | 1.00 (Reference) | 1.00 (Reference) | 1451/454,566 | 1.00 (Reference) | 1.00 (Reference) |
| One morbidity | 1736/54,3974 | 1.01 (0.95–1.08) | 1.00 (0.94–1.07) | 1361/408,943 | 1.02 (0.95–1.10) | 1 (0.93–1.08) |
| Multi-morbidities | 1459/428,635 | 1.04 (0.97–1.02) | 1.03 (0.96–1.11) | 1268/359,844 | 1.06 (0.98–1.14) | 1.02 (0.94–1.1) |
| Two morbidities | 911/266,831 | 1.05 (0.97–1.14) | 1.04 (0.96–1.13) | 786/218,780 | 1.08 (0.99–1.18) | 1.04 (0.95–1.14) |
| 3+ morbidities | 548/161,804 | 1.03 (0.93–1.13) | 1.01 (0.92–1.12) | 482/141,065 | 1.02 (092–1.14) | 0.97 (0.87–1.08) |
| Morbidity patterns | ||||||
| No-predominant morbidity | 3534/1,110,979 | 1.00 (Reference) | 1.00 (Reference) | 2670/798,572 | 1.00 (Reference) | 1.00 (Reference) |
| Psychiatric morbidities | 381/115,476 | 1.06 (0.95–1.18) | 1.04 (0.94–1.16) | 264/80,575 | 1.00 (0.88–1.14) | 0.98 (0.86–1.11) |
| Respiratory/immunological morbidities | 611/195,129 | 0.99 (0.91–1.08) | 0.98 (0.9–1.07) | 467/137,526 | 1.02 (0.92–1.12) | 1.01 (0.91–1.11) |
| Cardiovascular/metabolic morbidities | 246/75,843 | 0.94 (0.83–1.07) | 0.93 (0.81–1.06) | 232/69,252 | 0.96 (0.84–1.10) | 0.91 (0.79–1.05) |
| Unspecific morbidities | 554/173,957 | 0.98 (0.89–1.07) | 0.98 (0.89–1.07) | 447/137,429 | 0.96 (0.87–1.06) | 0.95 (0.86–1.05) |
| Event | Morbidity Pattern | Cases/Person-Years | Hazard Ratio (95%CI) |
|---|---|---|---|
| Breast cancer as first diagnosed cancer | |||
| No-predominant morbidity | 3534/1,110,979 | 1.00 (Reference) | |
| Psychiatric morbidities | 381/115,476 | 1.04 (0.94–1.16) | |
| Respiratory/immunological morbidities | 611/195,129 | 0.98 (0.90–1.07) | |
| Cardiovascular/metabolic morbidities | 246/758,423 | 0.93 (0.81–1.06) | |
| Unspecific morbidities | 554/173,957 | 0.98 (0.89–1.07) | |
| Non-breast cancer as first diagnosed cancer | |||
| No-predominant morbidity | 4964/1,110,979 | 1.00 (Reference) | |
| Psychiatric morbidities | 485/115,476 | 0.96 (0.88–1.06) | |
| Respiratory/immunological morbidities | 1041/195,129 | 1.18 (1.11–1.27) | |
| Cardiovascular/metabolic morbidities | 561/758,423 | 1.19 (1.09–1.30) | |
| Unspecific morbidities | 862/173,957 | 1.00 (0.93–1.07) | |
| Death | |||
| No-predominant morbidity | 645/1,110,979 | 1.00 (Reference) | |
| Psychiatric morbidities | 126/115,476 | 1.82 (1.50–2.21) | |
| Respiratory/immunological morbidities | 203/195,129 | 1.68 (1.44–1.97) | |
| Cardiovascular/metabolic morbidities | 242/758,423 | 3.06 (2.61–3.58) | |
| Unspecific morbidities | 205/173,957 | 1.65 (1.41–1.94) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henyoh, A.M.S.; Allodji, R.S.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N.M.Y.; Tran, T.-V.-T. Multi-Morbidity and Risk of Breast Cancer among Women in the UK Biobank Cohort. Cancers 2023, 15, 1165. https://doi.org/10.3390/cancers15041165
Henyoh AMS, Allodji RS, de Vathaire F, Boutron-Ruault M-C, Journy NMY, Tran T-V-T. Multi-Morbidity and Risk of Breast Cancer among Women in the UK Biobank Cohort. Cancers. 2023; 15(4):1165. https://doi.org/10.3390/cancers15041165
Chicago/Turabian StyleHenyoh, Afi Mawulawoe Sylvie, Rodrigue S. Allodji, Florent de Vathaire, Marie-Christine Boutron-Ruault, Neige M. Y. Journy, and Thi-Van-Trinh Tran. 2023. "Multi-Morbidity and Risk of Breast Cancer among Women in the UK Biobank Cohort" Cancers 15, no. 4: 1165. https://doi.org/10.3390/cancers15041165
APA StyleHenyoh, A. M. S., Allodji, R. S., de Vathaire, F., Boutron-Ruault, M.-C., Journy, N. M. Y., & Tran, T.-V.-T. (2023). Multi-Morbidity and Risk of Breast Cancer among Women in the UK Biobank Cohort. Cancers, 15(4), 1165. https://doi.org/10.3390/cancers15041165






