CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Patients
2.2. Immunohistochemistry and Interpretation
2.3. Cell Culture and Reagents
2.4. Transfection of siRNAs and cDNA
2.5. Lentiviral Infection and Generation of Stable Cell
2.6. Immunoblotting
2.7. The Sulforhodamine B Colorimetric (SRB) Assay
2.8. Colony Formation Assay
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Flow Cytometry
2.11. Tumorsphere Analysis
2.12. Orthotopic Xenografts
2.13. RNA Sequencing (RNA-Seq) Analysis
2.14. Public Data
2.15. Statistical Analysis
3. Results
3.1. Genetic Alteration of CTTN Is Associated with Poor Prognosis of HER2 Positive Breast Cancer Patients
3.2. CTTN Induces Cancer Stem Cell Properties and Resistance to Trastuzumab in HER2+ Breast Cancer
3.3. CTTN Downregulates DKK-1 Expression and Activates the Wnt Signaling Pathway
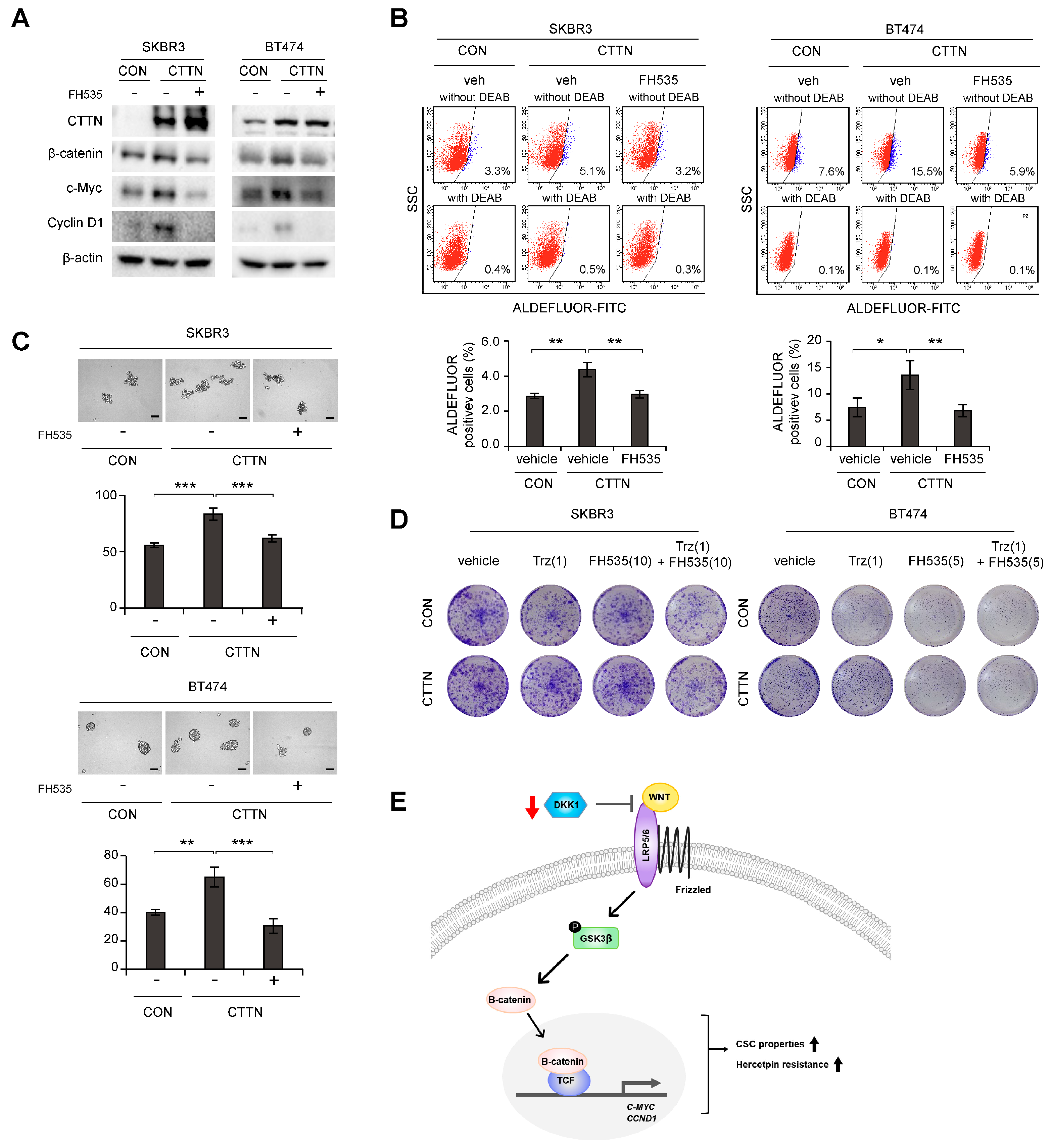
3.4. Inhibition of Wnt Signaling Reverses CTTN-Induced CSC-like Properties and Trastuzumab Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagini, S. Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Rezaei, Z.; Safarpour, H.; Sabri, M.; Mir, A.; Sanati, M.A.; Vahidian, F.; Gholamiyan Moghadam, A.; Aghadoukht, A.; Hajiasgharzadeh, K.; et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J. Cell. Physiol. 2020, 235, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The trastuzumab era: Current and upcoming targeted HER2+ breast cancer therapies. Am. J. Cancer Res. 2020, 10, 1045–1067. [Google Scholar]
- Schuuring, E. The involvement of the chromosome 11q13 region in human malignancies: Cyclin D1 and EMS1 are two new candidate oncogenes—A review. Gene 1995, 159, 83–96. [Google Scholar] [CrossRef]
- Dedes, K.J.; Lopez-Garcia, M.A.; Geyer, F.C.; Lambros, M.B.; Savage, K.; Vatcheva, R.; Wilkerson, P.; Wetterskog, D.; Lacroix-Triki, M.; Natrajan, R.; et al. Cortactin gene amplification and expression in breast cancer: A chromogenic in situ hybridisation and immunohistochemical study. Breast Cancer Res. Treat. 2010, 124, 653–666. [Google Scholar] [CrossRef]
- Jeannot, P.; Besson, A. Cortactin function in invadopodia. Small GTPases 2020, 11, 256–270. [Google Scholar] [CrossRef]
- Uzair, I.D.; Conte Grand, J.; Flamini, M.I.; Sanchez, A.M. Molecular Actions of Thyroid Hormone on Breast Cancer Cell Migration and Invasion via Cortactin/N-WASP. Front. Endocrinol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Lang, L.; Hou, Y.; Chen, Y.; Tu, G.; Tao, J.; Yang, D.; Xi, L.; Fu, L.; Sun, K.; Yin, J.; et al. ATM-Mediated Phosphorylation of Cortactin Involved in Actin Polymerization Promotes Breast Cancer Cells Migration and Invasion. Cell. Physiol. Biochem. 2018, 51, 2972–2988. [Google Scholar] [CrossRef]
- Hill, A.; McFarlane, S.; Mulligan, K.; Gillespie, H.; Draffin, J.E.; Trimble, A.; Ouhtit, A.; Johnston, P.G.; Harkin, D.P.; McCormick, D.; et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene 2006, 25, 6079–6091. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Zhang, T.; Wang, Z.; Qin, X.; Jing, X.; Wu, H.; Ji, X.; He, Y.; Zhao, R. Cortactin promotes colorectal cancer cell proliferation by activating the EGFR-MAPK pathway. Oncotarget 2017, 8, 1541–1554. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhu, M.X.; Yang, Y.W.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Lu, J.C.; Wang, L.; Deng, X.Y.; et al. Downregulation of RNF128 activates Wnt/beta-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J. Hematol. Oncol. 2019, 12, 21. [Google Scholar] [CrossRef]
- Timpson, P.; Wilson, A.S.; Lehrbach, G.M.; Sutherland, R.L.; Musgrove, E.A.; Daly, R.J. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res. 2007, 67, 9304–9314. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, L.; Liu, H.; Luo, X. Cancer stem cell-targeted therapeutic approaches for overcoming trastuzumab resistance in HER2-positive breast cancer. Stem Cells 2021, 39, 1125–1136. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Cancer stem cells: Implications for the progression and treatment of metastatic disease. J. Cell. Mol. Med. 2008, 12, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Lin, W.R.; Lim, S.M.; Nicholson, L.J. Cancer stem cells: In the line of fire. Cancer Treat. Rev. 2012, 38, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Bhola, N.E.; Sutton, C.; Ghosh, R.; Kuba, M.G.; Dave, B.; Chang, J.C.; Arteaga, C.L. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013, 73, 1190–1200. [Google Scholar] [CrossRef]
- Chihara, Y.; Shimoda, M.; Hori, A.; Ohara, A.; Naoi, Y.; Ikeda, J.I.; Kagara, N.; Tanei, T.; Shimomura, A.; Shimazu, K.; et al. A small-molecule inhibitor of SMAD3 attenuates resistance to anti-HER2 drugs in HER2-positive breast cancer cells. Breast Cancer Res. Treat. 2017, 166, 55–68. [Google Scholar] [CrossRef]
- Chung, S.S.; Giehl, N.; Wu, Y.; Vadgama, J.V. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int. J. Oncol. 2014, 44, 403–411. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, Y.; Oh, E.; Lee, N.; An, H.; Sung, D.; Cho, T.M.; Seo, J.H. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett. 2016, 379, 39–48. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, J.; Hu, Y.; Zhou, Y.; Guo, R.; Bai, J.; Zhang, S.; Zhang, H.; Zhang, J. The combination of NVP-BKM120 with trastuzumab or RAD001 synergistically inhibits the growth of breast cancer stem cells in vivo. Oncol. Rep. 2016, 36, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sung, D.; Oh, E.; Cho, Y.; Cho, T.M.; Farrand, L.; Seo, J.H.; Kim, J.Y. Flubendazole overcomes trastuzumab resistance by targeting cancer stem-like properties and HER2 signaling in HER2-positive breast cancer. Cancer Lett. 2018, 412, 118–130. [Google Scholar] [CrossRef]
- Park, H.; Jee, S.; Son, H.; Cha, H.; Bang, S.; Kim, H.; Shin, S.J.; Cha, C.; Chung, M.S.; Myung, J.; et al. Loss of Single-Stranded DNA Binding Protein 2 Expression Is Associated with Aggressiveness and Poor Overall Survival in Patients with Invasive Breast Carcinoma. Diagnostics 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Won, H.Y.; Park, J.H.; Kim, H.Y.; Choi, H.J.; Shin, D.H.; Kang, J.H.; Woo, J.K.; Oh, S.H.; Son, T.; et al. MEL-18 loss mediates estrogen receptor-alpha downregulation and hormone independence. J. Clin. Investig. 2015, 125, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Park, J.H.; Choi, H.J.; Park, M.K.; Won, H.Y.; Park, Y.J.; Lee, C.H.; Oh, S.H.; Song, Y.S.; Kim, H.S.; et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat. Commun. 2015, 6, 7821. [Google Scholar] [CrossRef]
- Choi, H.J.; Joo, H.S.; Won, H.Y.; Min, K.W.; Kim, H.Y.; Son, T.; Oh, Y.H.; Lee, J.Y.; Kong, G. Role of RBP2-Induced ER and IGF1R-ErbB Signaling in Tamoxifen Resistance in Breast Cancer. J. Natl. Cancer Inst. 2018, 110, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Choi, H.J.; Jin, S.; Cho, H.; Won, H.Y.; An, H.W.; Jeong, G.Y.; Park, Y.U.; Kim, H.Y.; Park, M.K.; Son, T.; et al. CDK12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS1-ErbB-PI3K signaling. EMBO Rep. 2019, 20, e48058. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, H.J.; Lee, J.Y.; Kong, G. Cancer Target Gene Screening: A web application for breast cancer target gene screening using multi-omics data analysis. Brief. Bioinform. 2020, 21, 663–675. [Google Scholar] [CrossRef]
- Castagnoli, L.; Iezzi, M.; Ghedini, G.C.; Ciravolo, V.; Marzano, G.; Lamolinara, A.; Zappasodi, R.; Gasparini, P.; Campiglio, M.; Amici, A.; et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014, 74, 6248–6259. [Google Scholar] [CrossRef] [PubMed]
- Triulzi, T.; De Cecco, L.; Sandri, M.; Prat, A.; Giussani, M.; Paolini, B.; Carcangiu, M.L.; Canevari, S.; Bottini, A.; Balsari, A.; et al. Whole-transcriptome analysis links trastuzumab sensitivity of breast tumors to both HER2 dependence and immune cell infiltration. Oncotarget 2015, 6, 28173–28182. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Bianchini, G.; Thomas, M.; Belousov, A.; Cheang, M.C.; Koehler, A.; Gomez, P.; Semiglazov, V.; Eiermann, W.; Tjulandin, S.; et al. Research-based PAM50 subtype predictor identifies higher responses and improved survival outcomes in HER2-positive breast cancer in the NOAH study. Clin. Cancer Res. 2014, 20, 511–521. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer stem cells behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/beta-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, J.; Liu, Z.; Zhang, J.; Chang, J. Label-Free Quantitative Proteomics Combined with Biological Validation Reveals Activation of Wnt/beta-Catenin Pathway Contributing to Trastuzumab Resistance in Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 1981. [Google Scholar] [CrossRef]
- Ormandy, C.J.; Musgrove, E.A.; Hui, R.; Daly, R.J.; Sutherland, R.L. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 2003, 78, 323–335. [Google Scholar] [CrossRef]
- Zaharieva, B.M.; Simon, R.; Diener, P.A.; Ackermann, D.; Maurer, R.; Alund, G.; Knonagel, H.; Rist, M.; Wilber, K.; Hering, F.; et al. High-throughput tissue microarray analysis of 11q13 gene amplification (CCND1, FGF3, FGF4, EMS1) in urinary bladder cancer. J. Pathol. 2003, 201, 603–608. [Google Scholar] [CrossRef]
- Borg, A.; Sigurdsson, H.; Clark, G.M.; Ferno, M.; Fuqua, S.A.; Olsson, H.; Killander, D.; McGurie, W.L. Association of INT2/HST1 coamplification in primary breast cancer with hormone-dependent phenotype and poor prognosis. Br. J. Cancer 1991, 63, 136–142. [Google Scholar] [CrossRef]
- Luo, M.L.; Shen, X.M.; Zhang, Y.; Wei, F.; Xu, X.; Cai, Y.; Zhang, X.; Sun, Y.T.; Zhan, Q.M.; Wu, M.; et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006, 66, 11690–11699. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cheng, X.; Ji, X.; He, Y.; Jing, X.; Wu, H.; Zhao, R. Cortactin contributes to the tumorigenicity of colorectal cancer by promoting cell proliferation. Oncol. Rep. 2016, 36, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012, 122, 1529–1540. [Google Scholar] [CrossRef] [PubMed]



| Cell Type | Cell Number for Injection | Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 Days | 32 Days | 39 Days | 47 Days | 53 Days | 60 Days | 62 Days | ||
| SKBR3 CON | 1000 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| 10,000 | 0/7 | 1/7 | 0/7 | 1/7 | 2/7 | 2/7 | 2/7 | |
| 100,000 | 0/7 | 2/7 | 3/7 | 3/7 | 5/7 | 5/7 | 6/7 | |
| TIC frequency | 1/221,488 | 1/204,950 | 1/151,387 | 1/65,006 | 1/65,006 | 1/45,613 | ||
| (1/69,236–1/708,542) | (1/66,312–1/633,440) | (1/55,072–1/416,146) | (1/28,464–1/148,458) | (1/28,464–1/148,458) | (1/20,146–1/103,269) | |||
| SKBR3 CTTN | 1000 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 1/7 |
| 10,000 | 0/7 | 1/7 | 4/7 | 5/7 | 5/7 | 5/7 | 6/7 | |
| 100,000 | 1/7 | 4/7 | 5/7 | 5/7 | 7/7 | 7/7 | 7/7 | |
| TIC frequency | 1/725,852 | 1/108,315 | 1/47,091 | 1/40,774 | 1/40,774 | 1/9538 | 1/5385 | |
| (1/102,732–1/5,128,513) | (1/43,489–1/269,776) | (1/20,824–1/106,490) | (1/17,876–1/93,000) | (1/17,876–1/93,000) | (1/3924–1/23,184) | (1/2361–1/12,282) | ||
| p-value | 0.343 | 0.039 | 0.049 | 0.433 | 0.002 | <0.001 | ||
| Cell Type | Cell Number for Injection | Days | ||||||
| 21 Days | 24 Days | 28 Days | 31 Days | |||||
| HCC-1954 shCON | 1000 | 0/7 | 0/7 | 1/7 | 4/7 | |||
| 10,000 | 4/7 | 6/7 | 7/7 | 7/7 | ||||
| 100,000 | 4/7 | 6/7 | 7/7 | 7/7 | ||||
| TIC frequency | 1/63,227 | 1/22,866 | 1/3213 | 1/1176 | ||||
| (1/27,750–1/144,061) | (1/9028–1/57,912) | (1/1339–1/7710) | (1/435–1/3183) | |||||
| HCC-1954 shCTTN#1 | 1000 | 0/7 | 0/7 | 0/7 | 0/7 | |||
| 10,000 | 0/7 | 1/7 | 2/7 | 2/7 | ||||
| 100,000 | 0/7 | 1/7 | 2/7 | 3/7 | ||||
| TIC frequency | 1/725,852 | 1/359,832 | 1/164,203 | 1/119,227 | ||||
| (1/86,771–1/1,492,202) | (1/58,039–1/464,560) | (1/46,688–1/304,468) | ||||||
| p-value | 0.0015 | 0.0001 | 0.0001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-J.; Choi, H.-J.; Kye, Y.-H.; Jeong, G.-Y.; Kim, H.-Y.; Myung, J.-K.; Kong, G. CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer. Cancers 2023, 15, 1168. https://doi.org/10.3390/cancers15041168
Moon S-J, Choi H-J, Kye Y-H, Jeong G-Y, Kim H-Y, Myung J-K, Kong G. CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer. Cancers. 2023; 15(4):1168. https://doi.org/10.3390/cancers15041168
Chicago/Turabian StyleMoon, So-Jeong, Hyung-Jun Choi, Young-Hyeon Kye, Ga-Young Jeong, Hyung-Yong Kim, Jae-Kyung Myung, and Gu Kong. 2023. "CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer" Cancers 15, no. 4: 1168. https://doi.org/10.3390/cancers15041168
APA StyleMoon, S.-J., Choi, H.-J., Kye, Y.-H., Jeong, G.-Y., Kim, H.-Y., Myung, J.-K., & Kong, G. (2023). CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer. Cancers, 15(4), 1168. https://doi.org/10.3390/cancers15041168







