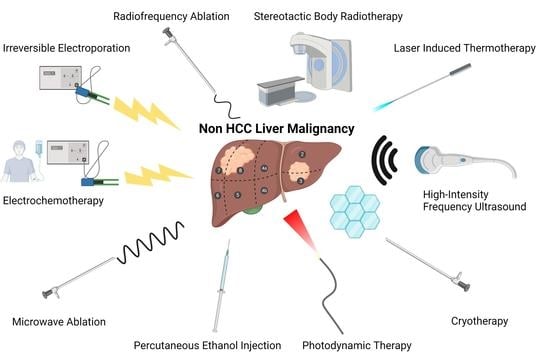
Ablative Therapy in Non-HCC Liver Malignancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Review Purpose
Materials and Methods
3. Impact of Non-HCC Primary Liver Malignancy on Prognosis
4. Impact of Metastatic Disease to the Liver on Prognosis
5. Radiofrequency Ablation
5.1. Technique
5.2. Radiofrequency Ablation in Non-HCC Primary Liver Malignancy
5.3. Radiofrequency Ablation in Metastatic Disease to the Liver
6. Microwave Ablation
6.1. Technique
6.2. Microwave Ablation in Non-HCC Primary Liver Malignancy
6.3. Microwave Ablation in Metastatic Disease to the Liver
7. Cryotherapy
7.1. Technique
7.2. Cryoablation in Non-HCC Primary Malignancy and Metastatic Disease to the Liver
8. Irreversible Electroporation
8.1. Technique
8.2. Irreversible Electroporation in Non-HCC Primary Liver Malignancy and Metastatic Disease to the Liver
9. High-Intensity Focused Ultrasound
9.1. Technique
9.2. High-Intensity Focused Ultrasound in Non-HCC Primary Liver Malignancy and Metastatic Disease to the Liver
10. Photodynamic Therapy
10.1. Technique
10.2. Photodynamic Therapy in Non-HCC Primary Liver Malignancy
10.3. Photodynamic Therapy in Metastatic Disease to the Liver
11. Stereotactic Body Radiotherapy
11.1. Technique
11.2. Stereotactic Body Radiotherapy in Non-HCC Primary Liver Malignancy
11.3. Stereotactic Body Radiotherapy in Metastatic Disease to the Liver
12. Laser-Induced Thermotherapy
12.1. Technique
12.2. Laser Induced Thermotherapy in Non-HCC Primary Liver Malignancy and Metastatic Disease to the Liver
13. Electrochemotherapy
13.1. Technique
13.2. Electrochemotherapy in Non-HCC Primary Liver Malignancy and Metastatic Disease to the Liver
14. Percutaneous Ethanol Injection
14.1. Technique
14.2. Percutaneous Ethanol Injection in Non-HCC Primary Liver Malignancy and Metastatic Disease to the Liver
15. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M. Zur elektrochirurgie. Arch. Klin. Chir. 1931, 167, 761–768. [Google Scholar]
- Taylor, L.S. Electromagnetic syringe. IEEE Trans. Biomed. Eng. 1978, 25, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, D.; Laurent, A.; Pawlik, T.M.; Lauwers, G.Y.; Vauthey, J.N.; Abdalla, E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br. J. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, N.; Takeda, A.; Tsurugai, Y.; Eriguchi, T. Role of stereotactic body radiotherapy in multidisciplinary management of liver metastases in patients with colorectal cancer. Jpn. J. Radiol. 2022, 40, 1009–1016. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Mueller, P.R. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am. J. Roentgenol. 2000, 174, 323–331. [Google Scholar] [CrossRef]
- Ben Ammar, M.; Nouri-Neuville, M.; Cornelis, F.H. Percutaneous image-guided therapies of primary liver tumors: Techniques and outcomes. Presse Med. 2019, 48(7–8 Pt. 2), e245–e250. [Google Scholar] [CrossRef]
- Covey, A.M.; Hussain, S.M. Liver-Directed Therapy for Hepatocellular Carcinoma: An Overview of Techniques, Outcomes, and Posttreatment Imaging Findings. Am. J. Roentgenol. 2017, 209, 67–76. [Google Scholar] [CrossRef]
- Horn, S.R.; Stoltzfus, K.C.; Lehrer, E.J.; Dawson, L.A.; Tchelebi, L.; Gusani, N.J.; Sharma, N.K.; Chen, H.; Trifiletti, D.M.; Zaorsky, N.G. Epidemiology of liver metastases. Cancer Epidemiol. 2020, 67, 101760. [Google Scholar] [CrossRef]
- Shaib, Y.; El-Serag, H.B. The epidemiology of cholangiocarcinoma. Semin. Liver. Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef]
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Bhadana, U.; Singh, R.A.; Ahuja, A. Primary hepatic angiosarcoma. Eur. J. Surg. Oncol. 2015, 41, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Crocetti, L.; Cioni, D.; Della Pina, C.; Bartolozzi, C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: Technique, indications, results, and new promises. Investig. Radiol. 2004, 39, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N.; Gazelle, G.S.; Halpern, E.F.; Rittman, W.J.; Mueller, P.R.; Rosenthal, D.I. Radiofrequency tissue ablation: Importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad. Radiol. 1996, 3, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.C.; Healey, T.T.; Dupuy, D.E. Microwave ablation devices for interventional oncology. Expert Rev. Med. Devices 2013, 10, 225–238. [Google Scholar] [CrossRef]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T., Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. S8), S192–S203. [Google Scholar] [CrossRef]
- Mansur, A.; Garg, T.; Shrigiriwar, A.; Etezadi, V.; Georgiades, C.; Habibollahi, P.; Huber, T.C.; Camacho, J.C.; Nour, S.G.; Sag, A.A.; et al. Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors. Diagnostics 2022, 12, 1300. [Google Scholar] [CrossRef]
- Arciero, C.A.; Sigurdson, E.R. Liver-directed therapies for patients with primary liver cancer and hepatic metastases. Curr. Treat Options Oncol. 2006, 7, 399–409. [Google Scholar] [CrossRef]
- Orlacchio, A.; Bazzocchi, G.; Pastorelli, D.; Bolacchi, F.; Angelico, M.; Almerighi, C.; Masala, S.; Simonetti, G. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: Initial experience. CardioVascular Interv. Radiol. 2008, 31, 587–594. [Google Scholar] [CrossRef]
- Qian, J. Interventional therapies of unresectable liver metastases. J. Cancer Res. Clin. Oncol. 2011, 137, 1763–1772. [Google Scholar] [CrossRef]
- Petre, E.N.; Sofocleous, C. Thermal Ablation in the Management of Colorectal Cancer Patients with Oligometastatic Liver Disease. Visc. Med. 2017, 33, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.R.; Kavnoudias, H.; Neal, R.E., 2nd. Introduction to Irreversible Electroporation--Principles and Techniques. Tech. Vasc. Interv. Radiol. 2015, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Yarmush, M.L. Nonthermal irreversible electroporation: Fundamentals, applications, and challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Quadri, S.A.; Waqas, M.; Khan, I.; Khan, M.A.; Suriya, S.S.; Farooqui, M.; Fiani, B. High-intensity focused ultrasound: Past, present, and future in neurosurgery. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef]
- Jagannathan, J.; Sanghvi, N.T.; Crum, L.A.; Yen, C.P.; Medel, R.; Dumont, A.S.; Sheehan, J.P.; Steiner, L.; Jolesz, F.; Kassell, F. High-intensity focused ultrasound surgery of the brain: Part 1—A historical perspective with modern applications. Neurosurgery 2009, 64, 201–211. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Ortner, M.E.; Caca, K.; Berr, F.; Liebetruth, J.; Mansmann, U.; Huster, D.; Voderholzer, W.; Schachschal, G.; Mössner, J.; Lochs, H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003, 125, 1355–1363. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Aitken, K.L.; Hawkins, M.A. Stereotactic body radiotherapy for liver metastases. Clin. Oncol. (R Coll. Radiol.). 2015, 27, 307–315. [Google Scholar] [CrossRef]
- Høyer, M.; Swaminath, A.; Bydder, S.; Lock, M.; Méndez Romero, A.; Kavanagh, B.; Goodman, K.A.; Okunieff, P.; Dawson, L.A. Radiotherapy for liver metastases: A review of evidence. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.W. Stereotactic body radiotherapy: Current strategies and future development. J. Thorac. Dis. 2016, 8 (Suppl. S6), S517–S527. [Google Scholar] [CrossRef]
- Mack, M.G.; Straub, R.; Eichler, K.; Engelmann, K.; Zangos, S.; Roggan, A.; Woitaschek, D.; Böttger, M.; Vogl, T.J. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom. Imaging 2001, 26, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Gadzijev, E.M.; Brecelj, E.; Miklavcic, D.; Kos, B.; Zupanic, A.; Mali, B.; Jarm, T.; Pavliha, D.; Marcan, M.; et al. Electrochemotherapy: A new technological approach in treatment of metastases in the liver. Technol. Cancer Res. Treat. 2011, 10, 475–485. [Google Scholar] [CrossRef]
- Ansari, D.; Andersson, R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J. Gastroenterol. 2012, 18, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Ruhl, C.E. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009, 136, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.H.; Kim, K.P.; Park, D.H.; Moon, S.H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Bekki, Y.; Von Ahrens, D.; Takahashi, H.; Schwartz, M.; Gunasekaran, G. Recurrent Intrahepatic Cholangiocarcinoma—Review. Front. Oncol. 2021, 11, 776863. [Google Scholar] [CrossRef]
- Bartsch, F.; Eberhard, J.; Rückert, F.; Schmelzle, M.; Lehwald-Tywuschik, N.; Fichtner-Feigl, S.; Gaedcke, J.; Oldhafer, K.J.; Oldhafer, F.; Diener, M.; et al. Repeated resection for recurrent intrahepatic cholangiocarcinoma: A retrospective German multicentre study. Liver Int. 2021, 41, 180–191. [Google Scholar] [CrossRef]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Quero, G.; Gassino, R.; Lapergola, A.; Gurriero, L.; Diana, M.; Vallan, A.; Perrone, G.; Schena, E.; Costamagna, G.; et al. Laser ablation of the biliary tree: In vivo proof of concept as potential treatment of unresectable cholangiocarcinoma. Int. J. Hyperthermia. 2018, 34, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, X.; Xue, P.; Chen, S.; Li, S.; Li, Z.; Pan, W.; Zhang, D. Long-term efficacy of percutaneous transhepatic cholangioscopy-guided photodynamic therapy for postoperative recurrent extrahepatic cholangiocarcinoma. Photodiagnosis Photodyn Ther. 2022, 40, 103122. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C.; Massagué, J. Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef]
- Joachim, C.; Macni, J.; Drame, M.; Pomier, A.; Escarmant, P.; Veronique-Baudin, J.; Vinh-Hung, V. Overall survival of colorectal cancer by stage at diagnosis: Data from the Martinique Cancer Registry. Medicine 2019, 98, e16941. [Google Scholar] [CrossRef]
- Pan, Z.; Peng, J.; Lin, J.; Chen, G.; Wu, X.; Lu, Z.; Deng, Y.; Zhao, Y.; Sui, Q.; Wan, D. Is there a survival benefit from adjuvant chemotherapy for patients with liver oligometastases from colorectal cancer after curative resection? Cancer Commun. 2018, 38, 29. [Google Scholar] [CrossRef]
- de Ridder, J.; de Wilt, J.H.; Simmer, F.; Overbeek, L.; Lemmens, V.; Nagtegaal, I. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 2016, 7, 55368–55376. [Google Scholar] [CrossRef]
- Hugen, N.; van de Velde, C.J.H.; de Wilt, J.H.W.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef]
- Xu, J.; Fan, J.; Qin, X.; Cai, J.; Gu, J.; Wang, S.; Wang, X.; Zhang, S.; Zhang, Z. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2018). J. Cancer Res. Clin. Oncol. 2019, 145, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Rothbarth, J.; van de Velde, C.J. Treatment of liver metastases of colorectal cancer. Ann. Oncol. 2005, 16 (Suppl. S2), ii144–ii149. [Google Scholar]
- Hagness, M.; Foss, A.; Line, P.D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, O.; Gladhaug, I.P.; Egge, T.S.; et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.D. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.M.; Essner, R.; Hughes, T.M.; Tang, P.C.; Bilchik, A.; Wanek, L.A.; Thompson, J.F.; Morton, D.L. Surgical resection for metastatic melanoma to the liver: The John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch. Surg. 2001, 136, 950–955. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Barber, B.; Farr, A.M.; Ivanov, B.; Novich, M. Overall survival in patients with metastatic melanoma. Curr. Med. Res. Opin. 2015, 31, 987–991. [Google Scholar] [CrossRef]
- Hameed, A.M.; Ng, E.E.; Johnston, E.; Hollands, M.J.; Richardson, A.J.; Pleass, H.C.; Lam, V.W.T. Hepatic resection for metastatic melanoma: A systematic review. Melanoma. Res. 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef]
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015, 3, 217–221. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Sun, T.; Zheng, X.; Li, J.; Sun, H.; Zhou, X.; Zhou, C.; Zhang, H.; Cheng, Z.; et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci. Rep. 2017, 7, 1339. [Google Scholar] [CrossRef]
- Risteski, M.; Crvenkova, S.; Atanasov, Z.; Isjanovska, R. Epidemiological analysis of progression-free survival (PFS) and overall survival (OS) in non-small-cell lung cancer patients in Republic of Macedonia. Pril. (Makedon. Akad. Nauk. Umet. Odd. Med. Nauki). 2013, 34, 49–61. [Google Scholar] [PubMed]
- Adam, R.; Aloia, T.; Krissat, J.; Bralet, M.P.; Paule, B.; Giacchetti, S.; Delvart, V.; Azoulay, D.; Bismuth, H.; Castaing, D. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann. Surg. 2006, 244, 897–907; discussion 907–908. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, P.; Meng, Z.; Chen, Z.; Yu, E.X.; Jin, H.; Chang, D.Z.; Liao, Z.; Cohen, L.; Liu, L. Multimodality treatment of pancreatic cancer with liver metastases using chemotherapy, radiation therapy, and/or Chinese herbal medicine. Pancreas 2011, 40, 120–125. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Sotiropoulos, G.C.; Li, J.; Kornasiewicz, O.; Plöckinger, U. Multimodal management of neuroendocrine liver metastases. HPB 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Mayo, S.C.; Herman, J.M.; Cosgrove, D.; Bhagat, N.; Kamel, I.; Geschwind, J.F.H.; Pawlik, T.M. Emerging approaches in the management of patients with neuroendocrine liver metastasis: Role of liver-directed and systemic therapies. J. Am. Coll. Surg. 2013, 216, 123–134. [Google Scholar] [CrossRef]
- Mazzaglia, P.J.; Berber, E.; Milas, M.; Siperstein, A.E. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: A 10-year experience evaluating predictors of survival. Surgery 2007, 142, 10–19. [Google Scholar] [CrossRef]
- Li, Y.; Feng, A.; Zheng, S.; Chen, C.; Lyu, J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control. 2022, 29, 10732748221099227. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Rong, Z.; Huang, C. Surgery Strategies for Gastric Cancer With Liver Metastasis. Front. Oncol. 2019, 9, 1353. [Google Scholar] [CrossRef]
- Bessen, T.; Caughey, G.E.; Shakib, S.; Potter, J.A.; Reid, J.; Farshid, G.; Roder, D.; Neuhaus, S.J. A population-based study of soft tissue sarcoma incidence and survival in Australia: An analysis of 26,970 cases. Cancer Epidemiol. 2019, 63, 101590. [Google Scholar] [CrossRef] [PubMed]
- Kawae, Y.; Matsuoka, M.; Onodera, T.; Yokota, I.; Iwasaki, K.; Hishimura, R.; Suzuki, Y.; Kondo, E.; Iwasaki, N. Liver metastasis in soft tissue sarcoma at initial presentation [published online ahead of print, 2022 July 6]. J. Surg. Oncol. 2022, 126, 1074–1079. [Google Scholar] [CrossRef]
- Dodd GD 3rd Frank, M.S.; Aribandi, M.; Chopra, S.; Chintapalli, K.N. Radiofrequency thermal ablation: Computer analysis of the size of the thermal injury created by overlapping ablations. Am. J. Roentgenol. 2001, 177, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Laeseke, P.F.; Frey, T.M.; Brace, C.L.; Sampson, L.A.; Winter TC 3rd Ketzler, J.R.; Lee, F.T., Jr. Multiple-electrode radiofrequency ablation of hepatic malignancies: Initial clinical experience. Am. J. Roentgenol. 2007, 188, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Schullian, P.; Haidu, M.; Bale, R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: Technique effectiveness, safety, and interoperator performance. Cardiovasc. Intervent. Radiol. 2012, 35, 570–580. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Hahn, P.F.; Tanabe, K.K.; Mueller, P.R.; Schima, W.; Athanasoulis, C.A.; Compton, C.C.; Solbiati, L.; Gazelle, G.S. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 1998, 9 Pt 1, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Garbagnati, F.; Lencioni, R.; Allgaier, H.P.; Marchianò, A.; Fornari, F.; Quaretti, P.; Tolla, G.D.; Ambrosi, C.; Mazzaferro, V.; et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000, 217, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.J.; Munoz-Juarez, M.; Farnell, M.B.; Donohue, J.H.; Que, F.G.; Crownhart, B.; Larson, D.; Nagorney, D.M. Major Hepatic Resection for Hilar Cholangiocarcinoma: Analysis of 46 Patients. Arch. Surg. 2004, 139, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.A.; Kim, P.N. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Shin, Y.M.; Won, H.J.; Kim, P.N. Percutaneous Radiofrequency Ablation for Recurrent Intrahepatic Cholangiocarcinoma After Curative Resection: Multivariable Analysis of Factors Predicting Survival Outcomes. Am. J. Roentgenol. 2021, 217, 426–432. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, P.N.; Lee, S.G.; Hwang, S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur. J. Radiol. 2011, 80, e221–e225. [Google Scholar] [CrossRef]
- Braunwarth, E.; Schullian, P.; Kummann, M.; Reider, S.; Putzer, D.; Primavesi, F.; Stättner, S.; Öfner, D.; Bale, R. Aggressive local treatment for recurrent intrahepatic cholangiocarcinoma-Stereotactic radiofrequency ablation as a valuable addition to hepatic resection. PLoS ONE 2022, 17, e0261136. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.J.; Lin, S.M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.P.; et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kudo, M. Radiofrequency ablation of liver metastases from colorectal cancer: A literature review. Gut Liver 2013, 7, 1–6. [Google Scholar] [CrossRef]
- Fan, X.X.; Lv, S.Y.; Zhang, M.W.; Dai, X.Y.; Zhao, J.P.; Mao, D.F.; Zhang, Y. Clinical analysis of ultrasound-guided radiofrequency ablation for recurrent colorectal liver metastases after hepatectomy. World J. Surg. Oncol. 2020, 18, 76. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, S.; Wu, H. Long-term outcomes after hepatic resection combined with radiofrequency ablation for initially unresectable multiple and bilobar liver malignancies. J. Surg. Res. 2014, 188, 14–20. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, Y.H.; Kim, J.; Chang, H.M.; Kim, T.W.; Kim, S.C.; Kim, P.N.; Han, D.J. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J. Vasc. Interv. Radiol. 2012, 23, 635–641. [Google Scholar] [CrossRef]
- Kang, H.; Han, S.Y.; Cho, J.H.; Kim, E.J.; Kim, D.U.; Yang, J.K.; Jeon, S.; Park, G.; Lee, T.H. Efficacy and safety of temperature-controlled intraductal radiofrequency ablation in advanced malignant hilar biliary obstruction: A pilot multicenter randomized comparative trial. J. Hepatobiliary Pancreat Sci. 2022, 29, 469–478. [Google Scholar] [CrossRef]
- Song, S.; Jin, H.; Cheng, Q.; Gong, S.; Lv, K.; Lei, T.; Tian, H.; Li, X.; Lei, C.; Yang, W.; et al. Local palliative therapies for unresectable malignant biliary obstruction: Radiofrequency ablation combined with stent or biliary stent alone? An updated meta-analysis of nineteen trials. Surg. Endosc. 2022, 36, 5559–5570. [Google Scholar] [CrossRef]
- Yu, M.A.; Liang, P.; Yu, X.L.; Cheng, Z.G.; Han, Z.Y.; Liu, F.Y.; Yu, J. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur. J. Radiol. 2011, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.A.; Kinsman, K.A.; Schmit, G.D.; Atwell, T.D.; Schmitz, J.J.; Welch, B.T.; Callstrom, M.R.; Geske, J.R.; Kurup, A.N. Thermal ablation of intrahepatic cholangiocarcinoma: Safety, efficacy, and factors affecting local tumor progression. Abdom. Radiol. 2018, 43, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Hu, P.; Wang, N.; Shen, Q.; Sun, A.I.; Kuang, M.; Qian, G.J. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2013, 20, 3596–3602. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, A.; Pegoraro, F.; Rhaiem, R.; Montalti, R.; Donadieu, A.; Tashkandi, A.; Al-Sadairi, A.R.; Kianmanesh, R.; Piardi, T. Microwave Ablation for Colorectal Liver Metastases: A Systematic Review and Pooled Oncological Analyses. Cancers 2022, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Stättner, S.; Jones, R.P.; Yip, V.S.; Buchanan, K.; Poston, G.J.; Malik, H.Z.; Fenwick, S.W. Microwave ablation with or without resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2013, 39, 844–849. [Google Scholar] [CrossRef]
- Shibata, T.; Niinobu, T.; Ogata, N.; Takami, M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000, 89, 276–284. [Google Scholar] [CrossRef]
- McEachron, K.R.; Ankeny, J.S.; Robbins, A.; Altman, A.M.; Marmor, S.; D’Souza, D.; Schat, R.; Spilseth, B.; Jensen, E.H. Surgical microwave ablation of otherwise non-resectable colorectal cancer liver metastases: Expanding opportunities for long term survival. Surg. Oncol. 2021, 36, 61–64. [Google Scholar] [CrossRef]
- Gruenberger, T.; Jourdan, J.L.; Zhao, J.; King, J.; Morris, D.L. Reduction in recurrence risk for involved or inadequate margins with edge cryotherapy after liver resection for colorectal metastases. Arch. Surg. 2001, 136, 1154–1157. [Google Scholar] [CrossRef]
- Ng, K.M.; Chua, T.C.; Saxena, A.; Zhao, J.; Chu, F.; Morris, D.L. Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann. Surg. Oncol. 2012, 19, 1276–1283. [Google Scholar] [CrossRef]
- Glazer, D.I.; Tatli, S.; Shyn, P.B.; Vangel, M.G.; Tuncali, K.; Silverman, S.G. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. Am. J. Roentgenol. 2017, 209, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Parikh, N.; El-Haddad, G.; Kis, B. Ablation of Intrahepatic Cholangiocarcinoma. Semin. Intervent. Radiol. 2019, 36, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.K.; Springer, A.; Baier, P.; Junginger, T. Liver resection or cryotherapy for colorectal liver metastases: A prospective case control study. Int. J. Colorectal. Dis. 2005, 20, 507–520. [Google Scholar] [CrossRef]
- Korpan, N.N. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann. Surg. 1997, 225, 193–201. [Google Scholar] [CrossRef]
- Bala, M.M.; Riemsma, R.P.; Wolff, R.; Pedziwiatr, M.; Mitus, J.W.; Storman, D.; Swierz, M.J.; Kleijnen, J. Cryotherapy for liver metastases. Cochrane Database Syst. Rev. 2019, 7, CD009058. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Leor, J.; Rubinsky, B. The effect of irreversible electroporation on blood vessels. Technol. Cancer Res. Treat. 2007, 6, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Silk, M.T.; Wimmer, T.; Lee, K.S.; Srimathveeravalli, G.; Brown, K.T.; Kingham, P.T.; Fong, Y.; Durack, J.C.; Sofocleous, C.T.; Solomon, S.B. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J. Vasc. Interv. Radiol. 2014, 25, 112–118. [Google Scholar] [CrossRef]
- Renzulli, M.; Ramai, D.; Singh, J.; Sinha, S.; Brandi, N.; Ierardi, A.M.; Albertini, E.; Sacco, R.; Facciorusso, A.; Golfieri, R. Locoregional Treatments in Cholangiocarcinoma and Combined Hepatocellular Cholangiocarcinoma. Cancers 2021, 13, 3336. [Google Scholar] [CrossRef]
- Tian, G.; Zhao, Q.; Chen, F.; Jiang, T.; Wang, W. Ablation of hepatic malignant tumors with irreversible electroporation: A systematic review and meta-analysis of outcomes. Oncotarget 2017, 8, 5853–5860. [Google Scholar] [CrossRef]
- Niessen, C.; Igl, J.; Pregler, B.; Beyer, L.; Noeva, E.; Dollinger, M.; Schreyer, A.G.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: A prospective single-center study. J. Vasc. Interv. Radiol. 2015, 26, 694–702. [Google Scholar] [CrossRef]
- Franken, L.C.; van Veldhuisen, E.; Ruarus, A.H.; Coelen, R.J.S.; Roos, E.; van Delden, O.M.; Besselink, M.G.; Klümpen, H.J.; van Lienden, K.P.; van Gulik, T.M.; et al. Outcomes of Irreversible Electroporation for Perihilar Cholangiocarcinoma: A Prospective Pilot Study. J. Vasc. Interv. Radiol. 2022, 33, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, M.; Zeman, F.; Niessen, C.; Lang, S.A.; Beyer, L.P.; Müller, M.; Stroszczynski, C.; Wiggermann, P. Bile Duct Injury after Irreversible Electroporation of Hepatic Malignancies: Evaluation of MR Imaging Findings and Laboratory Values. J. Vasc. Interv. Radiol. 2016, 27, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Barabasch, A.; Distelmaier, M.; Heil, P.; Krämer, N.A.; Kuhl, C.K.; Bruners, P. Magnetic Resonance Imaging Findings After Percutaneous Irreversible Electroporation of Liver Metastases: A Systematic Longitudinal Study. Investig. Radiol. 2017, 52, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, M.; Jung, E.M.; Beyer, L.; Niessen, C.; Scheer, F.; Müller-Wille, R.; Stroszczynski, C.; Wiggermann, P. Irreversible electroporation ablation of malignant hepatic tumors: Subacute and follow-up CT appearance of ablation zones. J. Vasc. Interv. Radiol. 2014, 25, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Kingham, T.P.; Karkar, A.M.; D’Angelica, M.I.; Allen, P.J.; Dematteo, R.P.; Getrajdman, G.I.; Sofocleous, C.T.; Solomon, S.B.; Jarnagin, W.R.; Fong, Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J. Am. Coll. Surg. 2012, 215, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef]
- Leslie, T.; Ritchie, R.; Illing, R.; Phillips, R.; Middleton, M.; Bch, B.; Wu, F.; Cranston, D. High-intensity focused ultrasound treatment of liver tumours: Post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br. J. Radiol. 2012, 85, 1363–1370. [Google Scholar] [CrossRef]
- Gu, L.; Shen, Z.; Ji, L.; Ng, D.M.; Du, N.; He, N.; Fan, X.; Yan, K.; Zheng, Z.; Chen, B.; et al. High-intensity focused ultrasound alone or combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with unsuitable indications for hepatectomy and radiofrequency ablation: A phase II clinical trial. Surg. Endosc. 2022, 36, 1857–1867. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Zhang, J.; Kong, X.R.; Zhu, H.; Wu, F.; Wang, Z. First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 2014, 59, 170–177. [Google Scholar] [CrossRef]
- Sehmbi, A.S.; Froghi, S.; Oliveira de Andrade, M.; Saffari, N.; Fuller, B.; Quaglia, A.; Davidson, B. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB 2021, 23, 187–196. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, J.; Zhu, L.; Zhu, Y.; Zhao, H. High-Intensity Focused Ultrasound Ablation for Unresectable Primary and Metastatic Liver Cancer: Real-World Research in a Chinese Tertiary Center With 275 Cases. Front. Oncol. 2020, 10, 519164. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, F.; Zhou, J.J.; Liu, X.; He, Q.; Wang, C.; Zheng, Y.W.; Wen, Y.; Xiong, L. Application of photodynamic therapy for liver malignancies. J. Gastrointest. Oncol. 2020, 11, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, B.R.; Zhu, Z.F.; Wang, S.Q.; Chai, C.X.; Shang, D.; Li, M. Photodynamic therapy: A next alternative treatment strategy for hepatocellular carcinoma? World J. Gastrointest. Surg. 2021, 13, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Quyn, A.J.; Ziyaie, D.; Polignano, F.M.; Tait, I.S. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB 2009, 11, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, L.; Wu, J.C.; Bie, L.K.; Gong, B. Efficacy and safety of photodynamic therapy for unresectable cholangiocarcinoma: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 718–724. [Google Scholar] [CrossRef]
- Moole, H.; Tathireddy, H.; Dharmapuri, S.; Moole, V.; Boddireddy, R.; Yedama, P.; Dharmapuri, S.; Uppu, A.; Bondalapati, N.; Duvvuri, A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Talreja, J.P.; Degaetani, M.; Ellen, K.; Schmitt, T.; Gaidhane, M.; Kahaleh, M. Photodynamic therapy in unresectable cholangiocarcinoma: Not for the uncommitted. Clin. Endosc. 2013, 46, 390–394. [Google Scholar] [CrossRef] [PubMed]
- van Duijnhoven, F.H.; Rovers, J.P.; Engelmann, K.; Krajina, Z.; Purkiss, S.F.; Zoetmulder, F.A.N.; Vogl, T.J.; Terpstra, O.T. Photodynamic therapy with 5,10,15,20-tetrakis(m-hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: Results from a phase I study. Ann. Surg. Oncol. 2005, 12, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.S.; Sahgal, A.; Chang, E.L.; Mayr, N.A.; Teh, B.S.; Huang, Z.; Schefter, T.E.; Yao, M.; Machtay, M.; Slotman, B.J.; et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. Clin. Oncol. (R Coll. Radiol.) 2013, 25, 378–387. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, M.S.; Cho, C.K.; Yoo, H.J.; Jang, W.I.; Seo, Y.S.; Paik, E.K.; Kim, K.B.; Han, C.J.; Kim, S.B. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat. Oncol. J. 2014, 32, 163–169. [Google Scholar] [CrossRef]
- Shen, Z.T.; Zhou, H.; Li, A.M.; Li, B.; Shen, J.S.; Zhu, X.X. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 93541–93550. [Google Scholar] [CrossRef] [PubMed]
- Sandler, K.A.; Veruttipong, D.; Agopian, V.G.; Finn, R.S.; Hong, J.C.; Kaldas, F.M.; Sadeghi, S.; Busuttli, R.W.; Lee, P. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv. Radiat. Oncol. 2016, 1, 237–243. [Google Scholar] [CrossRef]
- McPartlin, A.; Swaminath, A.; Wang, R.; Pentilie, M.; Brierley, J.; Kim, J.; Ringash, J.; Wong, R.; Dinniwell, R.; Craig, T.; et al. Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 388–395. [Google Scholar] [CrossRef]
- Scorsetti, M.; Comito, T.; Tozzi, A.; Navarria, P.; Fogliata, A.; Clerici, E.; Mancosu, P.; Reggiori, G.; Rimassa, L.; Torzilli, G.; et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Pedicini, V.; Comito, T.; Carnaghi, C.; Costa, G.; Poretti, D.; Franzese, C.; Personeni, N.; Del Fabbro, D.; Rimassa, L.; et al. Aggressive and Multidisciplinary Local Approach to Iterative Recurrences of Colorectal Liver Metastases. World J. Surg. 2018, 42, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Puls, R.; Langner, S.; Rosenberg, C.; Hegenscheid, K.; Kuehn, J.P.; Noeckler, K.; Hosten, N. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J. Vasc. Interv. Radiol. 2009, 20, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Straub, R.; Zangos, S.; Mack, M.G.; Eichler, K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: Experimental and clinical data. Int. J. Hyperthermia. 2004, 20, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Dommermuth, A.; Heinle, B.; Nour-Eldin, N.E.; Lehnert, T.; Eichler, K.; Zangos, S.; Bechstein, W.O.; Naguib, N.N. Colorectal cancer liver metastases: Long-term survival and progression-free survival after thermal ablation using magnetic resonance-guided laser-induced interstitial thermotherapy in 594 patients: Analysis of prognostic factors. Investig. Radiol. 2014, 49, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Trotovšek, B.; Djokić, M.; Čemažar, M.; Serša, G. New era of electrochemotherapy in treatment of liver tumors in conjunction with immunotherapies. World J. Gastroenterol. 2021, 27, 8216–8226. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Nasto, R.A.; Tarantino, P.; Fristachi, R.; Cacace, L.; Bortone, S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. Eur. J. Surg. Oncol. 2018, 44, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Music, M.M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Grcar Kuzmanov, B.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gasljevic, G.; Edhemovic, I.; Cemazar, M.; Brecelj, E.; Gadzijev, E.M.; Music, M.M.; Sersa, G. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLoS ONE. 2017, 12, e0180709. [Google Scholar] [CrossRef]
- Ebara, M.; Okabe, S.; Kita, K.; Sugiura, N.; Fukuda, H.; Yoshikawa, M.; Kondo, F.; Saisho, H. Percutaneous ethanol injection for small hepatocellular carcinoma: Therapeutic efficacy based on 20-year observation. J. Hepatol. 2005, 43, 458–464. [Google Scholar] [CrossRef]
- Livraghi, T.; Vettori, C.; Lazzaroni, S. Liver metastases: Results of percutaneous ethanol injection in 14 patients. Radiology 1991, 179, 709–712. [Google Scholar] [CrossRef]
- Weis, S.; Franke, A.; Berg, T.; Mössner, J.; Fleig, W.; Schoppmeyer, K. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst. Rev. 2015, 1, CD006745. [Google Scholar] [CrossRef] [PubMed]
- Shamimi-Noori, S.; Gonsalves, C.F.; Shaw, C.M. Metastatic Liver Disease: Indications for Locoregional Therapy and Supporting Data. Semin. Intervent. Radiol. 2017, 34, 145–166. [Google Scholar] [CrossRef]
- Mulcahy, M.F.; Mahvash, A.; Pracht, M.; Montazeri, A.H.; Bandula, S.; Martin, R.C.G., 2nd; Hermann, K.; Brown, E.; Zuckerman, D.; Wilson, G.; et al. Radioembolization With Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial. J. Clin. Oncol. 2021, 39, 3897–3907. [Google Scholar] [CrossRef]


| Ablative Therapy | Technique |
|---|---|
| Radiofrequency Ablation | A probe delivers electric current, which is alternated by a radiofrequency generator creating heat [6,13,14] |
| Microwave Ablation | A generator oscillates an electromagnetic field through an antenna, which causes molecules with dipole moments to feel electromagnetic force. The movement of molecules creates heat [15,16] |
| Cryotherapy | An applicator delivers argon gas or liquid nitrogen causing an ice ball to form adjacent to the probe [17,18,19,20,21] |
| Irreversible Electroporation | Electrodes are inserted into the target tissue and a high-voltage electric impulses created by the electrodes produce pores in the cellular membrane [17,21,22,23] |
| High-Intensity Frequency Ultrasound | A probe, similar to that which is used in conventional ultrasound, delivers ultrasonic waves at extremely high-frequency. These waves create pressure changes that cause gas or vapor-filled cavitations in the tissue. The cavitations oscillate and allow for the mechanical destruction of target tissue [24,25,26] |
| Photodynamic Therapy | Systemic administration of a fluorescent photosensitizing agent localizes to the tumor. A probe that emits light at a desired wavelength is directed toward the tumor. Activation of the agent by a specific wavelength of light creates free radical damage [27,28,29] |
| Stereotactic Body Radiotherapy | A linear accelerator or specialized device such as the Cyberknife delivers beams of radiation. A high degree of precision is achieved through patient immobilization, imaging, and flexible external placement of the radiation device [30,31,32] |
| Laser Induced Thermotherapy | Optical fiber applicators deliver light, which is absorbed by the target tissue creating heat [33] |
| Electrochemotherapy | Electrodes are inserted into the target tissue and chemotherapy is given systemically. An electric field is then created by the electrodes causing cells to become more porous and allowing for increased uptake of chemotherapy [34] |
| Percutaneous Ethanol Injection | A long needle is inserted and ethanol is injected into the target tissue [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, T.P.; Pebror, T.; Krosin, M.E.; Koniaris, L.G. Ablative Therapy in Non-HCC Liver Malignancy. Cancers 2023, 15, 1200. https://doi.org/10.3390/cancers15041200
Robinson TP, Pebror T, Krosin ME, Koniaris LG. Ablative Therapy in Non-HCC Liver Malignancy. Cancers. 2023; 15(4):1200. https://doi.org/10.3390/cancers15041200
Chicago/Turabian StyleRobinson, Tyler P., Travis Pebror, Matthew E. Krosin, and Leonidas G. Koniaris. 2023. "Ablative Therapy in Non-HCC Liver Malignancy" Cancers 15, no. 4: 1200. https://doi.org/10.3390/cancers15041200
APA StyleRobinson, T. P., Pebror, T., Krosin, M. E., & Koniaris, L. G. (2023). Ablative Therapy in Non-HCC Liver Malignancy. Cancers, 15(4), 1200. https://doi.org/10.3390/cancers15041200