Simple Summary
The incidence of hepatocellular carcinoma (HCC) in patients with non-alcoholic fatty liver disease (NAFLD) has increased in recent years. Compared to HCC caused by other chronic liver diseases, NAFLD-related HCC is often detected later, because it more commonly arises before cirrhosis has occurred. Because of this late diagnosis, NAFLD-related HCC is often more advanced at time of diagnosis, resulting in fewer curative treatment options. Most research in the pathogenesis of HCC has focused on the disease processes in hepatocytes, the most abundant type of liver cells. However, other cell types, such as cells of the immune system and cells that regulate connective tissue formation, also play an important role in the development of NAFLD-related HCC, both by contributing to the development of HCC itself and by interfering with the immune system’s ability to attack cancer cells. In this paper, we review the role of different cell types in the development of NAFLD-related HCC.
Abstract
Hepatocellular carcinoma (HCC) in the setting of non-alcoholic fatty liver disease (NAFLD)-related cirrhosis and even in the pre-cirrhotic state is increasing in incidence. NAFLD-related HCC has a poor clinical outcome as it is often advanced at diagnosis due to late diagnosis and systemic treatment response is poor due to reduced immune surveillance. Much of the focus of molecular research has been on the pathological changes in hepatocytes; however, immune cells, hepatic stellate cells, liver sinusoidal endothelial cells and the extracellular matrix may play important roles in the pathogenesis of NAFLD-related HCC as well. Here, we review the role of non-parenchymal cells in the liver in the pathogenesis of HCC in the context of NAFLD-NASH, with a particular focus on the innate and the adaptive immune system, fibrogenesis and angiogenesis. We review the key roles of macrophages, hepatic stellate cells (HSCs), T cells, natural killer (NK) cells, NKT cells and liver sinusoidal endothelial cells (LSECs) and the role of the extracellular matrix in hepatocarcinogenesis within the steatotic milieu.
1. Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common malignancy worldwide and the fourth most common cause of cancer-related mortality [1]. It occurs predominantly in patients with advanced stages of chronic liver disease [2,3]. Non-alcoholic fatty liver disease (NAFLD) is a rapidly growing cause of chronic liver disease which is closely associated with the epidemic of obesity, metabolic syndrome and type 2 diabetes mellitus [4,5,6]. In the global population, NAFLD, which is defined as the accumulation of intracellular fat in >5% of hepatocytes, reaches prevalence rates of over 25% [5] while in patients with obesity and/or type 2 diabetes mellitus prevalence rises to 60–80% [6,7]. The spectrum of NAFLD ranges from isolated steatosis, characterized by lipid accumulation in hepatocytes, to non-alcoholic steatohepatitis (NASH) with the addition of hepatic inflammation, and NASH-related fibrosis, cirrhosis and HCC [7,8]. Progression along the NAFLD disease spectrum often goes unnoticed until advanced stages of fibrosis or even cirrhosis or HCC occur. This becomes ever more clinically relevant since patients are living longer with more severe obesity and type 2 diabetes mellitus, driving the occurrence of advanced fibrotic stages of disease, which are in turn associated with increased liver-related mortality and all-cause mortality [9,10].
Currently, there are no amply sized prospective cohort studies that allow for the calculation of risk of developing HCC in patients with NAFLD. Data on the incidence of HCC in this setting are conflicting, but most reports agree that the incidence rate of HCC is generally lower in the setting of NAFLD compared to that of other common etiologies such as viral hepatitis [11,12]. Yet, in a U.S. population-based study involving 4406 patients with HCC, NAFLD was found to be the underlying disease in 59% of cases [13]. Recent estimates predict that the incidence of NAFLD-related HCC will increase dramatically by 2030, with expected increases of 117% and 122% in France and the United States, respectively [14]. In the United States, NAFLD is now the fastest growing underlying cause of HCC in liver transplant recipients and transplantation candidates on the waiting list [15].
The pathophysiology of NAFLD-NASH is complex, and has been extensively reviewed [16,17,18]. Briefly, obesity and insulin resistance are key drivers, the latter triggering an increased flux of circulating free fatty acids (FFAs) from insulin-resistant peripheral adipose tissue to the liver [19,20,21]. These FFAs are stored as triglycerides in lipid droplets, reducing hepatic insulin sensitivity and consequently increasing hepatic gluconeogenesis, which results in hyperglycemia and intrahepatic conversion of glucose to FFAs, further compounding intrahepatic fat accumulation [22]. Meanwhile, high plasma insulin levels increase de novo lipogenesis, producing even more triglycerides and further enhancing hepatic gluconeogenesis. When hepatic mechanisms for storage, secretion or beta-oxidation fall short, lipotoxicity occurs, causing mitochondrial dysfunction, resulting in the formation of reactive oxygen species (oxidative stress), necro-inflammation of hepatocytes and an influx of monocytes and lymphocytes [23,24,25,26,27,28]. Within this lipotoxic and inflammatory milieu and compounded by the inhibition of endothelial nitric oxide synthase (eNOS) mediated by liver sinusoidal endothelial cells (LSECs) [29], quiescent hepatic stellate cells (HSCs) differentiate into myofibroblasts which secrete a fibrotic matrix, rich in type I collagen [30,31] in a process of damage and repair which is maladaptive in the chronic state of NASH and thus leads to progressive fibrosis [16].
During this process of NASH and fibrogenesis, regenerative repair pathways are induced such as the Hedgehog (Hh) signaling pathway, which can ultimately induce hepatocarcinogenesis [18,32]. Most research into the development and subsequent progression of NAFLD-related HCC has focused on hepatocytes, the cell type of origin in HCC [33,34]. Yet, of note, the tumor microenvironment (TME) also consists of stromal cells, endothelial cells, immune cells, cytokines and extracellular matrix (ECM), and these may all play a key role in the initiation and progression of HCC [18,35]. Here, we review the roles of non-parenchymal cells, including macrophages, lymphocytes, LSECs and HSCs, in the initiation and progression of NAFLD-related HCC. Furthermore, we will assess the role of the ECM and matrix stiffness as drivers of NAFLD-related HCC. We will consider both the processes that underlie the initiation of NAFLD-related HCC and the processes that underlie its eventual progression.
2. Hepatocyte Injury in HCC Pathogenesis: A Brief Summary
First, we will briefly assess the pathogenesis underlying the initiation of NAFLD-related HCC, which has been extensively reviewed elsewhere [18,32,36]. Reactive oxygen species (ROS) are generated in response to elevated mitochondrial fatty acid oxidation and inadequate mitochondrial respiratory chain activity [32,37,38]. Moreover, when NAFLD progresses, macrophages and apoptotic hepatocytes are key contributors to ROS generation [37,39]. ROS can cause either point mutations or larger lesions in the genome, thus driving genomic damage and genetic instability [40,41]. This pro-carcinogenic effect is exacerbated as oxidative stress stimulates a DNA-damage response that results in error-prone DNA repair, further aiding genomic instability [18]. Additionally, ROS stimulates hepatocyte survival by stimulating the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling and increases the release of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6 [42,43,44,45]. This creates a self-propelling process where increased inflammation and oxidative stress exacerbate genomic instability [32,46,47].
In the setting of NAFLD, impaired autophagy further adds to oxidative stress in the liver [32]. Autophagy is a process aimed at the lysosomal degradation of damaged cell components [48,49]. As such, autophagy has antitumorigenic properties [50]. To counter this diminished autophagy and the rise in oxidative stress, hepatocytes induce the expression of the KEAP1-NRF2 pathway which has antioxidant properties [51]. However, this also allows for the expression of pro-survival genes and protects HCC-initiating cells from oxidative stress-induced death, thus contributing to hepatocarcinogenesis [32,50,52,53].
Hepatocyte apoptosis is significantly increased in NASH and constitutes a dual, yet opposing, role in the initiation of HCC [54,55,56,57]. On the one hand, it constitutes a protective mechanism by eliminating damaged hepatocytes, while on the other hand it increases liver regeneration and subsequent DNA replication stress [56]. This can ultimately lead to additional DNA damage and added genomic instability. Additionally, hepatocyte apoptosis adds to the pro-inflammatory environment and the recruitment of monocytes into the liver [58,59,60] and the release of hepatocyte apoptotic bodies can induce the activation of macrophages and HSCs [48,61].
Insulin resistance, one of the hallmarks of NAFLD [16,18], increases the secretion of insulin and insulin-like growth factor (IGF)-1. Binding of insulin or IGF-1 to their respective receptors triggers a signaling cascade that results in the activation of the downstream phosphoinositide-3 kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways [46]. These pathways play a significant role in hepatocarcinogenesis by the induction of hepatocyte proliferation and inhibition of apoptosis [46]. Pro-inflammatory cytokines, including TNF-α and IL-6, drive hepatocyte proliferation by activating stress-related signaling pathways, including c-Jun N-terminal kinase (JNK) and NF-κB signaling pathways, and signal transducer and activator of transcription (STAT)3 and extracellular signal-regulated kinase (ERK) signaling pathways, respectively. Furthermore, the Hh signaling pathway is activated in NASH because ballooned hepatocytes produce Hh molecules that are released upon hepatocyte injury [49,62]. Overstimulation of Hh signaling results in dysregulated cellular repair and subsequent malignant transformation [63].
In the next sections, we will assess the role of immune cells, LSECs and HSCs, in the initiation and progression of NAFLD-related HCC.
3. Inflammatory Pathways in the Pathogenesis of NAFLD and NAFLD-Related HCC
The liver has complex immunological functions, as it acts as the physiological connection between gut-derived molecules and the systemic circulation. Liver macrophages consists of Kupffer cells (KCs), which are self-renewing, liver resident cells that serve as sentinels for liver homeostasis, and monocytes, which are not liver-resident but infiltrate the liver from the peripheral blood and bone marrow following liver injury [64]. As an essential part of liver homeostasis, KCs are immunotolerogenic in nature, so as to avoid the induction of immunity against harmless substances such as gut-derived nutrients [64,65,66]. However, upon activation, liver KCs lose this immunotolerogenic nature, and subsequently secrete growth factors, pro-inflammatory cytokines, chemokines, such as chemokine ligand CCL2, and ROS [49,67,68]. CCL2, also referred to as monocyte chemoattractant protein 1 (MCP1), triggers chemotaxis, prompting monocytes derived from the peripheral blood or bone marrow to infiltrate into the liver and to differentiate into macrophages [69,70]. This pro-inflammatory environment is enhanced by the release of TNF-α and IL-6 locally in the liver, but is also caused by systemic chronic inflammation processes derived from metabolic tissues such as adipose tissue resulting from insulin resistance [71], and by high leptin but low adiponectin levels, which are typically found in people who are overweight [71,72]. In all, this triggering of the innate immune response marks the transition of simple steatosis to actual steatohepatitis [68,73] and plays a key part in the initiation of NAFLD-related HCC.
3.1. M1-Type Macrophages Drive Inflammation and Subsequent Hepatocarcinogenesis
Generally speaking, macrophages that encourage inflammation are called M1 macrophages, whereas those that decrease inflammation and encourage tissue repair are called M2 macrophages [26,68,74]. Yet, while anti-carcinogenic at first, chronic M1 macrophage activation actually induces hepatocarcinogenesis. This principle works as follows. Upon initial liver injury, pro-inflammatory and anti-carcinogenic M1 KCs are activated by stimuli such as lipopolysaccharides (LPS), IL-12, interferon (IFN)-γ, TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) [74]. M1 KCs subsequently secrete a number of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12, CCL2 and CCL5, and increased amounts of ROS and nitric oxide synthase (NOS) [68,74,75,76,77]. This yields a pro-inflammatory, anti-carcinogenic environment, characterized by a high IL-12, high IL-23, high NOS and low IL-10 phenotype [74]. In the context of NAFLD-NASH, the fatty acid palmitic acid (PA) induces M1 polarization through hypoxia-inducible factor (HIF)-1α [78]. The anti-carcinogenic properties of these macrophages consist of the trapping, phagocytosing and lysing of tumor cells [79]. Moreover, an enhanced tumor-antigen presenting ability of M1-type macrophages promotes cytotoxic functions by cytotoxic (CD8+) T cells and NK cells, which promote tumor cell apoptosis. Furthermore, M1 macrophages can trigger a T helper (Th)1 immune response [80] and exhibit strong anti-carcinogenic activity by the production of ROS and NOS, which, when expressed in high amounts, prompts autophagy and apoptosis of cancer cells [81,82,83]. Yet, side effects of chronic M1 macrophage activation actually induce hepatocarcinogenesis. These side effects include; hepatocyte (oxidative) DNA damage [38,84], the induction of damage-prone DNA-repair responses and dysplasia, hepatocyte proliferation by activation of intracellular signaling pathways and impaired tumor cell death through flawed apoptosis [18,32,85]. As such, chronic M1 macrophage activation actually promotes hepatocarcinogenesis.
3.2. Pro-Carcinogenic M2-Type Macrophages
When KCs are exposed to IL-4, IL-10, IL-13, IL-33, glucocorticoids or Toll-like receptor (TLR) ligands, they differentiate into anti-inflammatory and pro-carcinogenic M2 macrophages [74,76,80,83]. Moreover, in the setting of NAFLD-NASH, the unsaturated fatty acid oleic acid (OA) promotes M2 macrophage polarization [78]. M2 macrophages exhibit high phagocytic capacity and produce high levels of IL-4, IL-10, IL-12 and transforming growth factor (TGF)-β [75,76,77]. M2 macrophages promote a Th2 immune response and promote angiogenesis, tissue remodeling and repair [80,86]. Apart from the classically activated M1 phenotype and the alternatively activated M2 phenotype, the existence of more specific M2 subtypes underscores the diversity of macrophages [76], with the M1 and M2 phenotypes representing either end of the spectrum [76,87].
3.3. Switch from M1- to M2-Type Macrophages Stimulates Progression of NAFLD-Related HCC
The switch from M1- to M2-type macrophages plays an important role in the initiation of carcinogenesis and the progression of NAFLD-related HCC [64,88,89]. Several mechanisms facilitate transition from M1 to M2 macrophages. The upregulation of peroxisome proliferator-activated receptor (PPAR)-γ shifts lipid-induced macrophage polarization from M1- to M2-phenotype by interacting with NF-κB signaling induced by IL-4 and IL-13 [78,90]. NF-κB and STAT1 expression, on the other hand, shift macrophage polarization towards the M1-phenotype via the PI3K/Akt signaling pathway [91]. Insulin suppresses NF-κB and STAT1 expression while promoting PPAR-γ signaling and thus promoting M2 polarization [78,91,92]. Janus kinase (JAK)/STAT6 signaling, which is induced by IL-4, and IL-6/STAT3 and JAK3/STAT3 signaling are also involved in the polarization from M1 to M2 macrophages [90,91,93]. Moreover, TGF-β has been shown to promote M2 polarization [94,95] and IL-10, secreted by M2 macrophages has the ability to promote apoptosis of M1 macrophages, thus further tipping the balance towards an M2 phenotype [74,96]. These mechanisms, however, are not unique to NAFLD and can be seen across various chronic liver diseases. Additionally, numerous other factors, such as microRNAs, Notch signaling pathway [91], Nogo-B expression [97] and wingless-related integration sight (Wnt)/β-catenin signaling [98] are involved in M1 to M2 macrophage switching. The mechanisms that support macrophage polarization are manifold and the induction of polarization probably depends on the co-regulation of multiple signaling pathways [91]. Figure 1 shows a schematic summary of the opposing roles of M1- and M2-type macrophages and the mechanisms underlying polarization and phenotype switching in the context of NAFLD. There is extensive heterogeneity among the macrophage populations and a high proportion of macrophages share both M1- and M2-phenotype characteristics [77,83,89,99,100]. This underscores the complexity and heterogeneity of these processes and suggests the presence of additional pathways.

Figure 1.
Schematic summary of M1- and M2-type macrophages and the mechanisms underlying polarization and phenotype switching in the context of NAFLD.
3.4. Tumor-Associated Macrophages (TAMs)
Once HCC develops, the tumor microenvironment (TME) exacerbates the polarization of macrophages towards the M2-phenotype [68]. The TME, consisting of cancer-associated fibroblasts (CAFs), HSCs, endothelial cells, and immune cells, secretes CCL2 and macrophage colony-stimulating factor (M-CSF) which stimulates circulating monocytes to migrate into the liver and subsequently differentiate into tumor-associated macrophages (TAMs) [80,83]. TAMs are mainly polarized towards the M2-phenotype and secrete high levels of IL-10, whilst expressing low levels of pro-inflammatory cytokines, ROS and nitric oxide. Moreover, TAMs are poor antigen-presenting cells and drive tumor growth via the suppression of an effective cytotoxic response [80,83]. Recently, triggering receptor expressed on myeloid cells 2 (TREM2) has been identified as a specific marker of TAMs in different human cancer models including HCC [101]. Enrichment of TREM2+ TAMs is associated with poor clinical outcome in patients with HCC [102] and knock-out of TREM2 was found to suppress the growth of HCC in an in vivo murine model [103]. This is in line with the immunosuppressive role often attributed to TREM2+ TAMs [101]. On the other hand, several studies also point to a tumor suppressive role of TREM2+ TAMs in the context of hepatocarcinogenesis [104,105]. More research is warranted to elucidate the exact role TREM2+ TAMs play in hepatocarcinogenesis [101,103].
The location of TAMs within the tumor itself is indicative of their function. Soluble mediators secreted by tumor cells can trigger the early activation of monocytes in the peritumoral stroma whilst inducing immunosuppressive macrophages in the cancer nests [80,106]. Macrophages in the peritumoral stroma produce significant levels of pro-inflammatory cytokines. Of these, IL-1β, IL-6 and IL-23 promote Th17 cell expansion, whereas TNF-α and IL-10 promote the autocrine upregulation of programmed death ligand (PD-L)1 on the surface of those cells [107,108]. As such, tumor cells are able to reeducate macrophages; upon initial exposure to the TME, macrophages are driven towards a pro-inflammatory and anti-carcinogenic M1-phenotype, whilst macrophages in close proximity to tumor cells are driven towards an immunosuppressive phenotype, thus failing to trigger an effective antitumor immune response [80,106,109].
4. Fibrogenesis
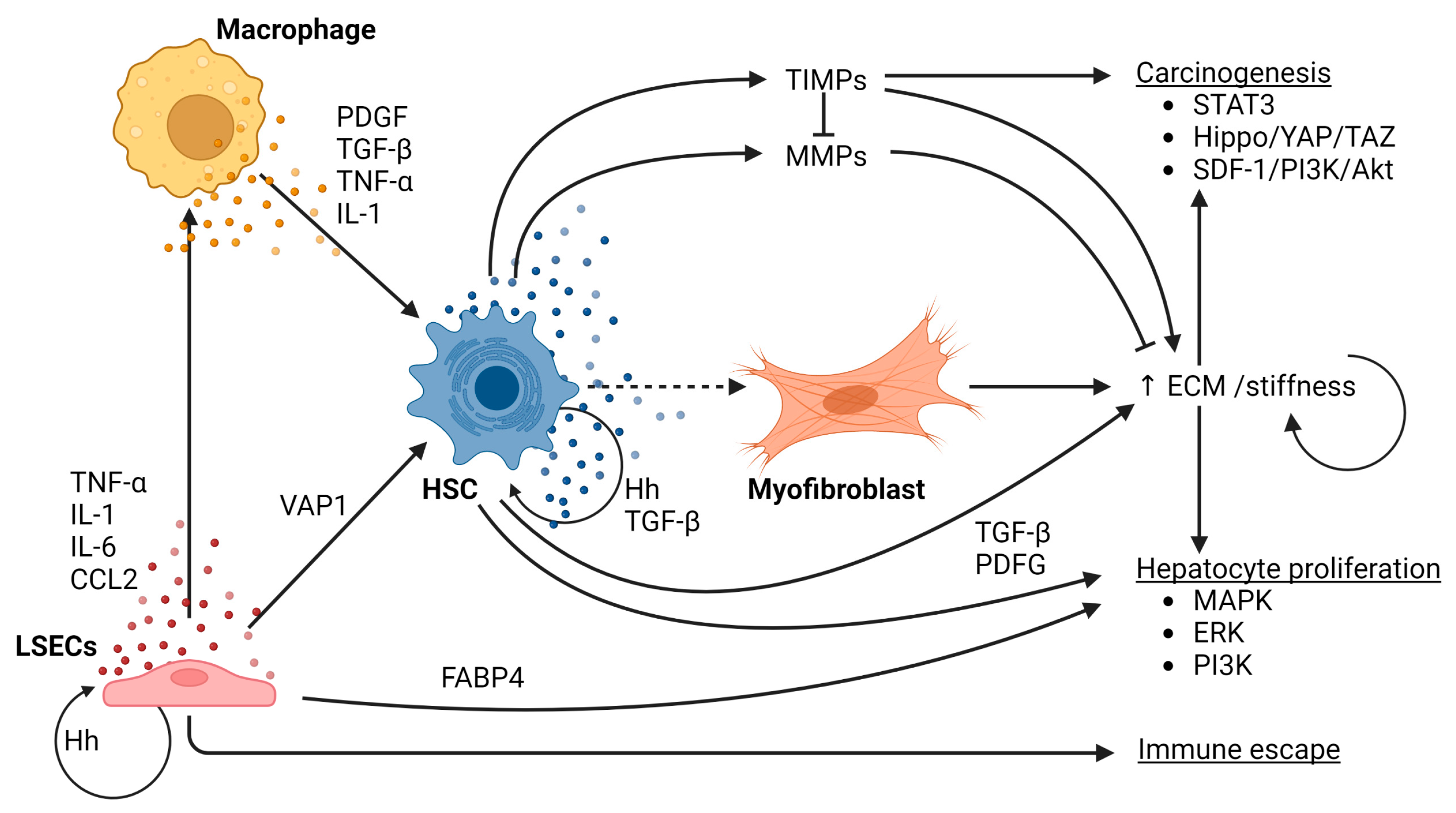
KC activation and liver injury drive activation of HSCs and subsequent fibrogenesis. In a healthy liver, HSCs are localized in the subendothelial space of Disse, representing ~8–10% of all resident liver cells [110]. The physiological roles of quiescent HSCs include storage of vitamin A, synthesis of ECM and matrix-degrading metalloproteinases, and the regulation of sinusoidal blood flow [111,112,113,114]. Many factors contribute to HSC activation in the context of NASH. Firstly, hepatocellular damage, either caused by metabolic stress, oxidative stress, inflammatory stimuli and qualitative and quantitative changes in ECM, are important drivers of HSC activation [115]. Secondly, the pro-inflammatory environment triggers the activation of HSCs. This is mediated by several pro-inflammatory cytokines, specifically platelet-derived growth factor (PDGF), TGF-β, TNF-α, IL-1 and several chemokines which originate mainly from activated KCs [49,77]. Single-cell RNA sequencing together with genetic ablation of subpopulation-enriched mediators revealed a dual role of specific subpopulations of HSCs [116]. Quiescent or weakly activated HSCs, characterized by the production of cytokines and growth factors including hepatocyte growth factor (HGF) exert a protective function against hepatocyte death and HCC development [116]. Yet, as liver injury continues, a dynamic shift between tumor-suppressive and tumor-promoting HSC subpopulations occurs, the latter of which is characterized by a highly active, myofibroblastic phenotype and leads to increased deposition of type I collagen and other matrix proteins, thus increasing matrix stiffness [116]. Moreover, these myofibroblastic HSCs promote the proliferation of hepatocytes and secrete increased amounts of pro-inflammatory as well as profibrogenic cytokines [110,116]. These profibrogenic cytokines/growth factors include TGF-β [110,117,118,119] and PDGF [110], two potent profibrogenic cytokines, whose production is not limited to HSCs and are also produced by macrophages [120,121]. Furthermore, activated TGF-β enhances the autocrine expression of TGF-β and prolongs the survival of activated HSCs by reducing apoptosis [122]. TNF-α and IL-1 stimulate the activation of HSCs [49] and pro-inflammatory cytokines produced by macrophages, including TNF-α and IL-1β, maintain HSC survival through the NF-κB pathway [77]. Integrins, which are cell surface receptors that act as mechanoreceptors by relaying the information from cell to cell, and from the ECM to cells, and vice versa, can interfere with TGF-β1 and PDGF and can modulate the proliferation and survival of hepatocytes and HSC [123]. This process relies on a variety of signaling pathways, among others, Hh signaling and MAPK and ERK signaling [49,124,125,126,127,128].
4.1. Matrix Stiffness Stimulates NAFLD-Related Hepatocarcinogenesis
Excessive collagen deposition increases the stiffness of the ECM, which in turn promotes the deposition of additional collagen by HSCs [129]. ECM stiffness can stimulate hepatocarcinogenesis through the activation of the Hippo-Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling pathway [115,129]. Once HCC has been established, matrix stiffness modulates HCC proliferation through a wide variety of signaling pathways [49,130]. ECM turnover is regulated by matrix metalloproteinases (MMPs), which are capable of degrading components of the ECM, and the tissue inhibitor of metalloproteinases (TIMPs), which acts as the endogenous inhibitor of MMPs [131,132]. HSCs are the main source of both MMPs and TIMPs, and their secretion is controlled by TGF-β1 and TNF-α [131]. MMPs and TIMPs are implicated in hepatocarcinogenesis [49,131]. TIMP-1, for instance, inhibits tumor apoptosis via stromal-derived factor (SDF)-1/PI3K/AKT signaling [133], and MMP-1 production enhances proliferation, invasion and fibrosis in NASH [49]. Moreover, increased matrix stiffness drives STAT3 activation [131]. These data underscore the role of MMPs and TIMPs in hepatocarcinogenesis [49]. Carcinoma-associated fibroblasts (CAFs), which are mainly derived from HSCs [134], play an important role in tumor growth and metastasis in HCC [135,136,137]. A number of oncogenic factors, including vascular-endothelial growth factor (VEGF), osteopontin (OPN), and TGF-β facilitate cancer development [49]. The positive feedforward loop in tumor cells and CAFs, in combination with oncogenic growth factors, exacerbates HCC progression [138].
4.2. Liver Sinusoidal Endothelial Cells (LSECs)
Liver sinusoidal endothelial cells (LSECs) form the main component of the liver endothelium and also play a key role in NAFLD-related hepatocarcinogenesis. LSECs are highly specialized endothelial cells situated between blood derived from the portal system, on the one side, and hepatocytes and HSCs, on the other [29]. During early stages of NAFLD, LSECs exhibit a downregulation of pro-inflammatory chemokines, including CCL2 through a MAPK-dependent pathway [139]. This may represent a compensatory mechanism to help prevent disease progression [139]. However, as NASH progresses, LSECs acquire a pro-inflammatory phenotype, characterized by the production of TNF-α, IL-1, IL-6 and CCL2 [29]. Thus, dysfunctional LSECs contribute to the inflammatory response and the activation of KCs, instead of maintaining KC quiescence [140]. Increased cytokines in the portal circulation, including TNF-α and IL-6, and increased intestinal permeability and consequently elevated LPS concentrations in the portal circulation contribute to the switch towards a pro-inflammatory phenotype [141,142,143]. Moreover, lipotoxicity stimulates ROS formation by LSECs, adding to the pro-carcinogenic environment [29,144]. Additionally, LSECs also stimulate fibrogenesis by overexpressing vascular adhesion protein (VAP)-1 in the context of NASH, which in turn leads to HSC activation [145], and increases the pro-inflammatory environment [29]. Moreover, capillarization, which is the loss of LSEC fenestrae, and LSEC dysfunction, characterized by the inability of LSECs to generate vasodilator agents in response to increased shear stress, both promote fibrogenesis [29]. Hh molecules released from ballooned hepatocytes in the context of NASH, lead to the activation of quiescent HSCs and the activation and capillarization of LSECs [146,147]. Activated HSCs and LSECs, in turn, secrete large volumes of Hh molecules, thus creating an autocrine and paracrine positive-feedback loop [148]. Eventually, this increases fibrogenesis in the liver. LSECs can also directly contribute to hepatocarcinogenesis via the expression of fatty acid-binding protein (FABP)4, which directly induces hepatocyte proliferation [149]. All in all, LSECs contribute to the initiation of NAFLD-related HCC, either directly or indirectly, by creating an environment that is susceptible for HCC development.
Figure 2 shows a schematic overview of the role of macrophages, LSECs and HSCs in the process of fibrogenesis and its role in hepatocarcinogenesis in the context of NAFLD.

Figure 2.
Schematic overview of the role of macrophages, LSECs and HSCs in the process of fibrogenesis and its role in hepatocarcinogenesis in the context of NAFLD.
4.3. Angiogenesis
Pathogenic angiogenesis increases with NASH [29,150,151]. This is mediated in a variety of ways. First of all, chronic inflammation promotes angiogenesis. In this regard, chronic inflammation and fibrosis sustain tissue hypoxia and, thus, induce the release of HIF-1α, a potent pro-angiogenic factor [151]. In addition, pro-inflammatory mediators, including TNF-α and IL-6, prompt a direct pro-angiogenic effect by inducing HIF-1α and VEGF [151], and cytokines and ROS released during NASH can activate the MAPK/ERK pathway, which is involved in cell migration and angiogenesis [29,151]. Activation of both macrophages and HSCs can increase angiogenesis. The activation of macrophages leads to the release of various factors that can induce angiogenesis, inducing ROS, NOS, TNF-α, HIF-1 and VEGF [152,153,154]. The activation of HSCs and the subsequent release of factors such as HGF, PDGF, VEGF and NO, also induces angiogenesis [151,155,156]. Moreover, in the context of NAFLD, the accumulation of FFAs causes damage to and swelling of hepatocytes. This swelling reduces sinusoidal perfusion thus adding to the pro-angiogenic response in the liver [150,151]. Moreover, the increased cellular stress caused by exposure to excessive amounts of FFAs and gut-derived LPS, prompts hepatocytes to release microvesicles containing pro-angiogenic factors [157].
LSECs also play a role in angiogenesis in NAFLD-NASH, as they are the main producers of angiopoetin-2, another driver of angiogenesis in inflammatory conditions [158,159]. Inflammation stimulates angiogenesis that in turn worsens inflammation, thus creating a self-propelling cycle. This is underscored by the anti-inflammatory effect of anti-angiogenic agents [29]. Moreover, tumor cells secrete a number of angiogenic growth factors, including VEGF, PDGF, placental growth factor (PlGF) and TGF-β1, to fulfill their need for high oxygen and nutrient supply [151]. However, these new vessels are marked by a disorganized vasculature, and consist of leaking, hemorrhagic and torturous vessels, which leads to poor oxygenation and free passage for tumor cells into the blood circulation, and hence, facilitate metastasis [151].
5. Immune Escape of Tumor Cells
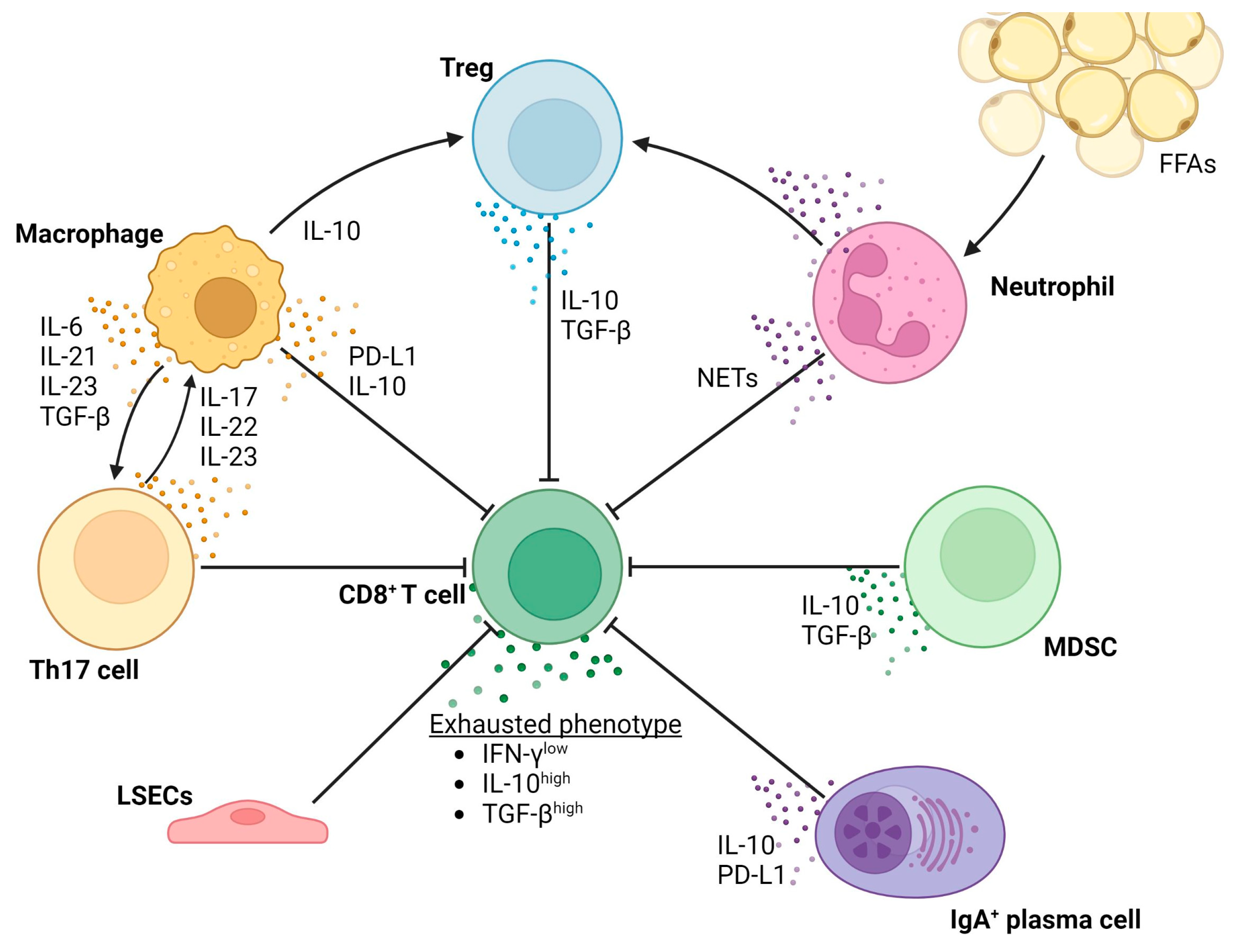
Macrophages and HSCs play an important role in tumor immune escape [160]. Both cell type are able to inhibit an effective cytotoxic T-cell response by expressing high levels of PD-L1 on their cell surface [77,107,160,161,162,163]. Additionally, HSCs promote T-cell apoptosis and inhibit T-cell proliferation through the C3 pathway [164], and both macrophages and HSCs are able to induce the expansion of myeloid-derived suppressor cells (MDSCs) [165] and Th17 cells [107,160]. MDSCs promote hepatocarcinogenesis in a variety of ways including the recruitment of regulatory T cells via the secretion of IL-10 and TGF-β and inhibition of cytotoxic T cells by stimulating the expression of PD-L1 on tumor cells, thus mediating immune evasion [166,167]. MDSCs also stimulate angiogenesis via the secretion of VEGF [168]. Th17 cell expansion, and its subsequent secretion of IL-17, IL-22 and IL-23, promotes NAFLD-related hepatocarcinogenesis [108,169]. Differentiation towards the Th17 phenotype is driven by multiple cytokines, including IL-6, TGF-β, IL-21 and IL-23, and IL-1β and TNF-α to a lesser extent [170]. Autocrine IL-17 stimulates the expression of PD-L1 on the surface of peritumoral macrophages and HSCs [171]. Apart from aiding immune escape, IL-17 stimulates the expression of IL-1β, IL-10 and TNF-α on macrophages [172,173], and directly induces type I collagen production by HSCs via activation of the STAT3 signaling pathway [174]. Additionally, Th17 cells are able to aggravate the influx of FFA in the liver and can, thus, exacerbate steatosis and induce additional DNA damage in hepatocytes [172]. The secretion of IL-22 is pro-carcinogenic as it stimulates the STAT3 signaling pathway in hepatocytes and, apart from Th17 cells, is also secreted by Th22 cells [170,175,176].
5.1. Role of the Adaptive Immune System
CD8+ T cells and CD4+ T cells are the main players in tumor immune escape in the context of NAFLD-related HCC. Tumor immune escape occurs in HCC with different etiologies, but some NAFLD-related specific methods are at play. In the context of NASH, macrophages actively participate in the recruitment and activation of CD8+ T cells and NKT cells. This is mediated by the release of ROS and cytokines [177]. Dendritic cells (DCs) also aid in the activation of CD8+ T cells by the secretion of cytokines, including IL-1β and TNF, and by acting as antigen-presenting cells (APCs) for CD8+ T cells [173,177,178]. Interestingly, in early stages of NAFLD, lipid-rich hepatic DCs are immunogenic, thereby activating T cells, NK cells and NKT cells, whereas lipid-poor DCs are more tolerogenic in nature and are able to induce Tregs [178,179].
5.2. CD8+ T Cells
CD8+ T cells have important cytotoxic effector functions and as such are responsible for killing cancerous or virally infected cells. CD8+ T cells are the main subset of tumor-infiltrating lymphocytes (TILs) and perform important anti-carcinogenic functions as they are able to kill target cells by the method of lying, which is partly regulated by IFN-γ [69,180,181]. Activated T cells produce HCC-inducing lymphotoxin-α and -β and other mitogenic cytokines, thus aiding hepatocarcinogenesis [182]. This can be seen in the light of tissue damage resulting from chronic inflammation and is consistent with that in other chronic inflammatory diseases [69]. As such, the antibody-mediated depletion of CD8+ T cells as well as pharmacological inhibition of the lymphotoxin-β receptor markedly delayed tumor development in mice with chronic liver injury [183]. Additionally, CD8+ T cells and NKT cells are capable of activating NF-κB and STAT3 signaling in hepatocytes, thus promoting hepatocarcinogenesis [183,184,185,186].
On the other hand, CD8+ T cells perform distinctive antitumor effector functions [180], as previously mentioned. In line with this view, large numbers of CD8+ T cells in human HCC correlate with improved overall survival, longer relapse-free survival and diminished disease progression [69,180,181]. Cytotoxic reactions are mainly mediated by IFN-γ secreting CD8+ T cells and a subset of NK cells [181]. However, in the setting of HCC, the anti-carcinogenic functions of CD8+ T cells and NK cells are impaired [69,180]. This phenomenon is called T-cell and NK-cell exhaustion, respectively. This has been demonstrated by an impaired ability of CD8+ T cells to secrete IFN-γ, thus hampering the lysing of tumor cells in human HCC with different etiologies, including NAFLD [180]. Moreover, chronic inflammation in the context of NASH is accompanied by the accumulation of liver-resident IgA-producing cells, which express PD-L1 and IL-10 [187]. As previously mentioned, this directly suppresses the function of CD8+ T cells, thus partially explaining the occurrence of T-cell exhaustion.
Regulatory B cells have been reported to promote HCC growth and invasiveness by directly interacting with hepatocytes through the CD40/CD154 signaling pathway [188]. In other respects, the role of the B cell in the development and progression of NAFLD-related HCC remains unclear [189]. T-cell exhaustion has also been linked to the function of neutrophils, which are able to generate neutrophil extracellular traps (NETs). These NETs enhance PD-L1 signaling and thus promote an immunosuppressive environment [190,191]. Interestingly, elevated FFAs stimulate the infiltration of neutrophils into the liver and stimulate the formation of NETs [190]. All in all, it can be stated that CD8+ T cells both cause liver damage and are needed for surveillance of pre-malignant and malignant cells in the liver [69]. Yet, during advanced stages of disease, TILs express markers of exhaustion, including PD-L1, and show increased IL-10 and TGF-β secretion [69]. Moreover, the recruitment of Tregs further diminishes the effectiveness of T cell-mediated tumor surveillance. Tumor-infiltrating Tregs mainly secrete IL-10 and TGF-β, both of which are key inhibitors of CD8+ T-cell function [69,192]. Tregs, in turn, are recruited into the TME by tumor-associated neutrophils and macrophages, partly by KC-derived IL-10 [193]. As such, an increased abundance of Tregs is correlated with poor survival and high recurrence rates [194].
5.3. CD4+ T Cells
While the number of Tregs increases in NASH, the number of CD4+ T cells decreases [177]. The interaction between neutrophils and naïve CD4+ T cells drives differentiation into Tregs [191]. In the setting of NAFLD, lipid metabolism dysregulation, the release of specific metabolites, including linoleic acids, and increased ROS induce a selective loss of CD4+ T cells [195]. This selective loss of CD4+ T cells accelerates hepatocarcinogenesis in NASH livers [195]. Although the total amount of CD4+ T cells is decreased in the setting of NASH and NAFLD-related HCC, specific subgroups of Th cells are increased [177]. As previously stated, Th17 cells are upregulated, aiding immune escape and aggravating the influx of FFAs and DNA damage [69,171,172]. Th1 cells secrete IL-2, TNF and IFN-γ, the latter of which stimulates CD8+ T cells and has anti-carcinogenic properties [69,170,191]. Th1 drives macrophages polarization towards the pro-inflammatory, anti-carcinogenic M1-phenotype. In early stages of disease, this (together with Th17 activity) represents an important mechanism supporting lobular inflammation during NASH evolution [173]. However, as disease progresses, the differentiation of naïve CD4+ T cells to Th1 cells, becomes inhibited [177]. The role of Th2 cells in the development of NAFLD-related HCC still remains unclear [177].
NK cells exhibit various anti-carcinogenic functions, including anti-fibrotic properties [196]. NK function, however, is diminished during HCC development by STAT3-mediated upregulation of IL-10 and TGF-β, thus inducing NK exhaustion [69,197,198].
5.4. Complex Interplay between the Innate and Adaptive Immune System
Increased amounts of NKT cells are found in human NASH, cirrhosis and HCC, but it remains unclear whether this propels or inhibits hepatocarcinogenesis [69,185]. NKT cells have both pro- and anti-inflammatory effector capacities through the secretion of IFN-γ, on the one hand, and IL-17 and LIGHT, on the other [173]. LIGHT, also known as tumor necrosis factor superfamily member (TNFSF)14, promotes steatosis by encouraging the uptake of lipids by hepatocytes [185]. Apart from NKT cells, it is also secreted by CD8+ T cells in early stages of disease [173]. Upregulation of LIGHT is seen in the liver of patients with NASH, but not in patients with ASH or chronic HCV infection, and may represent an NAFLD-specific way of disease progression [185]. Moreover, LIGHT has also been demonstrated to block TNF-α induced hepatocyte apoptosis, and stimulates NF-κB signaling in hepatocytes, thus stimulating proliferation [199]. In murine models, LIGHT deficiency prevented NASH and HCC development, and led to a strong reduction of NKT cells in the liver [185]. NKT cells also activate HSCs and thus promote fibrogenesis [200], adding to their pro-carcinogenic properties.
KCs influence tumor immune escape because of their tolerogenic nature. As such, KCs function as incomplete antigen-presenting cells (APCs) and can even inhibit DC-induced T-cell activation in murine models [201]. Moreover, in advanced HCC, the functions of DCs are impaired, thus preventing the proper activation of antigen-specific T cells [202].
Additionally, endothelial cells within HCC can also alter the tumor-associated immune response by promoting T-cell tolerance towards cancer-associated antigens and creating an immunosuppressive environment [203]. This is regulated by the uptake of tumor-cell apoptotic fragments by LSECs and the subsequent cross-presentation of this apoptotic cell material by LSECs to CD8+ T cells, which leads to the induction of CD8+ T-cell tolerance [203].
Figure 3 shows a schematic overview of the different cell types involved in and the processes resulting in T-cell exhaustion in the context of NAFLD-related HCC.

Figure 3.
Overview of the processes resulting in T-cell exhaustion in the context of NAFLD-related HCC.
5.5. Immunotherapy for NAFLD-Related HCC
A growing body of evidence highlights the difference in the effects of immunotherapy for HCC in the context of NAFLD-NASH to those of other etiologies [177,204,205]. In a mouse model of NAFLD-related HCC, immunotherapy with anti-PD1 led to an increase in the incidence of NAFLD-related HCC and in the number and size of tumor nodules. Interestingly, this increase was correlated with increased hepatic CD8+ programmed death (PD)+ T cells and TNF+ T cells and was prevented by depletion of CD8+ T cells or TNF neutralization. This suggests an important role of CD8+ T cells in the induction of NAFLD-related HCC [205]. This is in contrast to other mouse models with HCC from other, non-NAFLD, etiologies, where PD1 immunotherapy induces tumor regression [206]. Impaired tumor surveillance and enhanced T cell-mediated tissue damage are suspected to underlie this difference, adding to the risk of transition to HCC in mice with NASH [204,205]. These results are supported by a meta-analyses of three large randomized controlled phase III trials of immunotherapy in patients with advanced HCC [205]. While benefit is evident regardless of etiology, the meta-analysis suggests that patients with NAFLD etiology may benefit less after anti-PD1 treatment. A combined therapy using both anti-PD-L1 and anti-VEGF has been shown to prolong survival [207,208,209] and is now the recommended first-line medical therapy regardless of etiology, but further investigations are warranted to elucidate the underlying mechanisms relevant to variability in response rates [177,210].
6. Conclusions
In this review, we identified the key roles of non-parenchymal cells in both initiation and progression of NAFLD-related HCC. Upon activation, macrophages prompt an effective pro-inflammatory, anti-carcinogenic response. However, as inflammation becomes chronic, this pro-inflammatory response actually drives genomic instability via the secretion of ROS and hepatocyte proliferation, amongst others. Moreover, as inflammation continues, the pro-inflammatory, anti-carcinogenic M1 macrophage response shifts towards an anti-inflammatory, pro-carcinogenic M2 macrophage response. This further drives error-prone DNA repair and increases genomic instability and the chance of HCC initiation. Moreover, M2 macrophages suppress an effective cytotoxic response by secreting anti-inflammatory cytokines, such as IL-10, expression of PD-L1, and by acting as incomplete antigen-presenting cells, thus aiding immune escape. This is exacerbated by a combined effect of neutrophils, plasma cells, LSECs, MDSCs and regulatory T cells that drive CD8+ T-cell exhaustion, characterized by a IFN-γlow, IL-10high and TGF-βhigh phenotype.
The activation of HSCs by macrophages prompts their differentiation into myofibroblasts, which in turn secrete additional ECM. The increase in ECM stiffness contributes to hepatocarcinogenesis and promotes hepatocyte proliferation by modulating various signaling cascades. LSECs can also induce HSC activation and induce hepatocyte proliferation. This mechanism plays an important role in angiogenesis and increases the potential of metastasis.
The process of HCC initiation and subsequent progression is highly complex and depends on the underlying etiology. The differences in pathogenesis between NAFLD-related HCC and HCC induced by, for example, viral-induced HCC, warrants further research to elucidate the differences in treatment response. In this regard, impaired immune surveillance in NAFLD-related HCC may partially explain the difference in response to immune checkpoint inhibitors compared to viral-induced HCC. Specific molecular mechanisms leading to HCC may serve as potential new target agents. As such, therapies combating T-cell exhaustion, such as anti-PD1 treatment, could become viable treatment options for patients with NAFLD-related HCC.
Author Contributions
Writing-original draft preparation, K.C.v.S.; writing- review and editing, K.C.v.S., L.V., R.H., H.R., R.B.T., J.P.H.D., M.E.T., A.G.H.; visualization, K.C.v.S.; supervision, M.E.T., A.G.H.; project administration, K.C.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APC | Antigen-presenting cell |
| CAF | Carcinoma-associated fibroblast |
| DC | Dendritic cell |
| ECM | Extracellular matrix |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| FABP | Fatty acid-binding protein |
| FFA | Free fatty acid |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HCC | Hepatocellular carcinoma |
| HGF | Hepatocyte growth factor |
| Hh | Hedgehog |
| HIF | Hypoxia-inducible factor |
| HSC | Hepatic stellate cell |
| IFN | Interferon |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| KC | Kupffer cell |
| LPS | Lipopolysaccharides |
| LSEC | Liver sinusoidal endothelial cell |
| MAPK | Mitogen-activated protein kinase |
| M-CSF | Macrophage colony-stimulating factor |
| MDSC | Myeloid-derived suppressor cell |
| MMP | Matrix metalloproteinase |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NET | Neutrophil extracellular trap |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cell | Natural killer cell |
| NKT cell | Natural killer T cell |
| NOS | Nitric oxide synthase |
| OA | Oleic acid |
| OPN | Osteopontin |
| PA | Palmitic acid |
| PD | Programmed death |
| PD-L | Programmed death ligand |
| PI3K | Phosphoinositide-3 kinase |
| PlGF | Placental growth factor |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| SDF | Stromal-derived factor |
| STAT | Signal transducer and activator of transcription |
| TAM | Tumor-associated macrophage |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TGF | Transforming growth factor |
| Th | T helper |
| TIL | Tumor-infiltrating lymphocyte |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| TNF | Tumor necrosis factor |
| TNSMF | Tumor necrosis superfamily member |
| Treg cell | Regulatory T cell |
| VAP | Vascular adhesion protein |
| VEGF | Vascular-endothelial growth factor |
| Wnt | Wingless-related integration sight |
| YAP | Yes-associated protein |
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Ruissen, M.M.; Mak, A.L.; Beuers, U.; Tushuizen, M.E.; Holleboom, A.G. Non-alcoholic fatty liver disease: A multidisciplinary approach towards a cardiometabolic liver disease. Eur. J. Endocrinol. 2020, 183, R57–R73. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef]
- Fingas, C.D.; Best, J.; Sowa, J.-P.; Canbay, A. Epidemiology of nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin. Liver Dis. 2016, 8, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010, 26, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2018, 17, 748–755.e3. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.-M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef]
- Maeda Júnior, A.S.; Constantin, J.; Utsunomiya, K.S.; Gilglioni, E.H.; Gasparin, F.R.S.; Carreño, F.O.; de Moraes, S.M.F.; Rocha, M.; Natali, M.R.M.; Ghizoni, C.V.C.; et al. Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence. Nutrients 2018, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Nishikawa, T.; Nakajima, T.; Okada, Y.; Yamaguchi, K.; Mitsuyoshi, H.; Yasui, K.; Minami, M.; Iwai, M.; Kagawa, K.; et al. Oxidative stress is closely associated with tumor angiogenesis of hepatocellular carcinoma. J. Gastroenterol. 2011, 46, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Hafizi Abu Bakar, M.; Kian Kai, C.; Wan Hassan, W.N.; Sarmidi, M.R.; Yaakob, H.; Zaman Huri, H. Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: The roles of long chain fatty acids. Diabetes Metab. Res. Rev. 2015, 31, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver — from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 282–302.e8. [Google Scholar] [CrossRef]
- Hammoutene, A.; Rautou, P.-E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Helen, L.R.; Reeves, H.L.; Friedman, S.L. Activation of hepatic stellate cells—A key issue in liver fibrosis. Front. Biosci. 2002, 7, 808–826. [Google Scholar] [CrossRef]
- Angulo, P.; Machado, M.V.; Diehl, A.M. Fibrosis in Nonalcoholic Fatty Liver Disease: Mechanisms and Clinical Implications. Semin. Liver Dis. 2015, 35, 132–145. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Español-Suñer, R.; Mederacke, I.; Affò, S.; Manco, R.; Sempoux, C.; Lemaigre, F.P.; Adili, A.; Yuan, D.; Weber, A.; et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J. Clin. Investig. 2015, 125, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Cucarull, B.; Tutusaus, A.; Rider, P.; Hernáez-Alsina, T.; Cuño, C.; de Frutos, P.G.; Colell, A.; Marí, M.; Morales, A. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers 2022, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef] [PubMed]
- Zoller, H.; Tilg, H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 2016, 65, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.M.; Groothuis, G.M.M.; Olinga, P.; Boersema, M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Fu, Y.; Chung, F.-L. Oxidative stress and hepatocarcinogenesis. Hepatoma Res. 2018, 4, 39. [Google Scholar] [CrossRef]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Karin, M. NF-κB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Canli, Ö; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, O.; Kaleli, H.N.; Ozer, E. Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 8543763. [Google Scholar] [CrossRef]
- Gautheron, J.; Vucur, M.; Reisinger, F.; Cardenas, D.V.; Roderburg, C.; Koppe, C.; Kreggenwinkel, K.; Schneider, A.T.; Bartneck, M.; Neumann, U.P.; et al. A positive feedback loop between RIP 3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol. Med. 2014, 6, 1062–1074. [Google Scholar] [CrossRef]
- Sun, K.; Deng, W.; Zhang, S.; Cai, N.; Jiao, S.; Song, J.; Wei, L. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell Biosci. 2013, 3, 35. [Google Scholar] [CrossRef]
- Sircana, A.; Paschetta, E.; Saba, F.; Molinaro, F.; Musso, G. Recent Insight into the Role of Fibrosis in Nonalcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1745. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, B. The Role of Autophagy in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2015, 16, 26629–26643. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Umemura, A.; He, F.; Taniguchi, K.; Nakagawa, H.; Yamachika, S.; Font-Burgada, J.; Zhong, Z.; Subramaniam, S.; Raghunandan, S.; Duran, A.; et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell 2016, 29, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Gores, G.J. Death Receptor-Mediated Cell Death and Proinflammatory Signaling in Nonalcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef]
- Boege, Y.; Malehmir, M.; Healy, M.E.; Bettermann, K.; Lorentzen, A.; Vucur, M.; Ahuja, A.K.; Böhm, F.; Mertens, J.C.; Shimizu, Y.; et al. A Dual Role of Caspase-8 in Triggering and Sensing Proliferation-Associated DNA Damage, a Key Determinant of Liver Cancer Development. Cancer Cell 2017, 32, 342–359.e10. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Guicciardi, M.E.; Gores, G.J. Proapoptotic signaling induced by deletion of receptor-interacting kinase 1 and TNF receptor-associated factor 2 results in liver carcinogenesis. Hepatology 2017, 66, 983–985. [Google Scholar] [CrossRef]
- Cullen, S.P.; Henry, C.M.; Kearney, C.J.; Logue, S.E.; Feoktistova, M.; Tynan, G.A.; Lavelle, E.C.; Leverkus, M.; Martin, S.J. Fas/CD95-Induced Chemokines Can Serve as “Find-Me” Signals for Apoptotic Cells. Mol. Cell 2013, 49, 1034–1048. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2016, 63, 731–744. [Google Scholar] [CrossRef]
- Tomita, K.; Kabashima, A.; Freeman, B.L.; Bronk, S.F.; Hirsova, P.; Ibrahim, S.H. Mixed Lineage Kinase 3 Mediates the Induction of CXCL10 by a STAT1-Dependent Mechanism During Hepatocyte Lipotoxicity. J. Cell. Biochem. 2017, 118, 3249–3259. [Google Scholar] [CrossRef]
- Canbay, A.; Taimr, P.; Torok, N.; Higuchi, H.; Friedman, S.; Gores, G.J. Apoptotic Body Engulfment by a Human Stellate Cell Line Is Profibrogenic. Lab. Investig. 2003, 83, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zeng, W.; Gai, X.; Xu, Q.; Li, C.; Liang, Z.; Tuo, H.; Liu, Q. Role of the Hedgehog pathway in hepatocellular carcinoma (Review). Oncol. Rep. 2013, 30, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.R.; Nguyen, M.H.; Lim, J.K. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 8294–8303. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer Cell Metabolism and Function. J. Enzymol. Metab. 2015, 1, 101. [Google Scholar]
- Cha, J.-Y.; Kim, D.-H.; Chun, K.-H. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Lab. Anim. Res. 2018, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.; Szabo, G. Immune and inflammatory pathways in NASH. Hepatol. Int. 2013, 7 (Suppl. 2), S771–S781. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Seki, E. Hepatic Stellate Cell–Macrophage Crosstalk in Liver Fibrosis and Carcinogenesis. Semin. Liver Dis. 2020, 40, 307–320. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Gufler, S.; Seeboeck, R.; Schatz, C.; Haybaeck, J. The Translational Bridge between Inflammation and Hepatocarcinogenesis. Cells 2022, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef]
- Baffy, G. Kupffer cells in non-alcoholic fatty liver disease: The emerging view. J. Hepatol. 2009, 51, 212–223. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Bai, F.; Xu, Y.; Liang, X.; Ma, C.; Gao, L. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front. Immunol. 2021, 12, 708978. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediat. Inflamm. 2017, 2017, 9624760. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Shi, X.; He, X.; Gao, Y. Macrophage Phenotype and Function in Liver Disorder. Front. Immunol. 2020, 10, 3112. [Google Scholar] [CrossRef]
- Luo, W.; Xu, Q.; Wang, Q.; Wu, H.; Hua, J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 2017, 7, 44612. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages. BioMed. Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Koletsa, T.; Mitroulis, I.; Germanidis, G. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers 2022, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyanishi, K.; Kobune, M.; Kawano, Y.; Hoki, T.; Kubo, T.; Hayashi, T.; Sato, T.; Sato, Y.; Takimoto, R.; et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J. Gastroenterol. 2013, 48, 1249–1258. [Google Scholar] [CrossRef]
- Hirsova, P.; Bohm, F.; Dohnalkova, E.; Nozickova, B.; Heikenwalder, M.; Gores, G.J.; Weber, A. Hepatocyte apoptosis is tumor promoting in murine nonalcoholic steatohepatitis. Cell Death Dis. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Zhou, D.; Luan, J.; Huang, C.; Li, J. Tumor-Associated Macrophages in Hepatocellular Carcinoma: Friend or Foe? Gut Liver. 2021, 15, 500–516. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gao, M.; Yang, P.; Liu, D.; Wang, D.; Song, F.; Zhang, X.; Liu, Y. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J. Cell. Physiol. 2019, 234, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Y.; Zhang, Q.; Zhou, G.; Cao, F.; Yao, S. IL-4 Switches Microglia/macrophage M1/M2 Polarization and Alleviates Neurological Damage by Modulating the JAK1/STAT6 Pathway Following ICH. Neuroscience 2020, 437, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Sheppard, D. TGF-β Activation and Function in Immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Yuan, J.-H.; Ma, J.-Z.; Yang, W.-J.; Liu, X.-N.; Yin, Y.-P.; Liu, Y.; Pan, W.; Sun, S.-H. CTGF secreted by mesenchymal-like hepatocellular carcinoma cells plays a role in the polarization of macrophages in hepatocellular carcinoma progression. Biomed. Pharmacother. 2017, 95, 111–119. [Google Scholar] [CrossRef]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2013, 59, 130–142. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; You, Y.; Wen, D.; Feng, Z.; Zhou, Y.; Que, K.; Gong, J.; Liu, Z. Nogo-B fosters HCC progression by enhancing Yap/Taz-mediated tumor-associated macrophages M2 polarization. Exp. Cell Res. 2020, 391, 111979. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.-C.; Chen, Y.; Zhao, J.-L.; Gao, C.-C.; Han, H.; Liu, W.-C.; Qin, H.-Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Dong, L.-Q.; Peng, L.-H.; Ma, L.-J.; Liu, D.-B.; Zhang, S.; Luo, S.-Z.; Rao, J.-H.; Zhu, H.-W.; Yang, S.-X.; Xi, S.-J.; et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 2019, 72, 896–908. [Google Scholar] [CrossRef]
- Khantakova, D.; Brioschi, S.; Molgora, M. Exploring the Impact of TREM2 in Tumor-Associated Macrophages. Vaccines 2022, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, M.; Guo, H.; Hou, J.; Zhang, Y.; Li, M.; Wu, X.; Chen, X.; Wang, L. Integrated Analysis Highlights the Immunosuppressive Role of TREM2+ Macrophages in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 848367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, K.; Tan, D.; Liang, G. TREM2 knockdown improves the therapeutic effect of PD-1 blockade in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2022, 636 Pt 1, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lv, B.; Yang, B.; Chen, Y.; Yuan, F.; Ma, L.; Chen, S.; Zhang, S.; Xia, J. TREM2 acts as a tumor suppressor in hepatocellular carcinoma by targeting the PI3K/Akt/β-catenin pathway. Oncogenesis 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Baquer, A.; Labiano, I.; Sharif, O.; Agirre-Lizaso, A.; Oakley, F.; Rodrigues, P.M.; Zhuravleva, E.; O’Rourke, C.J.; Hijona, E.; Jimenez-Agüero, R.; et al. TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut 2021, 70, 1345–1361. [Google Scholar] [CrossRef]
- Kuang, D.-M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.-M.; Zheng, L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Kuang, D.-M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.-P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef]
- Kuang, D.-M.; Peng, C.; Zhao, Q.; Wu, Y.; Chen, M.-S.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010, 51, 154–164. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, L. Dynamic Education of Macrophages in Different Areas of Human Tumors. Cancer Microenviron. 2012, 5, 195–201. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Kent, G.; Gay, S.; Inouye, T.; Bahu, R.; Minick, O.T.; Popper, H. Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc. Natl. Acad. Sci. USA 1976, 73, 3719–3722. [Google Scholar] [CrossRef]
- Rojkind, M. Role of metalloproteinases in liver fibrosis. Alcohol. Clin. Exp. Res. 1999, 23, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Soon, R.K., Jr.; Yee, H.F., Jr. Stellate cell contraction: Role, regulation, and potential therapeutic target. Clin. Liver Dis. 2008, 12, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; O’Byrne, S.M.; Wongsiriroj, N.; Kluwe, J.; D’Ambrosio, D.M.; Jiang, H.; Schwabe, R.F.; Hillman, E.M.; Piantedosi, R.; Libien, J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2009, 1791, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Filliol, A.; Saito, Y.; Nair, A.; Dapito, D.H.; Yu, L.-X.; Ravichandra, A.; Bhattacharjee, S.; Affo, S.; Fujiwara, N.; Su, H.; et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 2022, 610, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf, K.; Godoy, P.; Ciuclan, L.; Singer, M.V.; Dooley, S. TGF-beta/Smad signaling in the injured liver. Z. Gastroenterol. 2006, 44, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P. TGF-β signalling and liver disease. Febs J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol. Clin. Exp. Res. 1999, 23, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Arnold, T.D.; Katamura, Y.; Giacomini, M.M.; Rodriguez, J.D.; McCarty, J.H.; Pellicoro, A.; Raschperger, E.; Betsholtz, C.; Ruminski, P.G.; et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013, 19, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Saile, B.; Matthes, N.; Knittel, T.; Ramadori, G. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology 1999, 30, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Patsenker, E.; Stickel, F. Role of integrins in fibrosing liver diseases. Am. J. Physiol. Liver Physiol. 2011, 301, G425–G434. [Google Scholar] [CrossRef]
- Ochoa, B.; Syn, W.-K.; Delgado, I.; Karaca, G.F.; Jung, Y.; Wang, J.; Zubiaga, A.M.; Fresnedo, O.; Omenetti, A.; Zdanowicz, M.; et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology 2010, 51, 1712–1723. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.; Aplin, A.E.; Alahari, S.K.; Juliano, R.L. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 1998, 10, 220–231. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, X.; Qiao, S.; Yu, L. Targeting integrins in hepatocellular carcinoma. Expert Opin. Ther. Targets 2011, 15, 421–437. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; Van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef]
- Wallace, M.C.; Friedman, S.L. Hepatic Fibrosis and the Microenvironment: Fertile Soil for Hepatocellular Carcinoma Development. Gene Expr. 2014, 16, 77–84. [Google Scholar] [CrossRef]
- Naim, A.; Pan, Q.; Baig, M.S. Matrix Metalloproteinases (MMPs) in Liver Diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Dou, C.; Jia, Y.; Tu, K.; Zheng, X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget 2015, 6, 12061–12079. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Coulouarn, C.; Clément, B. Stellate cells and the development of liver cancer: Therapeutic potential of targeting the stroma. J. Hepatol. 2014, 60, 1306–1309. [Google Scholar] [CrossRef]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, E.; Zhang, Y. Advances of cancer-associated fibroblasts in liver cancer. Biomark. Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, X.; Zuo, J.; Hu, B.; Yang, J.; Zhao, J.; Chen, J. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020, 111, 406–417. [Google Scholar] [CrossRef]
- McMahan, R.H.; Porsche, C.E.; Edwards, M.G.; Rosen, H.R. Free Fatty Acids Differentially Downregulate Chemokines in Liver Sinusoidal Endothelial Cells: Insights into Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0159217. [Google Scholar] [CrossRef] [PubMed]
- Tateya, S.; Rizzo, N.O.; Handa, P.; Cheng, A.M.; Morgan-Stevenson, V.; Daum, G.; Clowes, A.W.; Morton, G.J.; Schwartz, M.W.; Kim, F. Endothelial NO/cGMP/VASP Signaling Attenuates Kupffer Cell Activation and Hepatic Insulin Resistance Induced by High-Fat Feeding. Diabetes 2011, 60, 2792–2801. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral Fat Adipokine Secretion Is Associated With Systemic Inflammation in Obese Humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. 2010, 7, 15. [Google Scholar] [CrossRef]
- Peters, K.M.; Wilson, R.B.; Borradaile, N.M. Non-parenchymal hepatic cell lipotoxicity and the coordinated progression of non-alcoholic fatty liver disease and atherosclerosis. Curr. Opin. Lipidol. 2018, 29, 417–422. [Google Scholar] [CrossRef]
- Weston, C.J.; Shepherd, E.L.; Claridge, L.C.; Rantakari, P.; Curbishley, S.M.; Tomlinson, J.W.; Hübscher, S.G.; Reynolds, G.M.; Aalto, K.; Anstee, Q.M.; et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Investig. 2014, 125, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, F.; Guy, C.D.; Lu, J.; Suzuki, A.; Burchette, J.L.; Abdelmalek, M.F.; Chen, W.; Diehl, A.M. Increased production of sonic hedgehog by ballooned hepatocytes. J. Pathol. 2011, 224, 401–410. [Google Scholar] [CrossRef]
- Maslak, E.; Gregorius, A.; Chlopicki, S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol. Rep. 2015, 67, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Matz-Soja, M.; Gebhardt, R. The many faces of Hedgehog signalling in the liver: Recent progress reveals striking cellular diversity and the importance of microenvironments. J. Hepatol. 2014, 61, 1449–1450. [Google Scholar] [CrossRef]
- Laouirem, S.; Sannier, A.; Norkowski, E.; Cauchy, F.; Doblas, S.; Rautou, P.E.; Albuquerque, M.; Garteiser, P.; Sognigbé, L.; Raffenne, J.; et al. Endothelial fatty liver binding protein 4: A new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene 2019, 38, 3033–3046. [Google Scholar] [CrossRef]
- Coulon, S.; Francque, S.; Colle, I.; Verrijken, A.; Blomme, B.; Heindryckx, F.; De Munter, S.; Prawitt, J.; Caron, S.; Staels, B.; et al. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine 2012, 59, 442–449. [Google Scholar] [CrossRef]
- Coulon, S.; Heindryckx, F.; Geerts, A.; Van Steenkiste, C.; Colle, I.; Van Vlierberghe, H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2010, 31, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Wolf, R.; Russell, J.; Shanley, T.P.; Granger, D.N. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology 1997, 26, 1499–1505. [Google Scholar] [CrossRef]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Ehling, J.; Bartneck, M.; Wei, X.; Gremse, F.; Fech, V.; Möckel, D.; Baeck, C.; Hittatiya, K.; Eulberg, D.; Luedde, T.; et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 2014, 63, 1960–1971. [Google Scholar] [CrossRef]
- Aleffi, S.; Petrai, I.; Bertolani, C.; Parola, M.; Colombatto, S.; Novo, E.; Vizzutti, F.; Anania, F.A.; Milani, S.; Rombouts, K.; et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 2005, 42, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Ankoma-Sey, V.; Wang, Y.; Dai, Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 2000, 31, 141–148. [Google Scholar] [CrossRef]
- Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; De Mollerat Du Jeu, X.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-Induced Toxicity Stimulates Hepatocytes to Release Angiogenic Microparticles That Require Vanin-1 for Uptake by Endothelial Cells. Sci. Signal. 2013, 6, ra88. [Google Scholar] [CrossRef]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Lefere, S.; Van De Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 Promotes Pathological Angiogenesis and Is a Therapeutic Target in Murine Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Trailin, A.; Ambrozkiewicz, F.; Liška, V.; Hemminki, K. Activated Hepatic Stellate Cells in Hepatocellular Carcinoma: Their Role as a Potential Target for Future Therapies. Int. J. Mol. Sci. 2022, 23, 15292. [Google Scholar] [CrossRef]
- Wu, K.; Kryczek, I.; Chen, L.; Zou, W.; Welling, T.H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009, 69, 8067–8075. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Tacke, F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. HepatoBiliary Surg. Nutr. 2014, 3, 344–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-C.; Chen, C.-H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Xu, W.; Zheng, X.; Yi, X.; Huang, L.; Wang, Y.; Wu, K. Activated Hepatic Stellate Cells (HSCs) Exert Immunosuppressive Effects in Hepatocellular Carcinoma by Producing Complement C3. Onco Targets Ther. 2020, 13, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Wang, H.; Burke, L.J.; Bridle, K.R.; Li, X.; Zhao, C.-X.; Crawford, D.H.G.; Roberts, M.S.; Liang, X. Therapeutic modulators of hepatic stellate cells for hepatocellular carcinoma. Int. J. Cancer 2020, 147, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Redd, P.S.; Lee, J.R.; Savage, N.; Liu, K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1247135. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Cheng, J.-N.; Yuan, Y.-X.; Zhu, B.; Jia, Q. Myeloid-Derived Suppressor Cells: A Multifaceted Accomplice in Tumor Progression. Front. Cell Dev. Biol. 2021, 9, 740827. [Google Scholar] [CrossRef]
- Hirsova, P.; Bamidele, A.O.; Wang, H.; Povero, D.; Revelo, X.S. Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Front. Endocrinol. 2021, 12, 760860. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol. 2019, 10, 82. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiao, X.; Wu, Y.; Wei, Y.; Zhu, L.-Y.; Zhou, J.; Kuang, D.-M. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur. J. Immunol. 2011, 41, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.-P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Albano, E. Adaptive immunity: An emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef]
- Jiang, R.; Tan, Z.; Deng, L.; Chen, Y.; Xia, Y.; Gao, Y.; Wang, X.; Sun, B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011, 54, 900–909. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Ramadori, P.; Kam, S.; Heikenwalder, M. T cells: Friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology 2022, 75, 1038–1049. [Google Scholar] [CrossRef]
- Nati, M.; Chung, K.-J.; Chavakis, T. The Role of Innate Immune Cells in Nonalcoholic Fatty Liver Disease. J. Innate Immun. 2022, 14, 31–41. [Google Scholar] [CrossRef]
- Ibrahim, J.; Nguyen, A.H.; Rehman, A.; Ochi, A.; Jamal, M.; Graffeo, C.S.; Henning, J.R.; Zambirinis, C.P.; Fallon, N.C.; Barilla, R.; et al. Dendritic Cell Populations With Different Concentrations of Lipid Regulate Tolerance and Immunity in Mouse and Human Liver. Gastroenterology 2012, 143, 1061–1072. [Google Scholar] [CrossRef]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef]
- Haybaeck, J.; Zeller, N.; Wolf, M.J.; Weber, A.; Wagner, U.; Kurrer, M.O.; Bremer, J.; Iezzi, G.; Graf, R.; Clavien, P.-A.; et al. A Lymphotoxin-Driven Pathway to Hepatocellular Carcinoma. Cancer Cell 2009, 16, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Endig, J.; Buitrago-Molina, L.E.; Marhenke, S.; Reisinger, F.; Saborowski, A.; Schütt, J.; Limbourg, F.; Könecke, C.; Schreder, A.; Michael, A.; et al. Dual Role of the Adaptive Immune System in Liver Injury and Hepatocellular Carcinoma Development. Cancer Cell 2016, 30, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Won, G.L.-H.; Chim, A.M.-L.; Chu, W.C.-W.; Yeung, D.K.-W.; Li, K.C.-T.; Chan, H.L.-Y. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic Activation of Intrahepatic CD8+ T Cells and NKT Cells Causes Nonalcoholic Steatohepatitis and Liver Cancer via Cross-Talk with Hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Peiseler, M.; Tacke, F. Inflammatory Mechanisms Underlying Nonalcoholic Steatohepatitis and the Transition to Hepatocellular Carcinoma. Cancers 2021, 13, 730. [Google Scholar] [CrossRef]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef]
- Shao, Y.; Lo, C.M.; Ling, C.C.; Liu, X.B.; Ng, K.T.-P.; Chu, A.C.Y.; Ma, Y.Y.; Li, C.X.; Fan, S.T.; Man, K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014, 355, 264–272. [Google Scholar] [CrossRef]
- Barrow, F.; Khan, S.; Wang, H.; Revelo, X.S. The Emerging Role of B Cells in the Pathogenesis of NAFLD. Hepatology 2021, 74, 2277–2286. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef]
- Shen, Y.; Wei, Y.; Wang, Z.; Jing, Y.; He, H.; Yuan, J.; Li, R.; Zhao, Q.; Wei, L.; Yang, T.; et al. TGF-β Regulates Hepatocellular Carcinoma Progression by Inducing Treg Cell Polarization. Cell. Physiol. Biochem. 2015, 35, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Z.; Zhou, L.; Qi, Z.; Xing, S.; Lv, J.; Shi, J.; Fu, B.; Liu, Z.; Zhang, J.-Y.; et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2012, 58, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016, 531, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Doron, S.; Kfir-Erenfeld, S.; Horwitz, E.; Abu-Tair, L.; Safadi, R.; Mandelboim, O. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut 2011, 61, 885–893. [Google Scholar] [CrossRef]
- Sui, Q.; Zhang, J.; Sun, X.; Zhang, C.; Han, Q.; Tian, Z. NK Cells Are the Crucial Antitumor Mediators When STAT3-Mediated Immunosuppression Is Blocked in Hepatocellular Carcinoma. J. Immunol. 2014, 193, 2016–2023. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2016, 6, e1264562. [Google Scholar] [CrossRef]
- Matsui, H.; Hikichi, Y.; Tsuji, I.; Yamada, T.; Shintani, Y. LIGHT, a member of the tumor necrosis factor ligand superfamily, prevents tumor necrosis factor-alpha-mediated human primary hepatocyte apoptosis, but not Fas-mediated apoptosis. J Biol Chem. 2002, 277, 50054–50061. [Google Scholar] [CrossRef]
- Syn, W.-K.; Agboola, K.M.; Swiderska, M.; Michelotti, G.A.; Liaskou, E.; Pang, H.; Xie, G.; Philips, G.; Chan, I.S.; Karaca, G.F.; et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut 2012, 61, 1323–1329. [Google Scholar] [CrossRef]
- You, Q.; Cheng, L.; Kedl, R.M.; Ju, C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 2008, 48, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.-Y.; Park, E.-J.; Lee, C.-W. Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp. Mol. Med. 2020, 52, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Wingender, G.; Djandji, D.; Hegenbarth, S.; Momburg, F.; Hämmerling, G.; Limmer, A.; Knolle, P. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur. J. Immunol. 2006, 36, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Chung, A.S.; Mettlen, M.; Ganguly, D.; Lu, T.; Wang, T.; Brekken, R.A.; Hsiehchen, D.; Zhu, H. Immune Checkpoint Inhibition is Safe and Effective for Liver Cancer Prevention in a Mouse Model of Hepatocellular Carcinoma. Cancer Prev. Res. 2020, 13, 911–922. [Google Scholar] [CrossRef]
- Shen, W.; Chen, Y.; Lei, P.; Sheldon, M.; Sun, Y.; Yao, F.; Ma, L. Immunotherapeutic Approaches for Treating Hepatocellular Carcinoma. Cancers 2022, 14, 5013. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Salem, R.; Li, D.; Sommer, N.; Hernandez, S.; Verret, W.; Ding, B.; Lencioni, R. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: Results from the IMbrave150 trial. Cancer Med. 2021, 10, 5437–5447. [Google Scholar] [CrossRef]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).