The Value of Multiparametric Magnetic Resonance Imaging in the Preoperative Differential Diagnosis of Parotid Gland Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Data Sources Measurement
2.4. Statistical Methods
3. Results
3.1. Patient Demographics and Tumors Characteristics
3.2. Tumors Distribution According to the Histopathologic Subtype
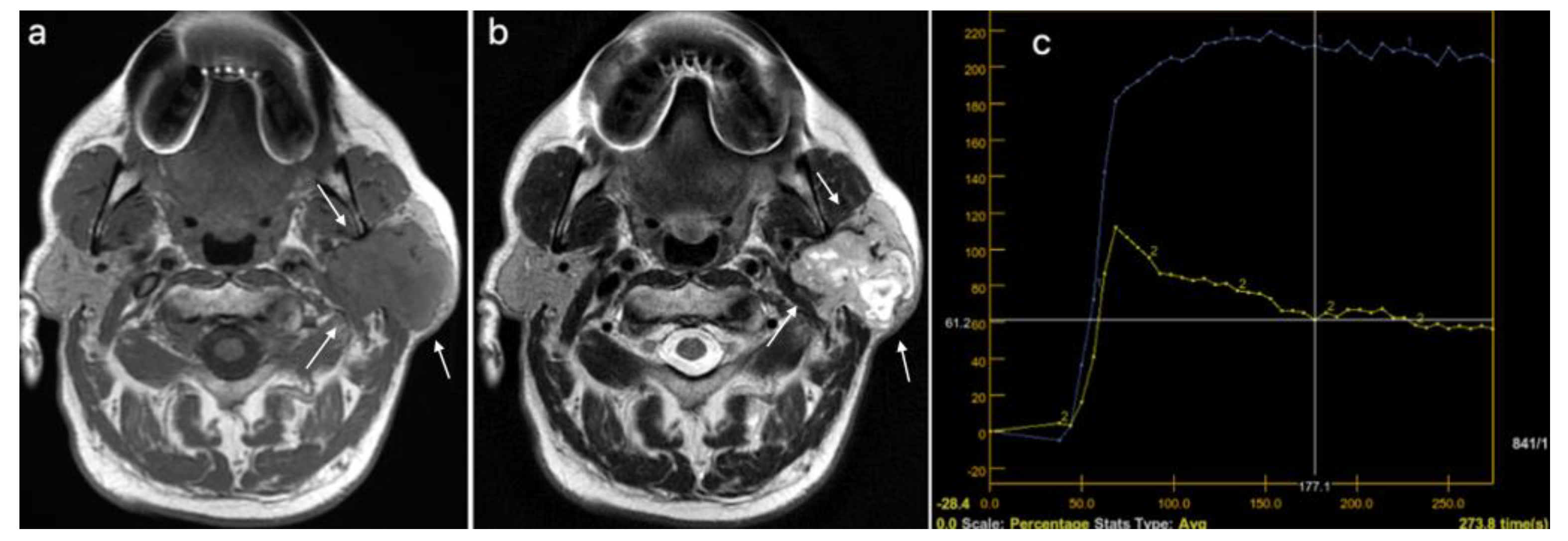
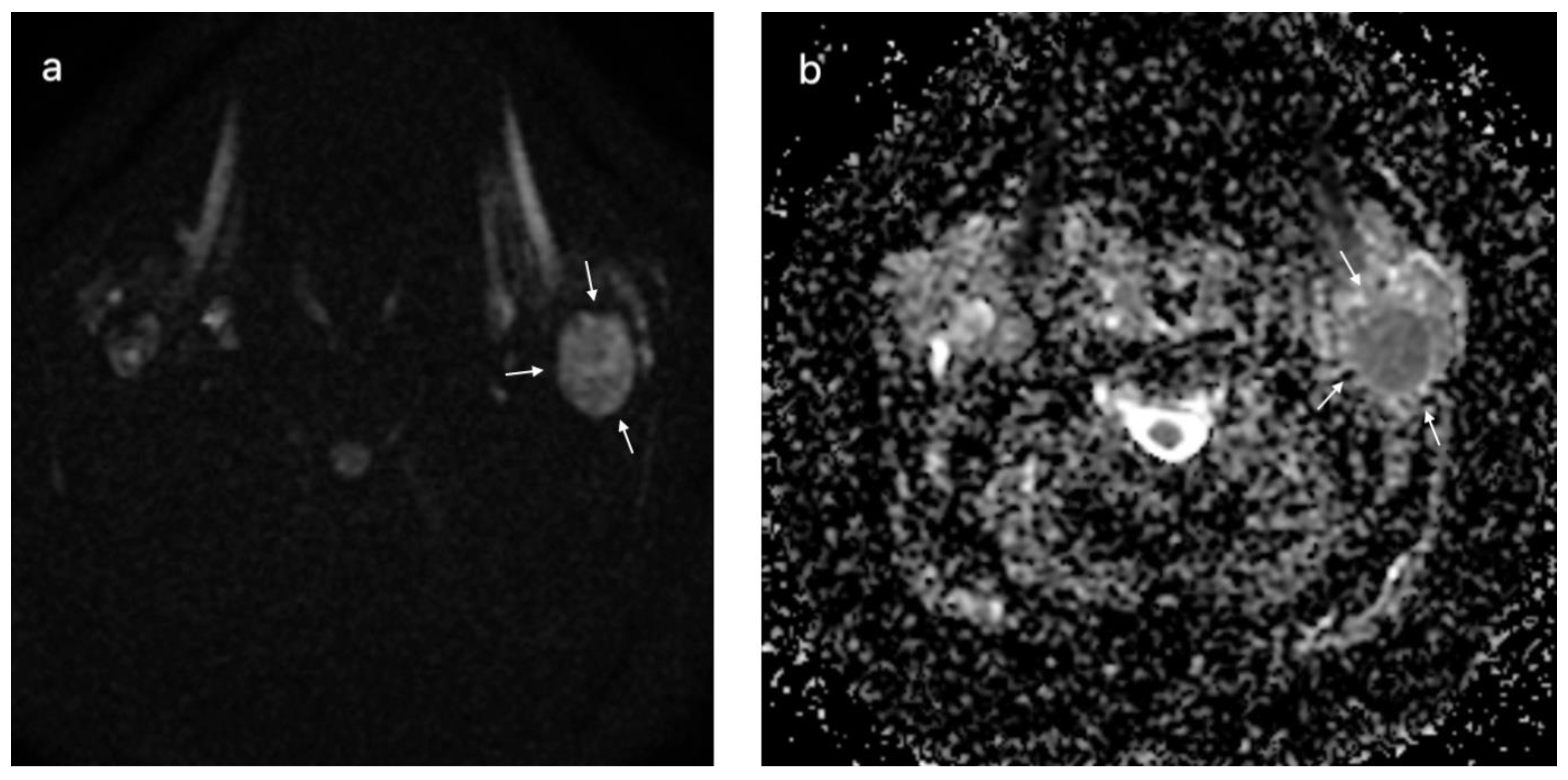
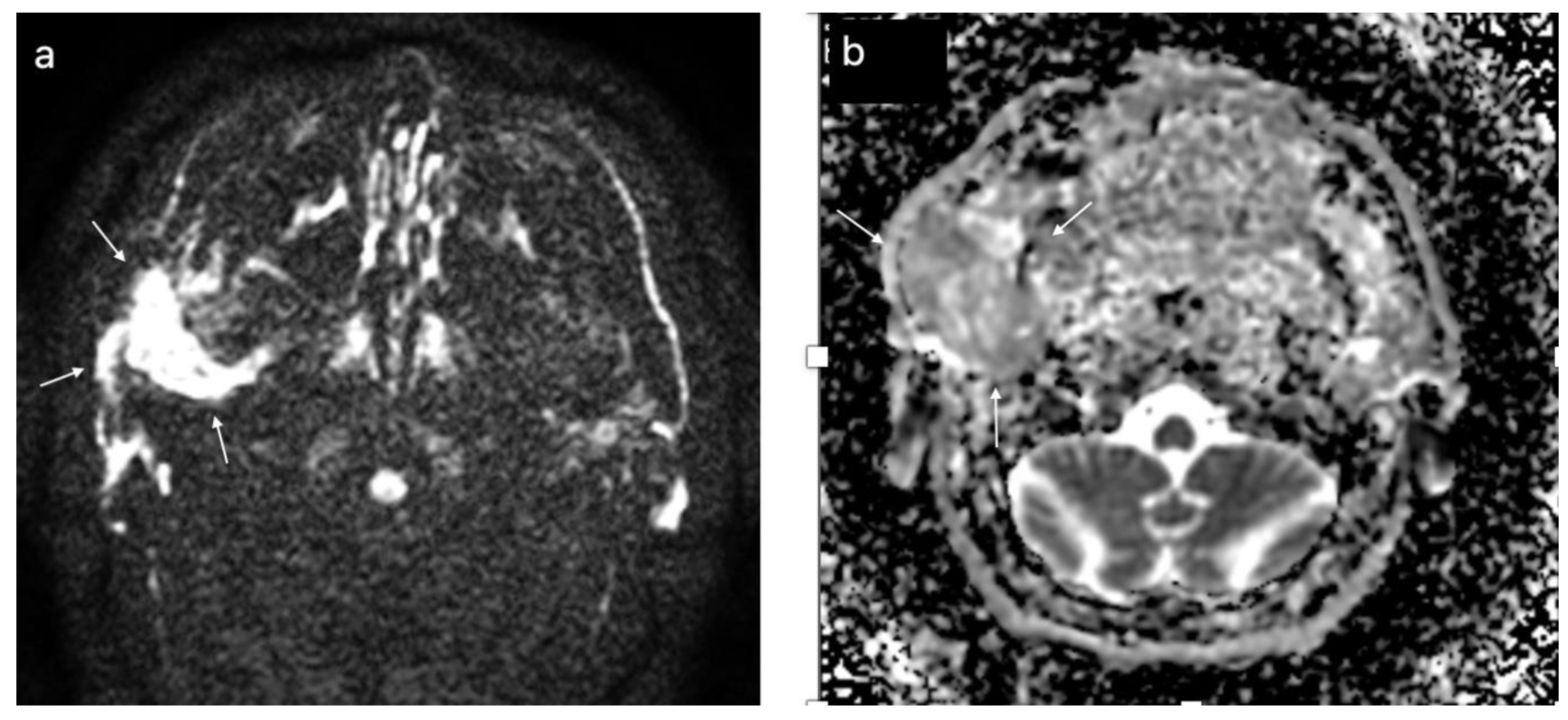
3.3. Multiparametric Magnetic Resonance Imaging Evaluation—Benign versus Malignant Parotid Tumors
3.4. Multiparametric Magnetic Resonance Imaging Evaluation—Pleomorphic Adenoma versus Warthin Tumor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, L.; Eveson, J.W.; Reichart, P.S.D. World Health Organization Classification of Tumors—Pathology and Genetics of Head and Neck Tumors; IARC: Lyon, France, 2005. [Google Scholar]
- Alsanie, I.; Rajab, S.; Cottom, H.; Adegun, O.; Agarwal, R.; Jay, A.; Graham, L.; James, J.; Barrett, A.W.; van Heerden, W.; et al. Distribution and Frequency of Salivary Gland Tumours: An International Multicenter Study. Head Neck Pathol. 2022, 16, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Lukšić, I.; Virag, M.; Manojlović, S.; Macan, D. Salivary gland tumours: 25 Years of experience from a single institution in Croatia. J. Cranio Maxillofac. Surg. 2012, 40, e75–e81. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hao, Y.; Huang, M.X.; Ma, D.Q.; Chen, Y.; Luo, H.Y.; Gao, Y.; Cao, Q.; Peng, X.; Yu, G.Y.; et al. Salivary gland tumours in a northern Chinese population: A 50-year retrospective study of 7190 cases. Int. J. Oral Maxillofac. Surg. 2017, 46, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Patella, F.; Franceschelli, G.; Petrillo, M.; Sansone, M.; Fusco, R.; Pesapane, F.; Pompili, G.; Ierardi, A.M.; Saibene, A.M.; Moneghini, L.; et al. A multiparametric analysis combining DCE-MRI- and IVIM- derived parameters to improve differentiation of parotid tumors: A pilot study. Futur. Oncol. 2018, 14, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; McGurk, M.; Vaz, F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S142–S149. [Google Scholar] [CrossRef] [PubMed]
- Bär, B.; Mantsopoulos, K.; Iro, H. Paradigm shift in surgery for benign parotid tumors: 19 years of experience with almost 3000 cases. Laryngoscope 2020, 130, 1941–1946. [Google Scholar] [CrossRef]
- Schapher, M.; Koch, M.; Goncalves, M.; Mantsopoulos, K.; Iro, H. Extracapsular Dissection in Pleomorphic Adenomas of the Parotid Gland: Results After 13 Years of Follow-up. Laryngoscope 2021, 131, E445–E451. [Google Scholar] [CrossRef]
- Wang, H.; Fundakowski, C.; Khurana, J.S.; Jhala, N. Fine-needle aspiration biopsy of salivary gland lesions. Arch. Pathol. Lab. Med. 2015, 139, 1491–1497. [Google Scholar] [CrossRef]
- Fundakowski, C.; Castaño, J.; Abouyared, M.; Lo, K.; Rivera, A.; Ojo, R.; Gomez-Fernandez, C.; Messinger, S.; Sargi, Z. The role of indeterminate fine-needle biopsy in the diagnosis of parotid malignancy. Laryngoscope 2014, 124, 678–681. [Google Scholar] [CrossRef]
- Yabuuchi, H.; Kamitani, T.; Sagiyama, K.; Yamasaki, Y.; Hida, T.; Matsuura, Y.; Hino, T.; Murayama, Y.; Yasumatsu, R.; Yamamoto, H. Characterization of parotid gland tumors: Added value of permeability MR imaging to DWI and DCE-MRI. Eur Radiol. 2020, 30, 6402–6412. [Google Scholar] [CrossRef]
- Stoia, S.; Băciuț, G.; Lenghel, M.; Badea, R.; Csutak, C.; Băciuț, G.M.R.; Tiberiu Tamaș, M.; Boțan, E.; Armencea, G.; Simion Bran, C.D. Cross-sectional imaging and cytologic investigations in the preoperative diagnosis of parotid gland tumors—An updated literature review. Bosn. J. BasicMed. Sci. 2021, 21, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Razek, A.A.K.A.; Samir, S.; Ashmalla, G.A. Characterization of parotid tumors with dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging and diffusion-weighted MR imaging. J. Comput. Assist. Tomogr. 2017, 41, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Yerli, H.; Aydin, E.; Haberal, N.; Harman, A.; Kaskati, T.; Alibek, S. Diagnosing common parotid tumours with magnetic resonance imaging including diffusion-weighted imaging vs. fine-needle aspiration cytology: A comparative study. Dentomaxillofacial Radiol. 2010, 39, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Elmokadem, A.H.; Abdel Khalek, A.M.; Abdel Wahab, R.M.; Tharwat, N.; Gaballa, G.M.; Elata, M.A.; Amer, T. Diagnostic Accuracy of Multiparametric Magnetic Resonance Imaging for Differentiation between Parotid Neoplasms. Can. Assoc. Radiol. J. 2019, 70, 264–272. [Google Scholar] [CrossRef]
- Li, Z.Q.; Gao, J.N.; Xu, S.; Shi, Y.; Liu, X.; Li, X.; Wan, J. Multimodal magnetic resonance imaging for the diagnosis of parotid gland malignancies: Systematic review and meta-analysis. Transl. Cancer Res. 2022, 11, 2275–2282. [Google Scholar] [CrossRef]
- Merino Domingo, F.; Martín Medina, P.; López Fernández, P.; Zafra Vallejo, V.; Salvador Álvarez, E.; Gutiérrez Díaz, R.; Aniceto, G.S.; Ramos, A. Advanced MRI Sequences (Diffusion and Perfusion): Its Value in Parotid Tumors. Int. Arch. Oral Maxillofac. Surg. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Orhan Soylemez, U.P.; Atalay, B. Differentiation of benign and malignant parotid gland tumors with mri and diffusion weighted imaging. Medeni Med. J. 2021, 36, 138–145. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.K.; Elkhamary, S.M.; Nada, N. Correlation of apparent diffusion coefficient with histopathological parameters of salivary gland cancer. Int. J. Oral Maxillofac. Surg. 2019, 48, 995–1000. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- de Oliveira, F.A.; Duarte, E.C.B.; Taveira, C.T.; Máximo, A.A.; de Aquino, É.C.; de Cássia Alencar, R.; Vencio, E.F. Salivary gland tumor: A review of 599 cases in a Brazilian population. Head Neck Pathol. 2009, 3, 271–275. [Google Scholar] [CrossRef]
- Faheem, M.H.; Shady, S.; Refaat, M.M. Role of magnetic resonance imaging (MRI) including diffusion weighted images (DWIs) in assessment of parotid gland masses with histopathological correlation. Egypt J. Radiol. Nucl. Med. 2018, 49, 368–373. [Google Scholar] [CrossRef]
- Psychogios, G.; Vlastos, I.; Thölken, R.; Zenk, J. Warthin’s tumour seems to be the most common benign neoplasm of the parotid gland in Germany. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Tunç, O.; Gönüldaş, B.; Arslanhan, Y.; Kanlıkama, M. Change in Warthin’s tumor incidence: A 20-year joinpoint trend analysis. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3431–3434. [Google Scholar] [CrossRef]
- Christe, A.; Waldherr, C.; Hallett, R.; Zbaeren, P.; Thoeny, H. MR imaging of parotid tumors: Typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. Am. J. Neuroradiol. 2011, 32, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C.Z.; Tanyeri, A.; Ozgur, R.; Ozturk, V.S. Parotid gland tumors: Comparison of conventional and diffusion-weighted MRI findings with histopathological results. Dentomaxillofac. Radiol. 2021, 50, 1–10. [Google Scholar] [CrossRef]
- Coudert, H.; Mirafzal, S.; Dissard, A.; Boyer, L.; Montoriol, P.F. Multiparametric magnetic resonance imaging of parotid tumors: A systematic review. Diagn. Interv. Imaging 2020, 102, 121–130. [Google Scholar] [CrossRef]
- Piludu, F.; Marzi, S.; Ravanelli, M.; Pellini, R.; Covello, R.; Terrenato, I.; Farina, D.; Campora, R.; Ferrazzoli, V.; Vidiri, A. MRI-Based Radiomics to Differentiate between Benign and Malignant Parotid Tumors with External Validation. Front Oncol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Gökçe, E. Multiparametric Magnetic Resonance Imaging for the Diagnosis and Differential Diagnosis of Parotid Gland Tumors. J. Magn. Reson. Imaging 2020, 52, 11–32. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, M.; Zheng, S.; Chen, S.; Xiao, J.; Hu, Z.; Lu, L.; Yang, Z.; Lin, D. Differential diagnosis of parotid gland tumours: Application of SWI combined with DWI and DCE-MRI. Eur. J. Radiol. 2022, 146, 110094. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S.; Pan, A.; Cheng, X.; Gao, M. A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2228–2234. [Google Scholar] [CrossRef]
- Eissa, L.; Abou Seif, S.; El Desooky, S.; Eid, M.; Koraitim, T. Accuracy assessment of combined diffusion weighed and dynamic gadolinium MR sequences in characterization of salivary gland tumors. Egypt J. Radiol. Nucl. Med. 2016, 47, 127–139. [Google Scholar] [CrossRef]
- Tsushima, Y.; Matsumoto, M.; Endo, K.; Aihara, T.; Nakajima, T. Characteristic bright signal of parotid pleomorphic adenomas on T2-weighted MR images with pathological correlation. Clin. Radiol. 1994, 49, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Assili, S.; Fathi Kazerooni, A.; Aghaghazvini, L.; Saligheh Rad, H.R.; Pirayesh Islamian, J. Dynamic contrast magnetic resonance imaging (DCE-MRI) and diffusion weighted MR imaging (DWI) for differentiation between benign and malignant salivary gland tumors. J. Biomed. Phys. Eng. 2015, 5, 157–168. [Google Scholar] [PubMed]
- Maraghelli, D.; Pietragalla, M.; Cordopatri, C.; Nardi, C.; Peired, A.J.; Maggiore, G.; Colagrande, S. Magnetic resonance imaging of salivary gland tumours: Key findings for imaging characterisation. Eur. J. Radiol. 2021, 139, 109716. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Matsuo, Y.; Kamitani, T.; Setoguchi, T.; Okafuji, T.; Soeda, H.; Sakai, S.; Hatakenaka, M.; Nakashima, T.; Oda, Y.; et al. Parotid gland tumors: Can addition of diffusion-weighted MR imaging to dynamic contrast—Enhanced MR imaging improve diagnostic accuracyin characterization? Radiology 2008, 249, 909–916. [Google Scholar] [CrossRef] [PubMed]





| Histopathology: | Benign | Malignant | p–Value |
|---|---|---|---|
| (n = 54) | (n = 11) | ||
| Age (years), median (IQR) | 54 (46–63) | 68 (64–69) | 0.003 |
| Gender (female), n (%) | 32 (58.18) | 5 (45.45) | 0.509 |
| Living environment (urban vs. rural), n (%) | 50 (92.59) | 5 (45.45) | <0.001 |
| Tumor localization (left vs. right), n (%) | 27 (50) | 2 (18.18) | 0.094 |
| Tumor localization within the parotid gland (deep lobe vs. superficial lobe), n (%) | 9 (16.36) | 5 (45.45) | 0.031 |
| Maximum tumor size (mm), median (IQR) | 25 (19–32.75) | 42 (27.5–48.5) | 0.029 |
| Time from onset to presentation (months), median (IQR) | 12 (6–24) | 24 (20–48) | 0.015 |
| Benign | Malignant | |
|---|---|---|
| (n = 54) | (n = 11) | |
| Histopathologic subtype | Warthin tumor: 29 (53.7) | |
| Pleomorphic adenoma 19 (35.18) | Mucoepidermoid carcinoma: 2 (18.18) | |
| Basal cell adenoma: 3 (5.55) | Ductal adenocarcinoma: 1 (9.09) | |
| Oncocytic papillary cystadenoma: 1 (1.85) | Basal cell carcinoma: 1 (9.09) | |
| Parotid gland cyst: 2 (3.7) | Acinic cell carcinoma: 1 (9.09) | |
| Salivary duct carcinoma: 1 (9.09) | ||
| Carcinoma ex pleomorphic adenoma: 1 (9.09) | ||
| Intraductal carcinoma: 1 (9.09) | ||
| Squamous cell carcinoma: 1 (9.09) | ||
| Intraparotid gland melanoma Lymph node metastasis: 1 (9.09) | ||
| Undifferentiated sarcoma: 1 (9.09) |
| MRI Parameters | Histology | ||
|---|---|---|---|
| Benign | Malignant | p–Value | |
| (n = 54) | (n = 11) | ||
| MRI diagnosis, n (%) | |||
| benign | 51 (94.44) | 2 (18.2) | |
| Malignant | 3 (5.56) | 9 (81.8) | |
| MRI, T1 weighted image, n (%) | 0.272 | ||
| Hyperintense signal | 9 (16.67) | 1 (9.09) | |
| Mixed signal intensity | 16 (29.63) | 1 (9.09) | |
| Hypointense signal | 29 (53.7) | 9 (81.82) | |
| MRI, T2 weighted image, n (%) | 0.004 | ||
| Hyperintense signal | 17 (31.48) | 0 (0) | |
| Mixed signal intensity | 27 (50) | 4 (36.36) | |
| Hypointense Signal | 10 (18.52) | 7 (63.64) | |
| DCE MRI*–TIC curve+, n (%) | 0.382 | ||
| Type A | 16 (29.63) | 1 (9.09) | |
| Type B | 18 (33.33) | 5 (45.45) | |
| Type C | 20 (37.04) | 5 (45.45) | |
| DCE MRI, TIC curve grouped (A, B vs. C), n (%) | 34 (62.96) | 6 (54.5S5) | 0.737 |
| DCE MR, TTP (ms), median (IQR) | 133.5 (87.15–203.75) | 143 (138–204.45) | 0.113 |
| DWI MRI, ADC × 10−3 mm2/s, median (IQR) | 1.03 (0.8–1.44) | 0.78 (0.68–0.99) | 0.024 |
| MRI Parameters | Histology | ||
|---|---|---|---|
| Pleomorphic Adenoma | Warthin Tumor | p-Value | |
| n = 19 | n = 29 | ||
| MRI, T1 weighted image, n (%) | 0.024 | ||
| Hyperintense signal | 1 (5.26) | 7 (24.14) | |
| Mixt signal intensity | 3 (15.79) | 11 (37.93) | |
| Hypointense signal | 15 (78.95) | 11 (37.93) | |
| MRI, T2 weighted image, n (%) | <0.001 | ||
| Hyperintense signal | 12 (63.16) | 3 (10.34) | |
| Mixed signal intensity | 6 (31.58) | 20 (68.97) | |
| Hypointense signal | 1 (5.26) | 6 (20.69) | |
| DCE MRI, TIC curve, n (%) | <0.001 | ||
| Type A | 14 (73.68) | 0 (0) | |
| Type A, C | 1 (5.26) | 0 (0) | |
| Type B | 3 (15.79) | 11 (37.93) | |
| Type C | 1 (5.26) | 18 (62.07) | |
| DCE MRI, TTP (ms), median (IQR) | 210 (166.15–228.9) | 88.5 (82–101.9) | <0.001 |
| DWI MRI, ADC × 10−3 mm2/s, median (IQR) | 1.5 (1.25–2.1) | 0.86 (0.73–1) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoia, S.; Lenghel, M.; Dinu, C.; Tamaș, T.; Bran, S.; Băciuț, M.; Boțan, E.; Leucuța, D.; Armencea, G.; Onișor, F.; et al. The Value of Multiparametric Magnetic Resonance Imaging in the Preoperative Differential Diagnosis of Parotid Gland Tumors. Cancers 2023, 15, 1325. https://doi.org/10.3390/cancers15041325
Stoia S, Lenghel M, Dinu C, Tamaș T, Bran S, Băciuț M, Boțan E, Leucuța D, Armencea G, Onișor F, et al. The Value of Multiparametric Magnetic Resonance Imaging in the Preoperative Differential Diagnosis of Parotid Gland Tumors. Cancers. 2023; 15(4):1325. https://doi.org/10.3390/cancers15041325
Chicago/Turabian StyleStoia, Sebastian, Manuela Lenghel, Cristian Dinu, Tiberiu Tamaș, Simion Bran, Mihaela Băciuț, Emil Boțan, Daniel Leucuța, Gabriel Armencea, Florin Onișor, and et al. 2023. "The Value of Multiparametric Magnetic Resonance Imaging in the Preoperative Differential Diagnosis of Parotid Gland Tumors" Cancers 15, no. 4: 1325. https://doi.org/10.3390/cancers15041325
APA StyleStoia, S., Lenghel, M., Dinu, C., Tamaș, T., Bran, S., Băciuț, M., Boțan, E., Leucuța, D., Armencea, G., Onișor, F., & Băciuț, G. (2023). The Value of Multiparametric Magnetic Resonance Imaging in the Preoperative Differential Diagnosis of Parotid Gland Tumors. Cancers, 15(4), 1325. https://doi.org/10.3390/cancers15041325









