Optimal Use of Novel Immunotherapeutics in B-Cell Precursor ALL
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immunotherapies
2.1. Blinatumomab
2.2. Inotuzumab Ozogamicin
2.3. Chimeric Antigen Receptor T Cells
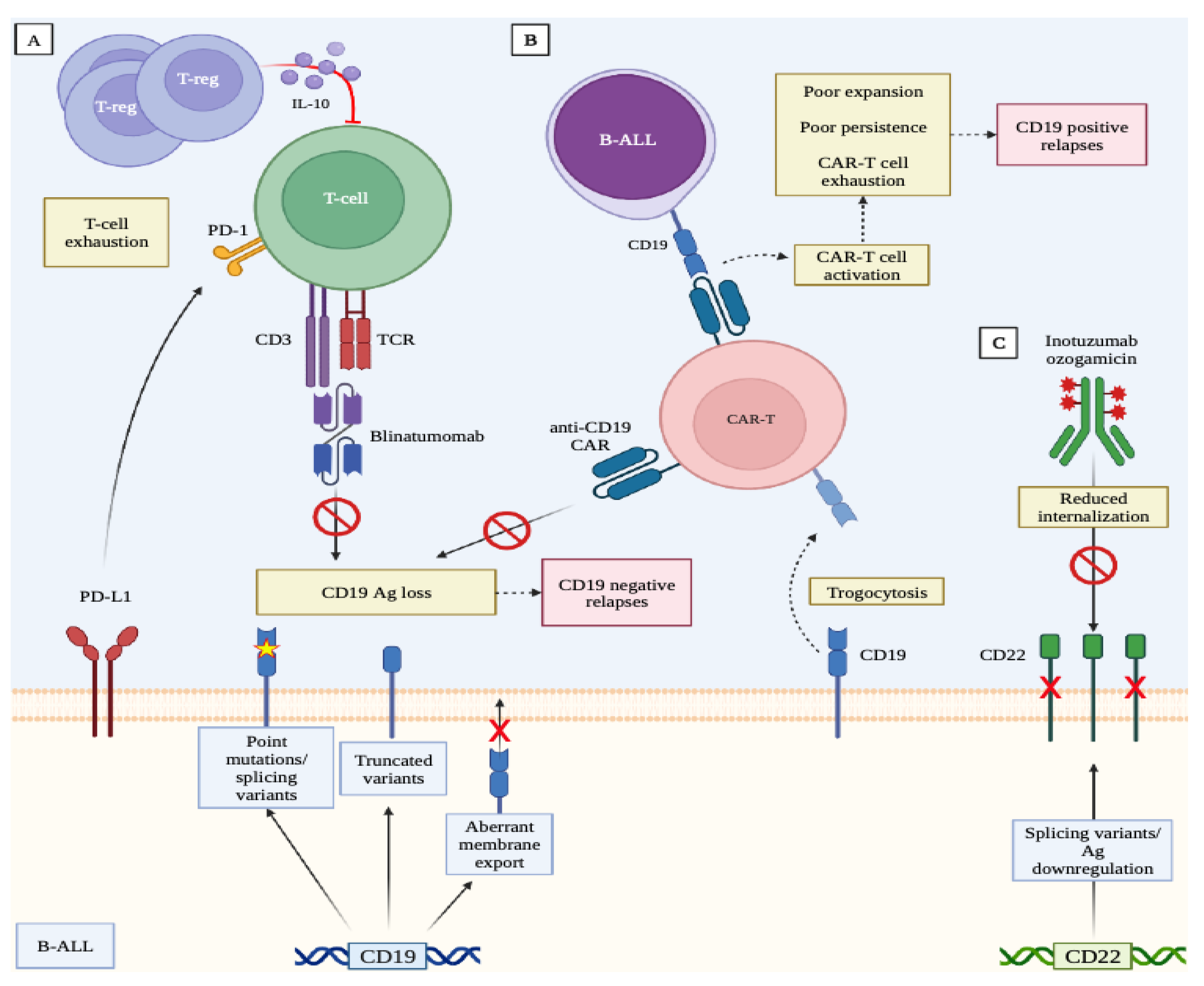
3. Mechanisms of Resistance to Immunotherapies
4. Optimal Use of the Different Immunotherapies According to Specific Clinical Conditions
4.1. Extramedullary Relapse
4.2. High-Burden Disease at Relapse
4.3. Blinatumomab, Inotuzumab, and CAR-T Cells: Do They Stand Alone or Bridge Treatments to Allogeneic Transplants?
5. Future Perspectives
5.1. Anti-CD19 and Anti-CD22 Immunotherapies as Frontline Treatments
5.2. Should Less Intensive Chemotherapy or a Chemo-Free Approach Be Used to Treat Old and Frail Patients?
5.3. The Use of Immunotherapies in Ph-Positive ALL
5.4. Combination with Checkpoint Inhibitors
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (Bispecific T-Cell Engager) Platform: Development and Future Potential of a Targeted Immuno-Oncology Therapy across Tumor Types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef]
- Topp, M.S.; Gokbuget, N.; Zugmaier, G.; Klappers, P.; Stelljes, M.; Neumann, S.; Viardot, A.; Marks, R.; Diedrich, H.; Faul, C.; et al. Phase II Trial of the Anti-CD19 Bispecific T Cell-Engager Blinatumomab Shows Hematologic and Molecular Remissions in Patients with Relapsed or Refractory B-Precursor Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2014, 32, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Gokbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and Activity of Blinatumomab for Adult Patients with Relapsed or Refractory B-Precursor Acute Lymphoblastic Leukaemia: A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Gore, L.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; O’Brien, M.M.; Bader, P.; Bhojwani, D.; Schlegel, P.-G.; Tuglus, C.A.; von Stackelberg, A. Survival after Blinatumomab Treatment in Pediatric Patients with Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Blood Cancer J. 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of Postreinduction Therapy Consolidation with Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults with First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Zugmaier, G.; Rizzari, C.; Morris, J.D.; Gruhn, B.; Klingebiel, T.; Parasole, R.; Linderkamp, C.; Flotho, C.; Petit, A.; et al. Effect of Blinatumomab vs. Chemotherapy on Event-Free Survival Among Children with High-Risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 843–854. [Google Scholar] [CrossRef]
- Martinelli, G.; Boissel, N.; Chevallier, P.; Ottmann, O.; Gokbuget, N.; Topp, M.S.; Fielding, A.K.; Rambaldi, A.; Ritchie, E.K.; Papayannidis, C.; et al. Complete Hematologic and Molecular Response in Adult Patients with Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment with Blinatumomab: Results from a Phase II, Single-Arm, Multicenter Study. J. Clin. Oncol. 2017, 35, 1795–1802. [Google Scholar] [CrossRef]
- Rambaldi, A.; Ribera, J.-M.; Kantarjian, H.M.; Dombret, H.; Ottmann, O.G.; Stein, A.S.; Tuglus, C.A.; Zhao, X.; Kim, C.; Martinelli, G. Blinatumomab Compared with Standard of Care for the Treatment of Adult Patients with Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia. Cancer 2020, 126, 304–310. [Google Scholar] [CrossRef]
- Gokbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Bruggemann, M.; Horst, H.-A.; et al. Blinatumomab for Minimal Residual Disease in Adults with B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef]
- Boissel, N.; Chiaretti, S.; Papayannidis, C.; Ribera, J.M.; Bassan, R.; Sokolov, A.N.; Alam, N.; Brescianini, A.; Pezzani, I.; Kreuzbauer, G.; et al. Real-World Use of Blinatumomab in Adult Patients with B-Cell Acute Lymphoblastic Leukemia in Clinical Practice: Results from the NEUF Study. Blood Cancer J. 2023, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Martínez Sánchez, P.; Zugmaier, G.; Gordon, P.; Jabbour, E.; Rifón Roca, J.J.; Schwartz, S.; Borlenghi, E.; Huguet, F.; Hernández-Rivas, J.M.; Lussana, F.; et al. Safety and Pharmacokinetics of Subcutaneous Blinatumomab (SC Blinatumomab) for the Treatment of Adults with Relapsed or Refractory B Cell Precursor Acute Lymphoblastic Leukemia (R/R B-ALL); Results from a Phase 1b Study. Blood 2022, 140, 6122–6124. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; Liang White, J.; et al. Inotuzumab Ozogamicin versus Standard of Care in Relapsed or Refractory Acute Lymphoblastic Leukemia: Final Report and Long-Term Survival Follow-up from the Randomized, Phase 3 INO-VATE Study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; Advani, A.S.; Marks, D.I.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; Jabbour, E.; Merchant, A.; Wang, T.; et al. Inotuzumab Ozogamicin for Relapsed/Refractory Acute Lymphoblastic Leukemia: Outcomes by Disease Burden. Blood Cancer J. 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kantarjian, H.; Ravandi, F.; Short, N.; Kebriaei, P.; Huang, X.; Jain, N.; Konopleva, M.; Garcia-Manero, G.; Champlin, R.; et al. Long-Term Follow-up of the Combination of Low-Intensity Chemotherapy Plus Inotuzumab Ozogamicin with or without Blinatumomab in Patients with Relapsed-Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Phase 2 Trial. Blood 2020, 136, 40–42. [Google Scholar] [CrossRef]
- Jabbour, E.; Sasaki, K.; Ravandi, F.; Huang, X.; Short, N.J.; Khouri, M.; Kebriaei, P.; Burger, J.; Khoury, J.; Jorgensen, J.; et al. Chemoimmunotherapy with Inotuzumab Ozogamicin Combined with Mini-Hyper-CVD, with or without Blinatumomab, Is Highly Effective in Patients with Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia in First Salvage. Cancer 2018, 124, 4044–4055. [Google Scholar] [CrossRef]
- Jabbour, E.; Sasaki, K.; Short, N.J.; Ravandi, F.; Huang, X.; Khoury, J.D.; Kanagal-Shamanna, R.; Jorgensen, J.; Khouri, I.F.; Kebriaei, P.; et al. Long-Term Follow-up of Salvage Therapy Using a Combination of Inotuzumab Ozogamicin and Mini-Hyper-CVD with or without Blinatumomab in Relapsed/Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia. Cancer 2021, 127, 2025–2038. [Google Scholar] [CrossRef]
- Jabbour, E.J.; Sasaki, K.; Ravandi, F.; Short, N.J.; Garcia-Manero, G.; Daver, N.; Kadia, T.; Konopleva, M.; Jain, N.; Cortes, J.; et al. Inotuzumab Ozogamicin in Combination with Low-Intensity Chemotherapy (Mini-HCVD) with or without Blinatumomab versus Standard Intensive Chemotherapy (HCVAD) as Frontline Therapy for Older Patients with Philadelphia Chromosome-Negative Acute Lymphoblastic. Cancer 2019, 125, 2579–2586. [Google Scholar] [CrossRef]
- Advani, A.S.; Moseley, A.; Liedtke, M.; O’Donnell, M.R.; Aldoss, I.; Mims, M.P.; O’Dwyer, K.M.; Othus, M.; Erba, H.P. SWOG 1312 Final Results: A Phase 1 Trial of Inotuzumab in Combination with CVP (Cyclophosphamide, Vincristine, Prednisone) for Relapsed/ Refractory CD22+ Acute Leukemia. Blood 2019, 134, 227. [Google Scholar] [CrossRef]
- O’Brien, M.M.; Ji, L.; Shah, N.N.; Rheingold, S.R.; Bhojwani, D.; Yuan, C.M.; Xu, X.; Yi, J.S.; Harris, A.C.; Brown, P.A.; et al. Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children’s Oncology Group Protocol AALL1621. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Teachey, D.T.; Porter, D.L.; Grupp, S.A. CD19-Targeted Chimeric Antigen Receptor T-Cell Therapy for Acute Lymphoblastic Leukemia. Blood 2015, 125, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Rives, S.; Maude, S.L.; Hiramatsu, H.; Baruchel, A.; Bader, P.; Bittencourt, H.; Buechner, J.; Laetsch, T.; De Moerloose, B.; Qayed, M.; et al. S112: Tisagenlecleucel In Pediatric and Young Adult Patients (Pts) With Relapsed/Refractory (R/R) B-Cell Acute Lymphoblastic Leukemia (B-All): Final Analyses from The Eliana Study. HemaSphere 2022, 6, 13–14. [Google Scholar] [CrossRef]
- Schultz, L.M.; Baggott, C.; Prabhu, S.; Pacenta, H.L.; Phillips, C.L.; Rossoff, J.; Stefanski, H.E.; Talano, J.-A.; Moskop, A.; Margossian, S.P.; et al. Disease Burden Affects Outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia After Commercial Tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 945–955. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Hu, Z.-H.; Curran, K.; Laetsch, T.; Locke, F.; Rouce, R.; Pulsipher, M.A.; Phillips, C.L.; Keating, A.; Frigault, M.J.; et al. Real-World Evidence of Tisagenlecleucel for Pediatric Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma. Blood Adv. 2020, 4, 5414–5424. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for Relapsed or Refractory Adult B-Cell Acute Lymphoblastic Leukaemia: Phase 2 Results of the Single-Arm, Open-Label, Multicentre ZUMA-3 Study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Grover, P.; Veilleux, O.; Tian, L.; Sun, R.; Previtera, M.; Curran, E.; Muffly, L. Chimeric Antigen Receptor T-Cell Therapy in Adults with B-Cell Acute Lymphoblastic Leukemia. Blood Adv. 2022, 6, 1608–1618. [Google Scholar] [CrossRef]
- Hay, K.A.; Gauthier, J.; Hirayama, A.V.; Voutsinas, J.M.; Wu, Q.; Li, D.; Gooley, T.A.; Cherian, S.; Chen, X.; Pender, B.S.; et al. Factors Associated with Durable EFS in Adult B-Cell ALL Patients Achieving MRD-Negative CR after CD19 CAR T-Cell Therapy. Blood 2019, 133, 1652–1663. [Google Scholar] [CrossRef]
- Frey, N.V.; Shaw, P.A.; Hexner, E.O.; Pequignot, E.; Gill, S.; Luger, S.M.; Mangan, J.K.; Loren, A.W.; Perl, A.E.; Maude, S.L.; et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults with Acute Lymphoblastic Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 415–422. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Yin, P.; Guo, T.; Liu, L.; Xia, L.; Wu, Y.; Zhou, F.; Ai, L.; Shi, W.; et al. Anti-CD19 Chimeric Antigen Receptor-Modified T-Cell Therapy Bridging to Allogeneic Hematopoietic Stem Cell Transplantation for Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia: An Open-Label Pragmatic Clinical Trial. Am. J. Hematol. 2019, 94, 1113–1122. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and Safety of Anti-CD19 CAR T-Cell Therapy in 110 Patients with B-Cell Acute Lymphoblastic Leukemia with High-Risk Features. Blood Adv. 2020, 4, 2325–2338. [Google Scholar] [CrossRef]
- Roddie, C.; Dias, J.; O’Reilly, M.A.; Abbasian, M.; Cadinanos-Garai, A.; Vispute, K.; Bosshard-Carter, L.; Mitsikakou, M.; Mehra, V.; Roddy, H.; et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3352–3363. [Google Scholar] [CrossRef]
- Yang, J.; He, J.; Zhang, X.; Li, J.; Wang, Z.; Zhang, Y.; Qiu, L.; Wu, Q.; Sun, Z.; Ye, X.; et al. Next-Day Manufacture of a Novel Anti-CD19 CAR-T Therapy for B-Cell Acute Lymphoblastic Leukemia: First-in-Human Clinical Study. Blood Cancer J. 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; Jaeger, U.; Shah, N.N.; Blaise, D.; Briones, J.; Shune, L.; Boissel, N.; Bondanza, A.; Lu, D.; Zhu, X.; et al. A First-in-Human Study of YTB323, a Novel, Autologous CD19-Directed CAR-T Cell Therapy Manufactured Using the Novel T-Charge TM Platform, for the Treatment of Patients (Pts) with Relapsed/Refractory (r/r) Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2021, 138, 740. [Google Scholar] [CrossRef]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; et al. Sleeping Beauty-Engineered CAR T Cells Achieve Antileukemic Activity without Severe Toxicities. J. Clin. Investig. 2020, 130, 6021–6033. [Google Scholar] [CrossRef]
- Lussana, F.; Magnani, C.F.; Gaipa, G.; Galimberti, S.; Gritti, G.; Belotti, D.; Napolitano, S.; Buracchi, C.; Borleri, G.M.; Rambaldi, B.; et al. Final Results of Phase I/II Study of Donor-Derived CAR T Cells Engineered with Sleeping Beauty in Pediatric and Adult Patients with B-Cell Acute Lymphoblastic Leukemia Relapsed Post-HSCT. Blood 2022, 140, 4568–4569. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a First-in-Class Allogeneic Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia (CALM): A Phase 1, Dose-Escalation Trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.N.; Liu, B.; Di Pierro, E.J.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef] [PubMed]
- Poels, R.; Drent, E.; Lameris, R.; Katsarou, A.; Themeli, M.; van der Vliet, H.J.; de Gruijl, T.D.; van de Donk, N.W.C.J.; Mutis, T. Preclinical Evaluation of Invariant Natural Killer T Cells Modified with CD38 or BCMA Chimeric Antigen Receptors for Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.; Gardner, R. Mechanisms of and Approaches to Overcoming Resistance to Immunotherapy. Hematol. Am. Soc. Hematol. Educ. Progr. 2019, 2019, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Forman, S.J. How I Treat Adults with Advanced Acute Lymphoblastic Leukemia Eligible for CD19-Targeted Immunotherapy. Blood 2020, 135, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Braig, F.; Brandt, A.; Goebeler, M.; Tony, H.-P.; Kurze, A.-K.; Nollau, P.; Bumm, T.; Bottcher, S.; Bargou, R.C.; Binder, M. Resistance to Anti-CD19/CD3 BiTE in Acute Lymphoblastic Leukemia May Be Mediated by Disrupted CD19 Membrane Trafficking. Blood 2017, 129, 100–104. [Google Scholar] [CrossRef]
- Zhao, Y.; Aldoss, I.; Qu, C.; Crawford, J.C.; Gu, Z.; Allen, E.K.; Zamora, A.E.; Alexander, T.B.; Wang, J.; Goto, H.; et al. Tumor-Intrinsic and -Extrinsic Determinants of Response to Blinatumomab in Adults with B-ALL. Blood 2021, 137, 471–484. [Google Scholar] [CrossRef]
- Aldoss, I.; Song, J.Y. Extramedullary Relapse of KMT2A(MLL)-Rearranged Acute Lymphoblastic Leukemia with Lineage Switch Following Blinatumomab. Blood 2018, 131, 2507. [Google Scholar] [CrossRef]
- Feucht, J.; Kayser, S.; Gorodezki, D.; Hamieh, M.; Doring, M.; Blaeschke, F.; Schlegel, P.; Bosmuller, H.; Quintanilla-Fend, L.; Ebinger, M.; et al. T-Cell Responses against CD19+ Pediatric Acute Lymphoblastic Leukemia Mediated by Bispecific T-Cell Engager (BiTE) Are Regulated Contrarily by PD-L1 and CD80/CD86 on Leukemic Blasts. Oncotarget 2016, 7, 76902–76919. [Google Scholar] [CrossRef]
- Duell, J.; Dittrich, M.; Bedke, T.; Mueller, T.; Eisele, F.; Rosenwald, A.; Rasche, L.; Hartmann, E.; Dandekar, T.; Einsele, H.; et al. Frequency of Regulatory T Cells Determines the Outcome of the T-Cell-Engaging Antibody Blinatumomab in Patients with B-Precursor ALL. Leukemia 2017, 31, 2181–2190. [Google Scholar] [CrossRef]
- Kohnke, T.; Krupka, C.; Tischer, J.; Knosel, T.; Subklewe, M. Increase of PD-L1 Expressing B-Precursor ALL Cells in a Patient Resistant to the CD19/CD3-Bispecific T Cell Engager Antibody Blinatumomab. J. Hematol. Oncol. 2015, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.; Papayannidis, C.; Lussana, F.; Fracchiolla, N.; Annunziata, M.; Sica, S.; Delia, M.; Foà, R.; Pizzolo, G.; Chiaretti, S. Real-World Multicenter Experience in Tumor Debulking Prior to Blinatumomab Administration in Adult Patients with Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 11, 804714. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Litzow, M.R. Management of Toxicities Associated with Novel Immunotherapy Agents in Acute Lymphoblastic Leukemia. Ther. Adv. Hematol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Lamble, A.; Myers, R.M.; Taraseviciute, A.; John, S.; Yates, B.; Steinberg, S.M.; Sheppard, J.D.; Kovach, A.E.; Wood, B.L.; Borowitz, M.J.; et al. Preinfusion Factors Impacting Relapse Immunophenotype Following CD19 CAR T Cells. Blood Adv. 2022. [Google Scholar] [CrossRef]
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic Mechanisms of Target Antigen Loss in CAR19 Therapy of Acute Lymphoblastic Leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef]
- Bueno, C.; Barrera, S.; Bataller, A.; Ortiz-Maldonado, V.; Elliot, N.; O’Byrne, S.; Wang, G.; Rovira, M.; Gutierrez-Agüera, F.; Trincado, J.L.; et al. CD34+CD19-CD22+ B-Cell Progenitors May Underlie Phenotypic Escape in Patients Treated with CD19-Directed Therapies. Blood 2022, 140, 38–44. [Google Scholar] [CrossRef]
- Fischer, J.; Paret, C.; El Malki, K.; Alt, F.; Wingerter, A.; Neu, M.A.; Kron, B.; Russo, A.; Lehmann, N.; Roth, L.; et al. CD19 Isoforms Enabling Resistance to CART-19 Immunotherapy Are Expressed in B-ALL Patients at Initial Diagnosis. J. Immunother. 2017, 40, 187–195. [Google Scholar] [CrossRef]
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of Resistance to Chimeric Antigen Receptor T Cell Therapy by Transduction of a Single Leukemic B Cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; van der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T Cell Trogocytosis and Cooperative Killing Regulate Tumour Antigen Escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Sadelain, M. Myeloid Leukemia Switch as Immune Escape from CD19 Chimeric Antigen Receptor (CAR) Therapy. Transl. Cancer Res. 2016, 5, S221–S225. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, E.; Nguyen, S.M.; Fountaine, T.J.; Welp, K.; Gryder, B.; Qin, H.; Yang, Y.; Chien, C.D.; Seif, A.E.; Lei, H.; et al. CD19 CAR Immune Pressure Induces B-Precursor Acute Lymphoblastic Leukaemia Lineage Switch Exposing Inherent Leukaemic Plasticity. Nat. Commun. 2016, 7, 12320. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.; Wu, D.; Cherian, S.; Fang, M.; Hanafi, L.-A.; Finney, O.; Smithers, H.; Jensen, M.C.; Riddell, S.R.; Maloney, D.G.; et al. Acquisition of a CD19-Negative Myeloid Phenotype Allows Immune Escape of MLL-Rearranged B-ALL from CD19 CAR-T-Cell Therapy. Blood 2016, 127, 2406–2410. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-Cell for Acute Lymphoblastic Leukemia (ALL) Treatment: A Review Study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef]
- Paul, M.R.; Wong, V.; Aristizabal, P.; Kuo, D.J. Treatment of Recurrent Refractory Pediatric Pre-B Acute Lymphoblastic Leukemia Using Inotuzumab Ozogamicin Monotherapy Resulting in CD22 Antigen Expression Loss as a Mechanism of Therapy Resistance. J. Pediatr. Hematol. Oncol. 2019, 41, e546–e549. [Google Scholar] [CrossRef]
- Reinert, J.; Beitzen-Heineke, A.; Wethmar, K.; Stelljes, M.; Fiedler, W.; Schwartz, S. Loss of CD22 Expression and Expansion of a CD22(Dim) Subpopulation in Adults with Relapsed/Refractory B-Lymphoblastic Leukaemia after Treatment with Inotuzumab-Ozogamicin. Ann. Hematol. 2021, 100, 2727–2732. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Stock, W.; Cassaday, R.D.; DeAngelo, D.J.; Jabbour, E.J.; O’Brien, S.M.; Stelljes, M.; Wang, T.; Liau, K.F.; Nguyen, K.; et al. Comparison of CD22 Expression between Baseline, End of Treatment, and Relapse Among Patients Treated with Inotuzumab Ozogamicin Who Responded and Subsequently Relapsed in Two Clinical Trials. Blood 2018, 132, 2699. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Stock, W.; Cassaday, R.D.; DeAngelo, D.J.; Jabbour, E.; O’Brien, S.M.; Stelljes, M.; Wang, T.; Paccagnella, M.L.; Nguyen, K.; et al. Inotuzumab Ozogamicin for Relapsed/Refractory Acute Lymphoblastic Leukemia in the INO-VATE Trial: CD22 Pharmacodynamics, Efficacy, and Safety by Baseline CD22. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2742–2754. [Google Scholar] [CrossRef]
- Zheng, S.; Gillespie, E.; Naqvi, A.S.; Hayer, K.E.; Ang, Z.; Torres-Diz, M.; Quesnel-Vallières, M.; Hottman, D.A.; Bagashev, A.; Chukinas, J.; et al. Modulation of CD22 Protein Expression in Childhood Leukemia by Pervasive Splicing Aberrations: Implications for CD22-Directed Immunotherapies. Blood Cancer Discov. 2022, 3, 103–115. [Google Scholar] [CrossRef]
- Aldoss, I.; Song, J.; Stiller, T.; Nguyen, T.; Palmer, J.; O’Donnell, M.; Stein, A.S.; Marcucci, G.; Forman, S.; Pullarkat, V. Correlates of Resistance and Relapse during Blinatumomab Therapy for Relapsed/Refractory Acute Lymphoblastic Leukemia. Am. J. Hematol. 2017, 92, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Otoukesh, S.; Zhang, J.; Mokhtari, S.; Ngo, D.; Mojtahedzadeh, M.; Al Malki, M.M.; Salhotra, A.; Ali, H.; Aribi, A.; et al. Extramedullary Disease Relapse and Progression after Blinatumomab Therapy for Treatment of Acute Lymphoblastic Leukemia. Cancer 2022, 128, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.M.; Yates, B.; Ling, A.; Yuan, C.M.; Wang, H.-W.; Stetler-Stevenson, M.; LaLoggia, M.; Molina, J.C.; Lichtenstein, D.A.; Lee, D.W.; et al. Characterization of Extramedullary Disease in B-ALL and Response to CAR T-Cell Therapy. Blood Adv. 2022, 6, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Frigault, M.J.; Dietrich, J.; Gallagher, K.; Roschewski, M.; Jordan, J.T.; Forst, D.; Plotkin, S.R.; Cook, D.; Casey, K.S.; Lindell, K.A.; et al. Safety and Efficacy of Tisagenlecleucel in Primary CNS Lymphoma: A Phase 1/2 Clinical Trial. Blood 2022, 139, 2306–2315. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, M.; Hu, Y.; Wang, Y.; Li, P.; Cao, J.; Shi, M.; Tan, J.; Zhang, M.; Xiao, X.; et al. Efficacy and Safety of CD19-Specific CAR T Cell–Based Therapy in B-Cell Acute Lymphoblastic Leukemia Patients with CNSL. Blood 2022, 139, 3376–3386. [Google Scholar] [CrossRef]
- Shah, N.N.; Lee, D.W.; Yates, B.; Yuan, C.M.; Shalabi, H.; Martin, S.; Wolters, P.L.; Steinberg, S.M.; Baker, E.H.; Delbrook, C.P.; et al. Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. J. Clin. Oncol. 2021, 39, 1650–1659. [Google Scholar] [CrossRef]
- Wei, A.H.; Ribera, J.-M.; Larson, R.A.; Ritchie, D.; Ghobadi, A.; Chen, Y.; Anderson, A.; Dos Santos, C.E.; Franklin, J.; Kantarjian, H. Biomarkers Associated with Blinatumomab Outcomes in Acute Lymphoblastic Leukemia. Leukemia 2021, 35, 2220–2231. [Google Scholar] [CrossRef]
- Cabannes-Hamy, A.; Brissot, E.; Leguay, T.; Huguet, F.; Chevallier, P.; Hunault, M.; Escoffre-Barbe, M.; Cluzeau, T.; Balsat, M.; Nguyen, S.; et al. High Tumor Burden before Blinatumomab Has a Negative Impact on the Outcome of Adult Patients with B-Cell Precursor Acute Lymphoblastic Leukemia. A Real-World Study by the GRAALL. Haematologica 2022, 107, 2072–2080. [Google Scholar] [CrossRef]
- King, A.C.; Bolanos, R.; Velasco, K.; Tu, H.; Zaman, F.; Geyer, M.B.; Park, J.H. Real World Chart Review of Blinatumomab to Treat Patients with High Disease Burden of Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2019, 134, 5079. [Google Scholar] [CrossRef]
- Myers, R.M.; Taraseviciute, A.; Steinberg, S.M.; Lamble, A.J.; Sheppard, J.; Yates, B.; Kovach, A.E.; Wood, B.; Borowitz, M.J.; Stetler-Stevenson, M.; et al. Blinatumomab Nonresponse and High-Disease Burden Are Associated with Inferior Outcomes After CD19-CAR for B-ALL. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 932–944. [Google Scholar] [CrossRef]
- Jabbour, E.J.; Gokbuget, N.; Kantarjian, H.M.; Thomas, X.; Larson, R.A.; Yoon, S.-S.; Ghobadi, A.; Topp, M.S.; Tran, Q.; Franklin, J.L.; et al. Transplantation in Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia Who Are Treated with Blinatumomab from a Phase 3 Study. Cancer 2019, 125, 4181–4192. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.; Stein, A.S.; Zugmaier, G.; Dombret, H.; Brueggemann, M.; Bonifacio, M.; Dong, X.; Kantarjian, H.M.; Bargou, R.C.; Gökbuget, N. Long-Term Survival of Adults with B-Cell Precursor (BCP) Acute Lymphoblastic Leukemia (ALL) after Treatment with Blinatumomab and Subsequent Allogeneic Hematopoietic Stem Cell Transplantation (HSCT). J. Clin. Oncol. 2018, 36, 7044. [Google Scholar] [CrossRef]
- Rambaldi, A.; Huguet, F.; Zak, P.; Cannell, P.; Tran, Q.; Franklin, J.; Topp, M.S. Blinatumomab Consolidation and Maintenance Therapy in Adults with Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia. Blood Adv. 2020, 4, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Yang, D.; Mokhtari, S.; Malki, M.M.A.; Ali, H.; Sandhu, K.S.; Aribi, A.; Khaled, S.; Mei, M.; Budde, E.; et al. Outcomes of Allogeneic Hematopoietic Cell Transplantation after Salvage Therapy with Blinatumomab in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. Biol. Blood Marrow Transplant. 2020, 26, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.; Martinelli, G.; Stelljes, M.; DeAngelo, D.J.; Gökbuget, N.; Advani, A.S.; O’Brien, S.; Liedtke, M.; Merchant, A.A.; Cassaday, R.D.; et al. Efficacy of Inotuzumab Ozogamicin in Patients with Philadelphia Chromosome-Positive Relapsed/Refractory Acute Lymphoblastic Leukemia. Cancer 2021, 127, 905–913. [Google Scholar] [CrossRef]
- Jabbour, E.; Gökbuget, N.; Advani, A.; Stelljes, M.; Stock, W.; Liedtke, M.; Martinelli, G.; O’Brien, S.; Wang, T.; Laird, A.D.; et al. Impact of Minimal Residual Disease Status in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia Treated with Inotuzumab Ozogamicin in the Phase III INO-VATE Trial. Leuk. Res. 2020, 88, 106283. [Google Scholar] [CrossRef]
- Marks, D.I.; Kebriaei, P.; Stelljes, M.; Gökbuget, N.; Kantarjian, H.; Advani, A.S.; Merchant, A.; Stock, W.; Cassaday, R.D.; Wang, T.; et al. Outcomes of Allogeneic Stem Cell Transplantation after Inotuzumab Ozogamicin Treatment for Relapsed or Refractory Acute Lymphoblastic Leukemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, 1720–1729. [Google Scholar] [CrossRef]
- Lee III, D.W.; Stetler-Stevenson, M.; Yuan, C.M.; Shah, N.N.; Delbrook, C.; Yates, B.; Zhang, H.; Zhang, L.; Kochenderfer, J.N.; Rosenberg, S.A.; et al. Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL Are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation. Blood 2016, 128, 218. [Google Scholar] [CrossRef]
- Bassan, R.; Chiaretti, S.; Starza, I.D.; Spinelli, O.; Santoro, A.; Elia, L.; Vitale, A.; Taherinasab, A.; Piccini, M.; Ferrara, F.; et al. Preliminary Results of the GIMEMA LAL2317 Sequential Chemotherapy-Blinatumomab Front-Line Trial for Newly Diagnosed Adult PH-Negative B-Lineage ALL Patients. EHA Libr. 2021, 5, S114. [Google Scholar]
- Jabbour, E.; Short, N.J.; Jain, N.; Thompson, P.A.; Kadia, T.M.; Ferrajoli, A.; Huang, X.; Yilmaz, M.; Alvarado, Y.; Patel, K.P.; et al. Hyper-CVAD and Sequential Blinatumomab for Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphocytic Leukaemia: A Single-Arm, Single-Centre, Phase 2 Trial. Lancet. Haematol. 2022, 9, e878–e885. [Google Scholar] [CrossRef]
- Litzow, M.R.; Sun, Z.; Paietta, E.; Mattison, R.J.; Lazarus, H.M.; Rowe, J.M.; Arber, D.A.; Mullighan, C.G.; Willman, C.L.; Zhang, Y.; et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase 3 Nationa. Blood 2022, 140, LBA-1. [Google Scholar] [CrossRef]
- Aldoss, I.; Forman, S.J.; Pullarkat, V. Acute Lymphoblastic Leukemia in the Older Adult. J. Oncol. Pract. 2019, 15, 67–75. [Google Scholar] [CrossRef]
- Short, N.J.; Kantarjian, H.M.; Ravandi, F.; Huang, X.; Jain, N.; Kadia, T.M.; Khoury, J.D.; Jorgensen, J.L.; Wang, S.A.; Alvarado, Y.; et al. Reduced-Intensity Chemotherapy with Mini-Hyper-CVD Plus Inotuzumab Ozogamicin, with or without Blinatumomab, in Older Adults with Newly Diagnosed Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: Results from a Phase II Study. Blood 2020, 136, 15–17. [Google Scholar] [CrossRef]
- Stelljes, M.; Raffel, S.; Wäsch, R.; Scholl, S.; Kondakci, M.; Rank, A.; Haenel, M.; Martin, S.; Schwab, K.; Knaden, J.; et al. First Results of an Open Label Phase II Study to Evaluate the Efficacy and Safety of Inotuzumab Ozogamicin for Induction Therapy Followed by a Conventional Chemotherapy Based Consolidation and Maintenance Therapy in Patients Aged 56 Years and Older with AAcute Lymphoblastic Leukemia (INITIAL-1 trial). Blood 2020, 136, 12–13. [Google Scholar] [CrossRef]
- Advani, A.S.; Moseley, A.; O’Dwyer, K.M.; Wood, B.L.; Fang, M.; Wieduwilt, M.J.; Aldoss, I.; Park, J.H.; Klisovic, R.B.; Baer, M.R.; et al. SWOG 1318: A Phase II Trial of Blinatumomab Followed by POMP Maintenance in Older Patients with Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1574–1582. [Google Scholar] [CrossRef]
- Vignetti, M.; Fazi, P.; Cimino, G.; Martinelli, G.; Di Raimondo, F.; Ferrara, F.; Meloni, G.; Ambrosetti, A.; Quarta, G.; Pagano, L.; et al. Imatinib plus Steroids Induces Complete Remissions and Prolonged Survival in Elderly Philadelphia Chromosome-Positive Patients with Acute Lymphoblastic Leukemia without Additional Chemotherapy: Results of the Gruppo Italiano Malattie Ematologiche Dell’Ad. Blood 2007, 109, 3676–3678. [Google Scholar] [CrossRef]
- Foà, R.; Vitale, A.; Vignetti, M.; Meloni, G.; Guarini, A.; De Propris, M.S.; Elia, L.; Paoloni, F.; Fazi, P.; Cimino, G.; et al. Dasatinib as First-Line Treatment for Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Blood 2011, 118, 6521–6528. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Papayannidis, C.; Piciocchi, A.; Robustelli, V.; Soverini, S.; Terragna, C.; Marconi, G.; Lemoli, R.M.; Guolo, F.; Fornaro, A.; et al. INCB84344-201: Ponatinib and Steroids in Frontline Therapy for Unfit Patients with Ph+ Acute Lymphoblastic Leukemia. Blood Adv. 2022, 6, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Short, N.J.; Jain, N.; Huang, X.; Montalban-Bravo, G.; Banerjee, P.; Rezvani, K.; Jiang, X.; Kim, K.H.; Kanagal-Shamanna, R.; et al. Ponatinib and Blinatumomab for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia: A US, Single-Centre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2023, 10, e24–e34. [Google Scholar] [CrossRef]
- Webster, J.; Luskin, M.R.; Prince, G.T.; DeZern, A.E.; DeAngelo, D.J.; Levis, M.J.; Blackford, A.; Sharon, E.; Streicher, H.; Luznik, L.; et al. Blinatumomab in Combination with Immune Checkpoint Inhibitors of PD-1 and CTLA-4 in Adult Patients with Relapsed/Refractory (R/R) CD19 Positive B-Cell Acute Lymphoblastic Leukemia (ALL): Preliminary Results of a Phase I Study. Blood 2018, 132, 557. [Google Scholar] [CrossRef]
- Schwartz, M.; Damon, L.E.; Jeyakumar, D.; Costello, C.L.; Tzachanis, D.; Schiller, G.J.; Reiner, J.; Wieduwilt, M.J. Blinatumomab in Combination with Pembrolizumab Is Safe for Adults with Relapsed or Refractory B-Lineage Acute Lymphoblastic Leukemia: University of California Hematologic Malignancies Consortium Study 1504. Blood 2019, 134, 3880. [Google Scholar] [CrossRef]
- Ghorashian, S.; Kramer, A.M.; Onuoha, S.; Wright, G.; Bartram, J.; Richardson, R.; Albon, S.J.; Casanovas-Company, J.; Castro, F.; Popova, B.; et al. Enhanced CAR T Cell Expansion and Prolonged Persistence in Pediatric Patients with ALL Treated with a Low-Affinity CD19 CAR. Nat. Med. 2019, 25, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Amrolia, P.J.; Wynn, R.; Hough, R.E.; Vora, A.; Bonney, D.; Veys, P.; Chiesa, R.; Rao, K.; Clark, L.; Al-Hajj, M.; et al. Phase I Study of AUTO3, a Bicistronic Chimeric Antigen Receptor (CAR) T-Cell Therapy Targeting CD19 and CD22, in Pediatric Patients with Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia (r/r B-ALL): Amelia Study. Blood 2019, 134, 2620. [Google Scholar] [CrossRef]
- Kantarjian, H.; Jabbour, E. Incorporating Immunotherapy into the Treatment Strategies of B-Cell Adult Acute Lymphoblastic Leukemia: The Role of Blinatumomab and Inotuzumab Ozogamicin. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, M.R.; Banerjee, P.; Milton, D.R.; Jiang, X.; Ganesh, C.; Khazal, S.; Nandivada, V.; Islam, S.; Kaplan, M.; Daher, M.; et al. Blinatumomab Maintenance after Allogeneic Hematopoietic Cell Transplantation for B-Lineage Acute Lymphoblastic Leukemia. Blood 2022, 139, 1908–1919. [Google Scholar] [CrossRef] [PubMed]


| Trial/Study [Reference] | Setting | Study Design | Total Patients | Immunotherapeutic Treatment | Main Results |
|---|---|---|---|---|---|
| Tower 2017 [13] | R/R Ph-negative adults | Phase III randomized trial | 271 blinatumomab vs. 134 ChT | Blinatumomab 9 µg/day first week then 28 µg/day c.i. | CR: blinatumomab 34% vs. ChT 16%. Molecular response: blinatumomab 76% vs. ChT 48%. Median RFS: blinatumomab 7.3 vs. ChT 4.6 months. OS: blinatumomab 7.7 vs. ChT 4.0 months. |
| AALL1331 [6] | R/R Ph-negative children and young adults (1–30 years) | Phase III randomized trial | 107 blinatumomab vs. 109 ChT | Blinatumomab 15 μg/m2 day c.i. for 28 days | Molecular response: blinatumomab 75% vs. 32% ChT. RFS: blinatumomab 54% vs. ChT 39%. OS: blinatumomab 71% vs. ChT 58%. |
| Locatelli et al. [7] | High risk first relapse Ph-negative children | Phase III randomized trial | 54 blinatumomab vs. 54 ChT for the 3rd consolidation before alloHSCT | Blinatumomab 15 μg/m2 day c.i. for 28 days | Minimal residual disease remission: blinatumomab 90% vs. ChT 54%. EFS: blinatumomab 66% vs. ChT 27%. OS: blinatumomab 85% vs. ChT 70%. |
| ALCANTARA [8] | R/R Ph-positive adults | Phase II trial | 45 | Blinatumomab 9 µg/day first week then 28 µg/day c.i. | CR: 36%. Molecular response: 88%. Median RFS: 6.7 months. OS: 7.1 months. |
| Blast [10] | MRD positive after induction | Phase II trial | 113 | Blinatumomab 15 µg/m2/day c.i. | Molecular response: 78%. Medina RFS: 18.9 months. OS: 36.5 months. |
| INO-VATE ALL [14] | R/R Ph-negative or -positive adults | Phase III randomized trial | 109 inotuzumab vs. 109 ChT | Inotuzumab 0.8 mg/m2 on day 1 of each cycle and 0.5 mg/m2 on days 8 and 15 | CR: inotuzumab 81% vs. ChT 29%. Molecular response: inotuzumab 78% vs. ChT 28%. Median RFS: inotuzumab 5 months vs. ChT 1.8 months. OS: inotuzumab 7.7 vs. ChT 6.7 months. VOD: inotuzumab 13% vs. ChT 1% (most cases occurred after alloHSCT). |
| AALL1621 [21] | R/R Ph- and Ph+ children and adolescents (age of 1–21 years) | Phase II trial | 48 | Inotuzumab dosing was 0.8 mg/m2 intravenously on day 1 and 0.5 mg/m2 on days 8 and 15 of a 28-day cycle | CR: 58%. MRD response: 67%. Minimal residual disease measured by flow cytometry: 18 (66.7%) had minimal residual disease < 0.01%. VOD: 29% among 21 patients undergoing alloHSCT. Partial CD22 expression and lower CD22 site density were associated with a lower likelihood of response to inotuzumab. |
| Eliana study [23,24,25] | R/R Ph- and Ph+ children and young adults (age of 3–21) | Phase II trial | 79 | Single infusion of tisagenlecleucel | CR rate: 82%. MRD negativity: 100%. 5-year RFS: 49%. 5y OS: 55%. |
| Tisagenlecleucel real-world study [28] | R/R Ph- and Ph+ children and young adults (age of 1–26) | Real-world setting | 255 | Single infusion of commercial tisagenlecleucel | CR: 86%. DOR: 61%. EFS: 52%. OS: 77%. |
| ZUMA-3 trial [29] | R/R Ph- and Ph+ adults | Phase II trial | 55 | Single infusion of brexucabtagene autoleucel | CR: 71%. MRD response: 97%. Median DOR: 13 months. Median RFS: 12 months. Median OS: 18 months. |
| Condition | Our Approach |
|---|---|
| Extramedullary relapse |
|
| High-burden disease at relapse |
|
| Consolidation treatment after CR achievement with or without alloHSCT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lussana, F.; Cavallaro, G.; De Simone, P.; Rambaldi, A. Optimal Use of Novel Immunotherapeutics in B-Cell Precursor ALL. Cancers 2023, 15, 1349. https://doi.org/10.3390/cancers15041349
Lussana F, Cavallaro G, De Simone P, Rambaldi A. Optimal Use of Novel Immunotherapeutics in B-Cell Precursor ALL. Cancers. 2023; 15(4):1349. https://doi.org/10.3390/cancers15041349
Chicago/Turabian StyleLussana, Federico, Gianluca Cavallaro, Pantaleo De Simone, and Alessandro Rambaldi. 2023. "Optimal Use of Novel Immunotherapeutics in B-Cell Precursor ALL" Cancers 15, no. 4: 1349. https://doi.org/10.3390/cancers15041349
APA StyleLussana, F., Cavallaro, G., De Simone, P., & Rambaldi, A. (2023). Optimal Use of Novel Immunotherapeutics in B-Cell Precursor ALL. Cancers, 15(4), 1349. https://doi.org/10.3390/cancers15041349







