USP15 Represses Hepatocellular Carcinoma Progression by Regulation of Pathways of Cell Proliferation and Cell Migration: A System Biology Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor-Normal Comparison of Publicly Available Data of GTEx/TCGA
2.2. Patient Cohort and Tissue Samples
2.3. Cell Culture
2.4. Plasmid Constructs
2.5. Lentivirus Production and Transduction
2.6. Quantitative Real-Time PCR Analysis
2.7. Western Blot and Antibodies
2.8. Immunohistochemical Staining
2.9. Tissue Scoring
2.10. Cell Migration Assay
2.11. Wound Healing Assay
2.12. Cell Growth Assay
2.13. Tumor Formation in Nude Mice
2.14. Protein–Protein Interaction Network
2.15. HCC Oncology Gene Collection and USP15 Cancer Gene List
2.16. Next-Generation Sequencing
2.17. Network of Pathway Hierarchies and Mapping of Functions
2.18. Sub-Networks of Pathway Hierarchies
2.19. Statistical Analysis
3. Results
3.1. Expression of USP15 Was Low in Tumor Tissue; High Expression of USP15 Correlated with Decreased Risk for Mortality and Cancer Relapse
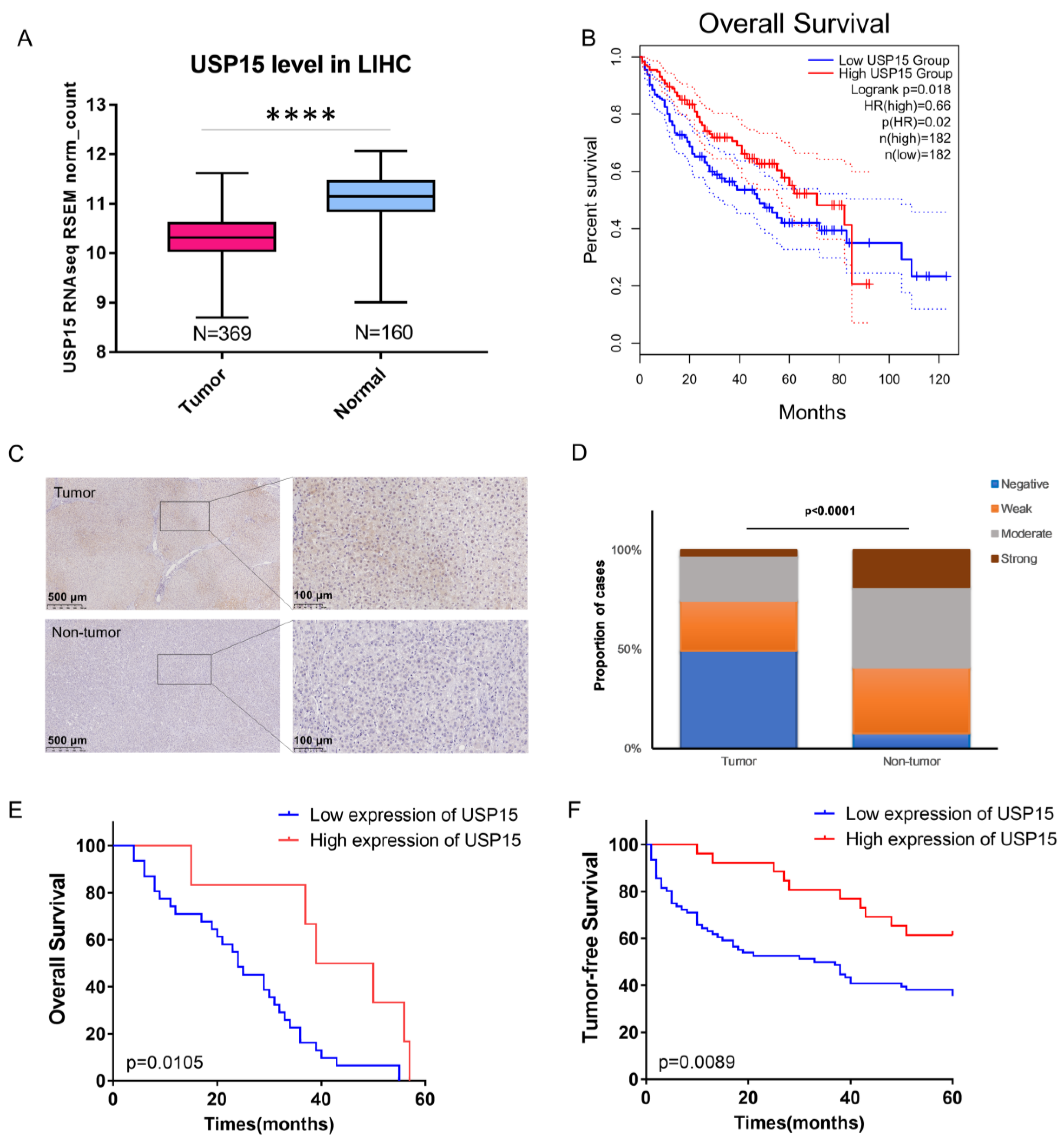
3.2. USP15 Expression Was Increased in HCC Cell Lines
3.3. Overexpressing USP15 Correlated with Slow Proliferation and Slow Migration
3.4. Knockout of USP15 Correlated with High Proliferation and High Migration
3.5. Overexpression of USP15 Correlated with Slow Tumor Growth in Mice
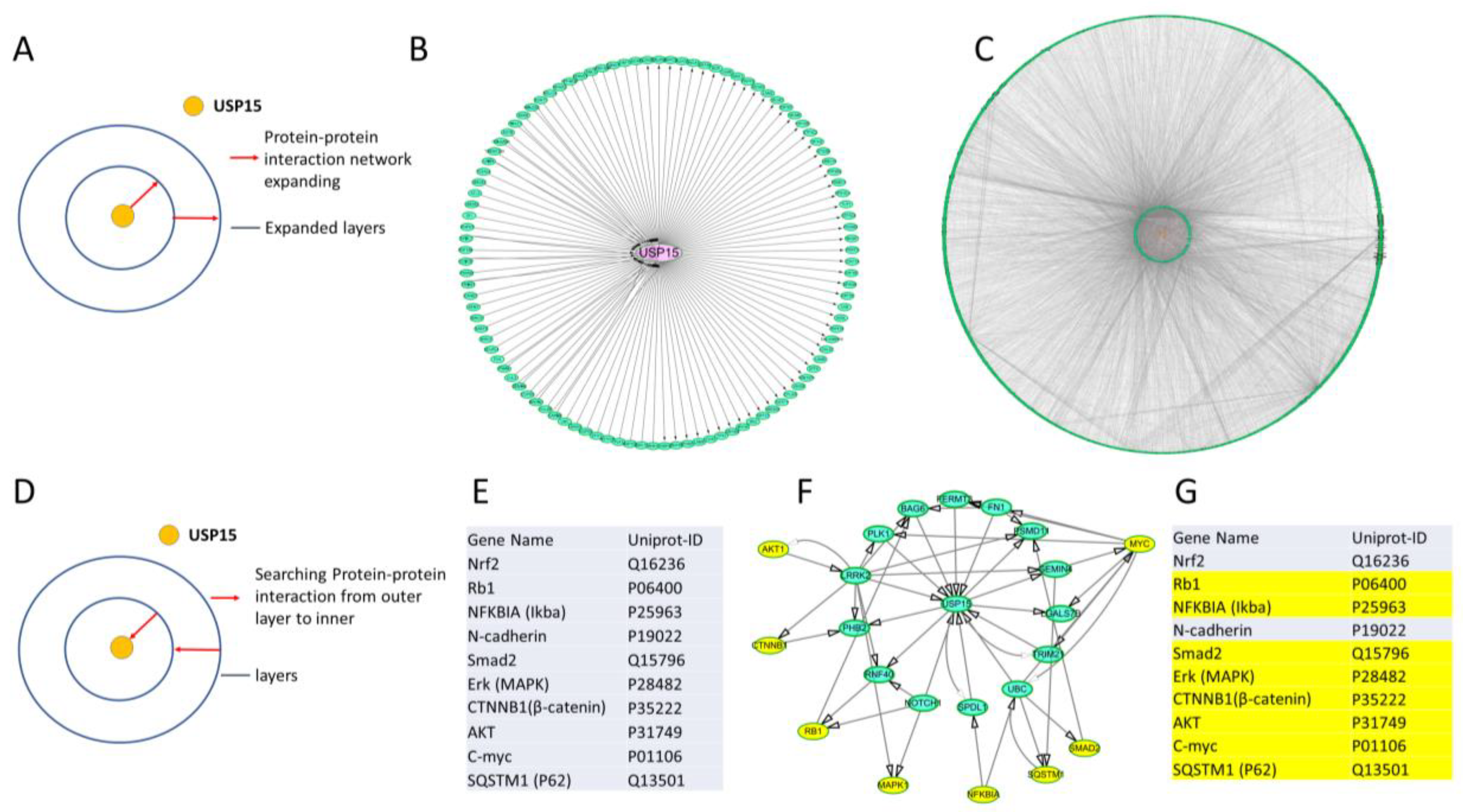
3.6. Tumor-Related Proteins Were Indirectly Regulated by USP15 via Interactions Mediated by Other Proteins
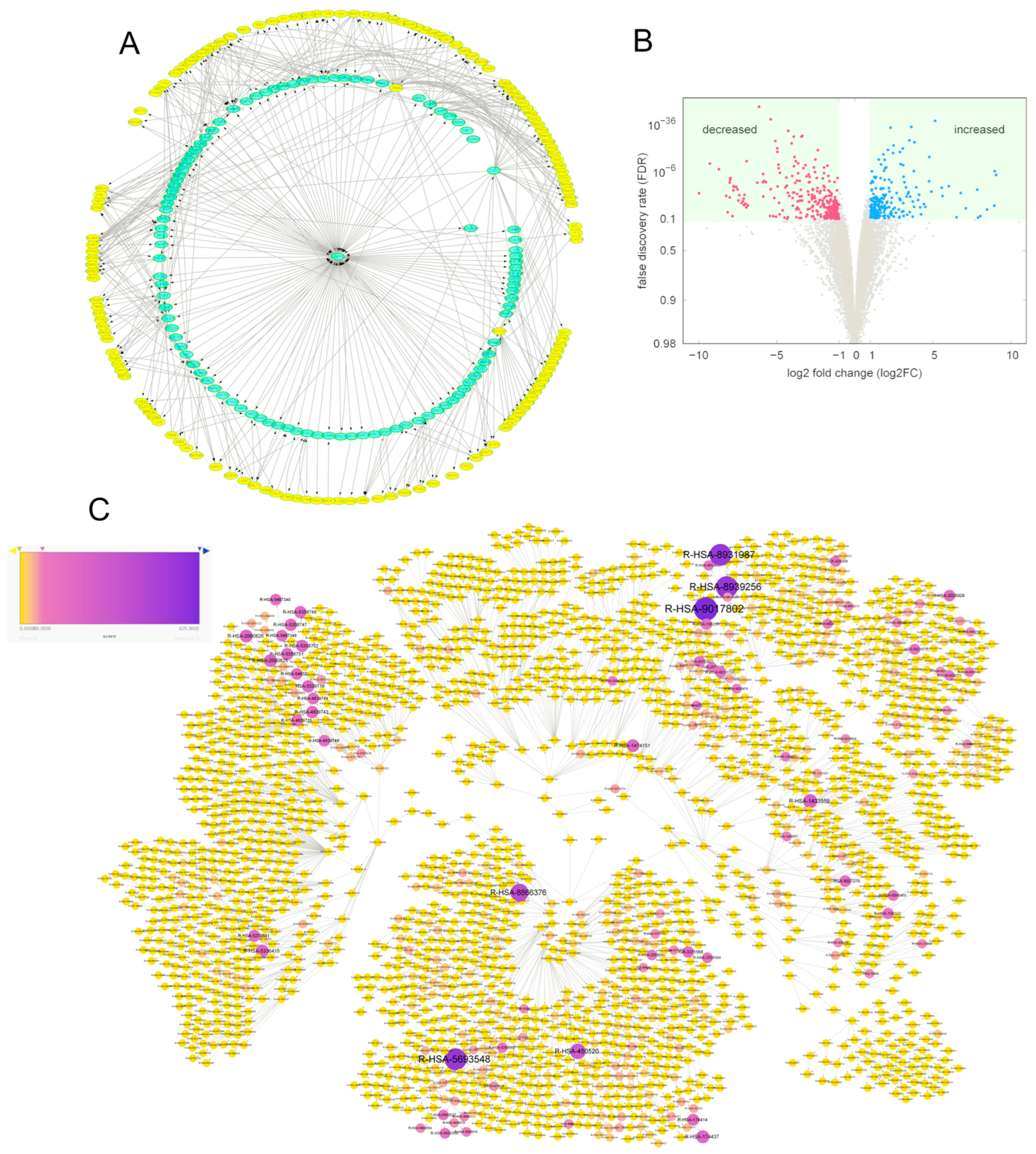
3.7. Regulatory Role of USP15 in HCC: Relevant Pathway Hierarchies
3.8. Regulatory Roles of USP15 in HCC Were Cell Proliferation and Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| BFS | breadth-first search |
| BCLC | Barcelona Clinic Liver Cancer |
| CI | confidence interval |
| DSBs | DNA double-strand breaks |
| EMT | epithelial-mesenchymal transition |
| HCC | hepatocellular carcinoma |
| IHC | immunohistochemical staining |
| KO | knockout |
| MET | mesenchymal-epithelial transition |
| NHEJ | non-homologous end joining |
| NGS | next-generation sequencing |
| OE | overexpressing |
| PVDF | polyvinylidene difluoride |
| PPI | protein–protein interaction |
| Ub | ubiquitin |
| USPs | ubiquitin-specific proteases |
| USP15 | ubiquitin carboxyl-terminal hydrolase 15 |
References
- Vogel, A.; Saborowski, A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat. Rev. 2019, 82, 101946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, L.; Zheng, H.; Abrams, T.A.; Miksad, R.; Bullock, A.J.; Allen, J.N.; Yurgelun, M.B.; Clark, J.W.; Kambadakone, A.; Muzikansky, A.; et al. A Phase II and Biomarker Study of Sorafenib Combined with Modified FOLFOX in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Ferenci, P.; Fried, M.; Labrecque, D.; Bruix, J.; Sherman, M.; Omata, M.; Heathcote, J.; Piratsivuth, T.; Kew, M.; Otegbayo, J.A.; et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): A global perspective. J. Gastrointest. Liver Dis. 2010, 19, 311–317. [Google Scholar]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hunter, T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle 2010, 9, 2342–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Hao, Q.; Wang, J.; Li, J.; Huang, C.; Zhang, Y.; Wu, X.; Lu, H.; Zhou, X. Ubiquitin ligase TRIM71 suppresses ovarian tumorigenesis by de-grading mutant p53. Cell Death Dis. 2019, 10, 737. [Google Scholar] [CrossRef] [Green Version]
- Paul, I.; Batth, T.S.; Iglesias-Gato, D.; Al-Araimi, A.; Al-Haddabi, I.; Alkharusi, A.; Norstedt, G.; Olsen, J.V.; Zadjali, F.; Flores-Morales, A. The ubiquitin ligase Cullin5(SOCS2) regulates NDR1/STK38 stability and NF-kappaB transactivation. Sci Rep. 2017, 7, 42800. [Google Scholar] [CrossRef]
- Shirahashi, H.; Sakaida, I.; Terai, S.; Hironaka, K.; Kusano, N.; Okita, K. Ubiquitin and hepatocellular carcinoma. Liver Int. 2002, 22, 413–418. [Google Scholar] [CrossRef]
- Hershko, A. Ubiquitin: Roles in protein modification and breakdown. Cell 1983, 34, 11–12. [Google Scholar] [CrossRef]
- Mevissen, T.E.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [Green Version]
- Mansilla, A.A.; Martín, F.A.; Martín, D.; Ferrús, A.A. Ligand-independent requirements of steroid receptors EcR and USP for cell survival. Cell Death Differ. 2015, 23, 405–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.P.; Chen, J.; Tse, W.K.F. Role of Deubiquitinases in Human Cancers: Potential Targeted Therapy. Int. J. Mol. Sci. 2020, 21, 2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Tcherpakov, M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell 2010, 143, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, R.T.; Wang, X.-W.; Woollatt, E.; White, J.A.; Sutherland, G.R. Identification, Functional Characterization, and Chromosomal Localization of USP15, a Novel Human Ubiquitin-Specific Protease Related to the UNP Oncoprotein, and a Systematic Nomenclature for Human Ubiquitin-Specific Proteases. Genomics 1999, 59, 264–274. [Google Scholar] [CrossRef]
- Eichhorn, P.J.; Rodón, L.; Gonzàlez-Juncà, A.; Dirac, A.; Gili, M.; Martínez-Sáez, E.; Aura, C.; Barba, I.; Peg, V.; Prat, A.; et al. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat Med. 2012, 18, 429–435. [Google Scholar] [CrossRef]
- Zou, Q.; Jin, J.; Hu, H.; Li, H.S.; Romano, S.; Xiao, Y.; Nakaya, M.; Zhou, X.; Cheng, X.; Yang, P.; et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit anti-tumor T cell responses. Nat. Immunol. 2014, 15, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Oikonomaki, M.; Bady, P.; Hegi, M.E. Ubiquitin Specific Peptidase 15 (USP15) suppresses glioblastoma cell growth via stabili-zation of HECTD1 E3 ligase attenuating WNT pathway activity. Oncotarget 2017, 8, 110490–110502. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Liao, Q.; Tan, W.; Peng, C.; Hu, Z.; Chen, Y.; Li, Z.; Li, J.; Zhen, B.; Zhu, W.; et al. The deubiquitylating enzyme USP15 regulates homologous recombi-nation repair and cancer cell response to PARP inhibitors. Nat. Commun. 2019, 10, 1224. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Meyer, T.; Hart, I. Mechanisms of tumour metastasis. Eur. J. Cancer 1998, 34, 214–221. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving hu-man subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agresti, A. A Survey of Exact Inference for Contingency Tables: Rejoinder. Stat. Sci. 1992, 7, 131–153. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, J.; Gao, S.; Ji, T.; Song, T.; Li, Y.; Wang, J.; Geng, C.; Long, M.; Chen, J.; et al. Restriction of hepatitis B virus replication by c-Abl–induced proteasomal degradation of the viral polymerase. Sci. Adv. 2019, 5, eaau7130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017, PO.17.00011. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardoni, G.; Petterlini, M.; Laudanna, C. Analyzing biological network parameters with CentiScaPe. Bioinformatics 2009, 25, 2857–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [Green Version]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; Del-Toro, N.; et al. The MIntAct project--IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014, 42, D358–D363. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.; Qu, C.; Zhang, S.; Zeng, H.; Sun, K.; Gu, X.; Xia, C.; Yang, Z.; Li, H.; Wei, W.; et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018, 30, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Cavard, C.; Colnot, S.; Audard, V.; Benhamouche, S.; Finzi, L.; Torre, C.; Grimber, G.; Godard, C.; Terris, B.; Perret, C. Wnt/β-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Futur. Oncol. 2008, 4, 647–660. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Wang, Z.; Sarkar, F.H. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers 2011, 3, 716–729. [Google Scholar] [CrossRef]
- Gaillard, H.; Garcia-Muse, T.; Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 2015, 15, 276–289. [Google Scholar] [CrossRef]
- Gorgoulis, V.G.; Vassiliou, L.V.F.; Karakaidos, P.; Zacharatos, P.; Kotsinas, A.; Liloglou, T.; Venere, M.; Ditullio, R.A., Jr.; Kastrinakis, N.G.; Levy, B.; et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005, 434, 907–913. [Google Scholar] [CrossRef]
- Bartkova, J.; Hořejší, Z.; Koed, K.; Krämer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Irminger-Finger, I.; Jefford, C.E. Is there more to BARD1 than BRCA1? Nat. Rev. Cancer 2006, 6, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-Y.; Kim, E.-H.; Lee, H.-J.; Gil, N.-Y.; Cha, H.-J.; Lee, Y.-S. Heat shock factor 1, an inhibitor of non-homologous end joining repair. Oncotarget 2015, 6, 29712–29724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sishc, B.J.; Davis, A.J. The Role of the Core Non-Homologous End Joining Factors in Carcinogenesis and Cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Asano, Y.; Kawase, T.; Okabe, A.; Tsutsumi, S.; Ichikawa, H.; Tatebe, S.; Kitabayashi, I.; Tashiro, F.; Namiki, H.; Kondo, T.; et al. IER5 generates a novel hypo-phosphorylated active form of HSF1 and contributes to tumorigenesis. Sci. Rep. 2016, 6, 19174. [Google Scholar] [CrossRef]
- Karlseder, J.; Broccoli, D.; Dai, Y.; Hardy, S.; de Lange, T. p53- and ATM-Dependent Apoptosis Induced by Telomeres Lacking TRF2. Science 1999, 283, 1321–1325. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, B.; Yang, J.; Wang, H.; Yang, G.; Xu, R.; You, L.; Zhao, Y. The role of histone methylation in the development of digestive cancers: A potential direction for cancer management. Signal Transduct. Target. Ther. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Wei, L.; Chiu, D.K.-C.; Tsang, F.H.-C.; Law, C.-T.; Cheng, C.L.-H.; Au, S.L.-K.; Lee, J.M.-F.; Wong, C.C.L.; Ng, I.O.-L.; Wong, C.-M. Histone methyltransferase G9a promotes liver cancer development by epigenetic silencing of tumor suppressor gene RARRES3. J. Hepatol. 2017, 67, 758–769. [Google Scholar] [CrossRef]
- Huang, M.; Hou, J.; Wang, Y.; Xie, M.; Wei, C.; Nie, F.; Wang, Z.; Sun, M. Long noncoding RNA LINC00673 is activated by SP1 and exerts on-cogenic properties by interacting with LSD1 and EZH2 in gastric cancer. Mol. Ther. 2017, 25, 1014–1026. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Qi, G.; Tang, F.; Yuan, S.; Wang, Z.; Liang, X.; Li, B.; Yu, S.; Liu, J.; Huang, Q.; et al. Aberrant JMJD3 Expression Upregulates Slug to Promote Migration, Invasion, and Stem Cell–Like Behaviors in Hepatocellular Carcinoma. Cancer Res. 2016, 76, 6520–6532. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Total | USP15 Expression | p-Value | ||
|---|---|---|---|---|
| Low (%) | High (%) | |||
| Age | ||||
| <53 | 47 | 37.25 | 8.83 | 0.254 |
| ≥53 | 55 | 37.25 | 16.67 | |
| Gender | ||||
| Male | 85 | 63.73 | 19.61 | 0.363 |
| Female | 17 | 10.78 | 5.88 | |
| AFP (ng/mL) | ||||
| <400 | 63 | 45.10 | 16.67 | 0.816 |
| ≥400 | 39 | 29.41 | 8.82 | |
| HBsAg | ||||
| Negative | 16 | 12.75 | 2.94 | 0.755 |
| Positive | 86 | 61.76 | 22.55 | |
| Tumor size | ||||
| <5 cm | 61 | 44.12 | 15.69 | 1.0 |
| <5 cm | 41 | 30.39 | 9.80 | |
| ≥5 cm | ||||
| Tumor number | ||||
| Single | 89 | 63.73 | 23.53 | 0.507 |
| Multiple | 13 | 10.78 | 1.96 | |
| Liver cirrhosis | ||||
| No | 45 | 29.41 | 14.71 | 0.116 |
| Yes | 57 | 45.10 | 10.78 | |
| Name | Sequences (5′–3′) |
|---|---|
| SgRNA-1 | F: 5′-CACCGCGTCGCGATGTCAGACCGC-3′ |
| SgRNA-1 | R: 5′-AAACGCGGTCTGACATCGCGACGC-3′ |
| EcoRI-USP15 | F: 5′-ATTACGCTGAATTCATGGCGGAAGGCGGAGCGGCGGAT-3′ |
| BamHI-USP15 | R: 5′-CGACTCTAGAGGATCCTA TTAGTTAGTGTGCATACAGT-3′ |
| Cluster | #Nodes |
|---|---|
| Hemostasis | 39 |
| Intrinsic pathway for Apoptosis | 20 |
| Neuronal System | 78 |
| Developmental Biology | 59 |
| Metabolism | 325 |
| Reproduction | 8 |
| Extracellular matrix organization | 19 |
| Cell–Cell communication | 13 |
| Signal Transduction | 382 |
| Cell Cycle | 122 |
| Disease | 517 |
| Immune System | 197 |
| Organelle biogenesis and maintenance | 13 |
| Transport of small molecules | 69 |
| Metabolism of proteins | 124 |
| Muscle contraction | 11 |
| Circadian Clock | 4 |
| Chromatin organization | 7 |
| Programmed Cell Death | 21 |
| Vesicle-mediated transport | 38 |
| DNA Replication | 19 |
| DNA Repair | 61 |
| Gene expression (Transcription) | 125 |
| Metabolism of RNA | 51 |
| Cellular responses to external stimuli | 24 |
| Digestion and absorption | 7 |
| Protein localization | 6 |
| Autophagy | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Song, Z.; Rieser, J.; Ackermann, J.; Koch, I.; Lv, X.; Ji, T.; Cai, X. USP15 Represses Hepatocellular Carcinoma Progression by Regulation of Pathways of Cell Proliferation and Cell Migration: A System Biology Analysis. Cancers 2023, 15, 1371. https://doi.org/10.3390/cancers15051371
Ren Y, Song Z, Rieser J, Ackermann J, Koch I, Lv X, Ji T, Cai X. USP15 Represses Hepatocellular Carcinoma Progression by Regulation of Pathways of Cell Proliferation and Cell Migration: A System Biology Analysis. Cancers. 2023; 15(5):1371. https://doi.org/10.3390/cancers15051371
Chicago/Turabian StyleRen, Yiyue, Zhen Song, Jens Rieser, Jörg Ackermann, Ina Koch, Xingyu Lv, Tong Ji, and Xiujun Cai. 2023. "USP15 Represses Hepatocellular Carcinoma Progression by Regulation of Pathways of Cell Proliferation and Cell Migration: A System Biology Analysis" Cancers 15, no. 5: 1371. https://doi.org/10.3390/cancers15051371






