From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
- Hyperthermic radio-sensitization: This condition is generally believed to be the result of a “physiological vasodilation”, which increases tumor blood flow (“reperfusion”), and oxygen levels (“reoxygenation”), occasionally lasting up to 24–48 h post-HT [12].
- Hyperthermia enhances cytotoxicity of anticancer drugs: Besides direct sensitization to a series of anticancer agents (e.g., Cisplatin, Carboplatin, Oxaliplatin, Bleomycin, Doxorubicin), HT can improve the blood-borne delivery (via an increase in heat-induced tumor perfusion and/or a homogenization of blood flow), and enhanced extravasation in the leaky microvasculature of malignant tumors or a temperature-triggered drug release from thermo-sensitive liposomes for localized thermo-chemotherapy.
- Hyperthermia affects radio-immuno-oncology: It is known that hypoxia compromises anticancer immune responses such as reducing the survival, cytolytic, and migratory activity of key effector cells such as natural killer (NK) cells and NK-like T cells, as well as CD4+ helper and CD8+ cytotoxic T cells, reduces the production of essential “effector” cytokines, as well as fostering an immunosuppressive environment by supporting immunoregulatory Treg cells, myeloid-derived suppressor cells (MDSCs) and inducing the expression of immune checkpoint inhibitors (reviewed in [20]). HT-induced improvements of tumor oxygenation status (“reversal of tumor hypoxia”) and the increased perfusion triggered by mild HT enhances the trafficking of immune cells, and intra-tumoral access to crucial immune regulators such as antibodies and cytokines, all of which are needed to generate effective antitumor immune responses. Hyperthermia is also known to be an effective immune modulator that has multiple effects on the innate and adaptive immune systems (reviewed in [21]).
- Hyperthermia and the innate immune system: With respect to the innate immune system, hyperthermia increases the expression of activation receptors such as NKG2D and MHC class I-related chain A (MICA) on the surface of natural killer (NK) cells, thereby enhancing their antitumor potential [21]. This is confirmed by findings that NK cells are important mediators of antitumor immunity after radiotherapy and hyperthermia [22] and that cells of the innate immune system in patients recover faster when hyperthermia and radio-chemotherapy are combined [23].
- Hyperthermia and the adaptive immune system: With regards to the adaptive immune system, hyperthermia influences all aspects of adaptive antitumor immunity, from the function and antigen presentation capacity of antigen-presenting cells (APCs) to the responsiveness of CD4+ and CD8+ T-cell populations [23]. Combining hyperthermia with radiotherapy promotes the infiltration of dendritic cells—crucial antigen-presenting cells and initiators of adaptive immune cells—into solid tumors [23], as well as inducing the maturation of DCs and the release of pro-inflammatory cytokines from DCs and macrophages [24,25]. In addition to direct effects on cellular immunity, combining hyperthermia and radiotherapy has also been shown to mediate immune effects via multiple mechanisms, including the release of Danger Associated Molecular Pattern (DAMP) signals such as heat shock proteins (HSPs) and HMGB1 [24,25].
2. Methods, Search Strategies and Sources of Information
3. Results: Assessment of Reliable Experimental and Clinical Data
3.1. Transiently Improved Tumor Blood Flow upon Localized Mild Hyperthermia: Potential Mechanisms Involved
3.1.1. Tumor Vascularization and Blood Flow Are Decisive Parameters Critically Affecting Efficacy of Localized Hyperthermia
3.1.2. Prime Role of Tumor Blood Flow in Hyperthermia Treatments
- Primary dilation of co-opted vessels within tumors;
- Thermoregulatory dilation of upstream blood vessels in the normal tissue adjacent to the growing tumor, a regulation that leads to secondary flow increases (“re-perfusion”) through downstream tumor vessels in series with the normal tissue vascular bed [39];
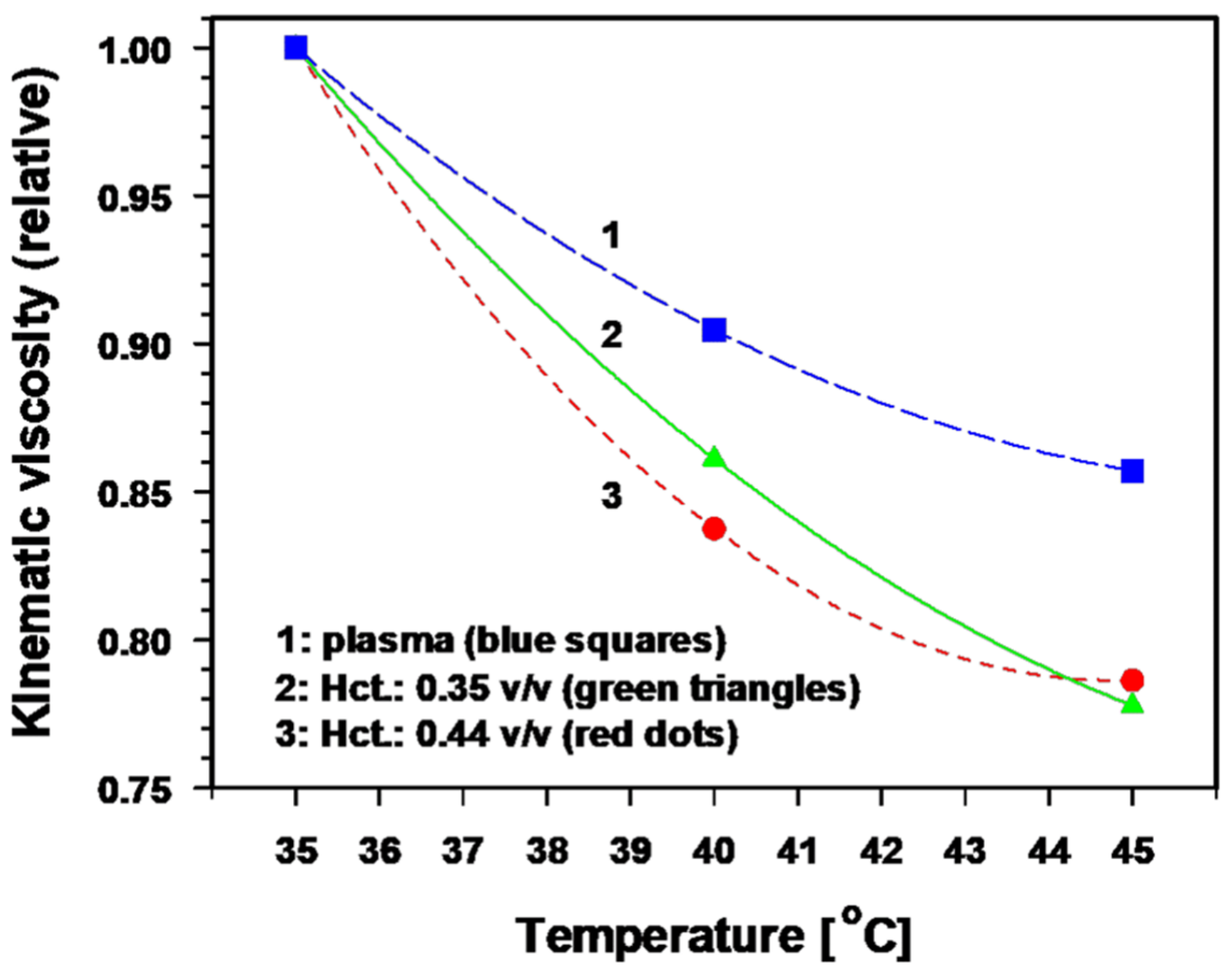
- Distinct reduction of viscous resistance to flow due to significant improvements in the key rheological parameters that determine blood flow behavior [40]. In vitro, a temperature rise of 1 K significantly decreases the blood viscosity by ≈ 3.5% and the plasma viscosity by ≈ 2.5% [41,42,43]. The relative kinematic viscosity of blood (at different hematocrit values) and of plasma as a function of temperature is shown in Figure 3 [40]. Taken together, these mHT-induced changes in key parameters clearly improve the blood flow behaviour.
3.2. Enhanced Tumor Oxygenation Status upon Localized Mild Hyperthermia
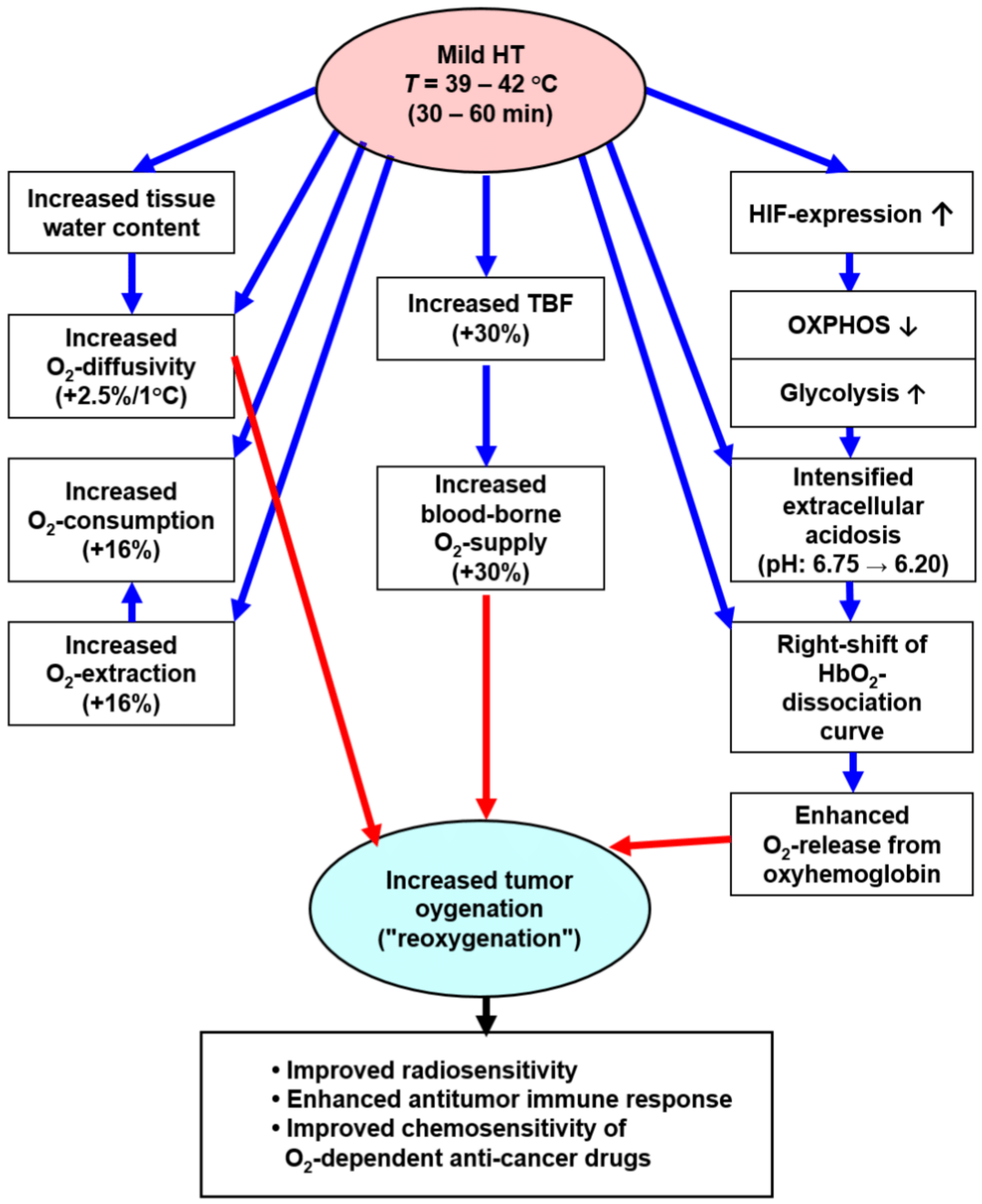
3.2.1. A Multifactorial, Complex Scenario Is Involved in the Transient Improvement of Tumor Oxygenation upon Mild Hyperthermia
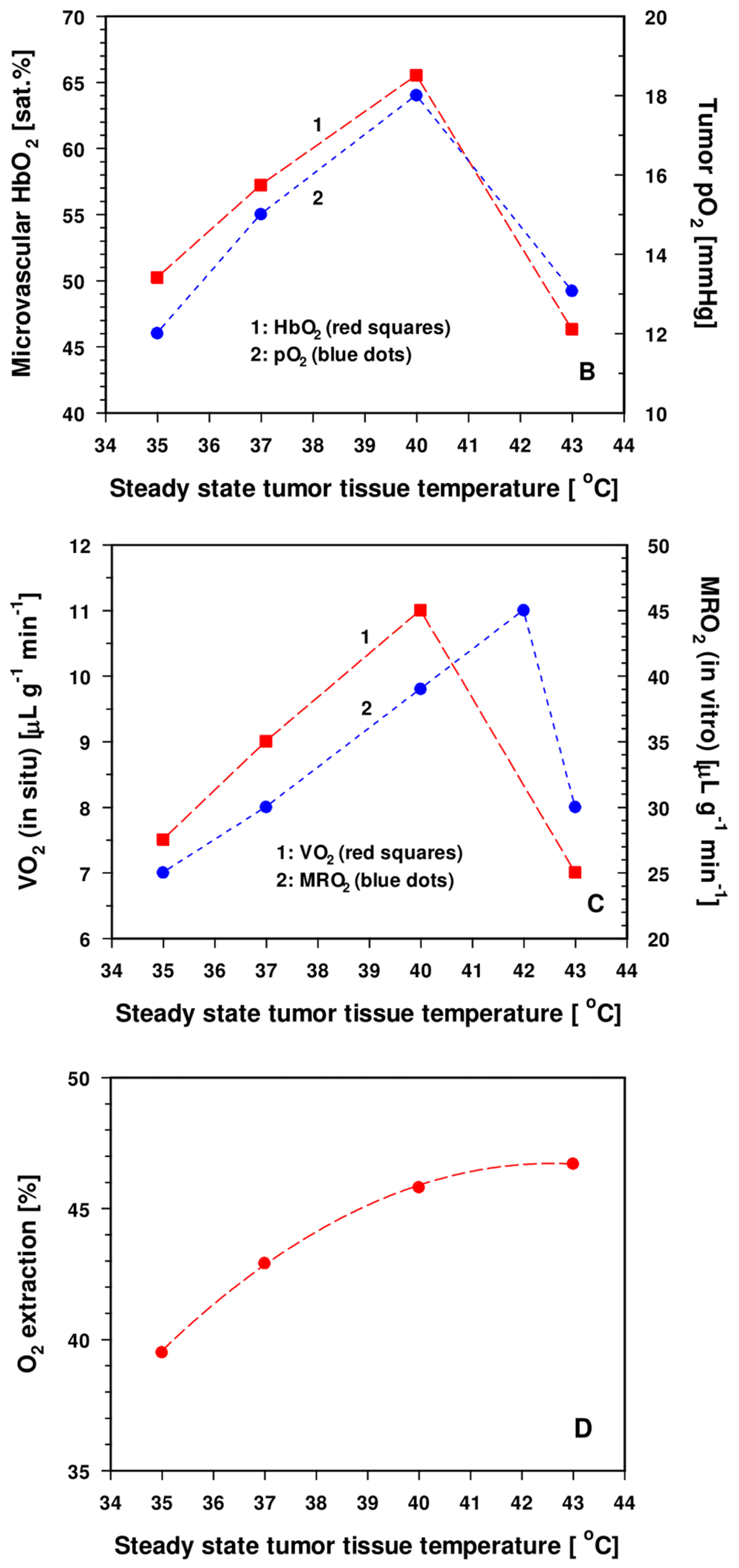
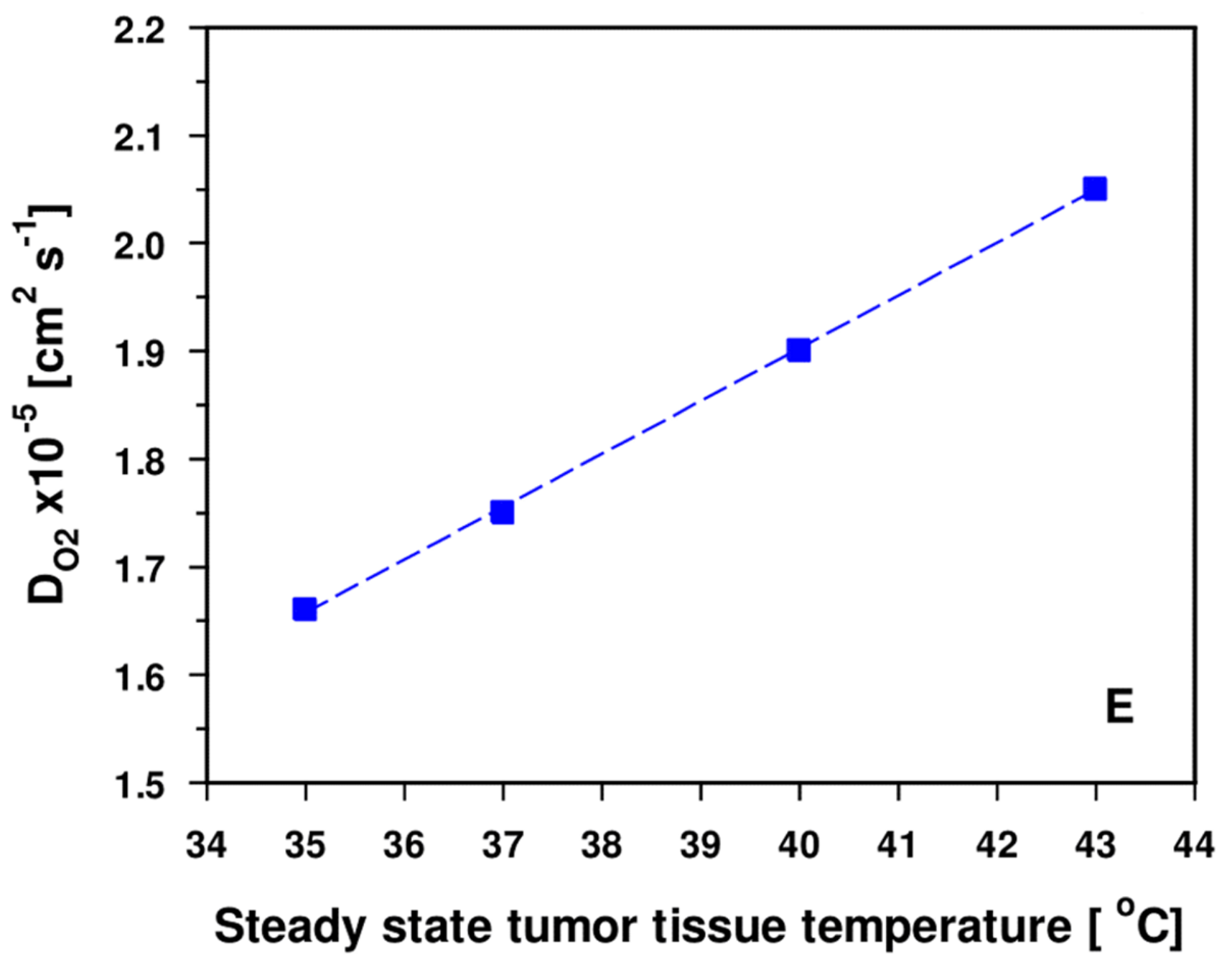
- DO2 values (O2 diffusivities) for tumors are increased by a factor of ≈ 1.9 compared to the tissues of origin [54]. Additional edema formation is often seen upon HT [9], thus increasing hyperhydration and—as a result—further improving O2 diffusivity, finally supporting re-oxygenation of the tumor upon mHT.
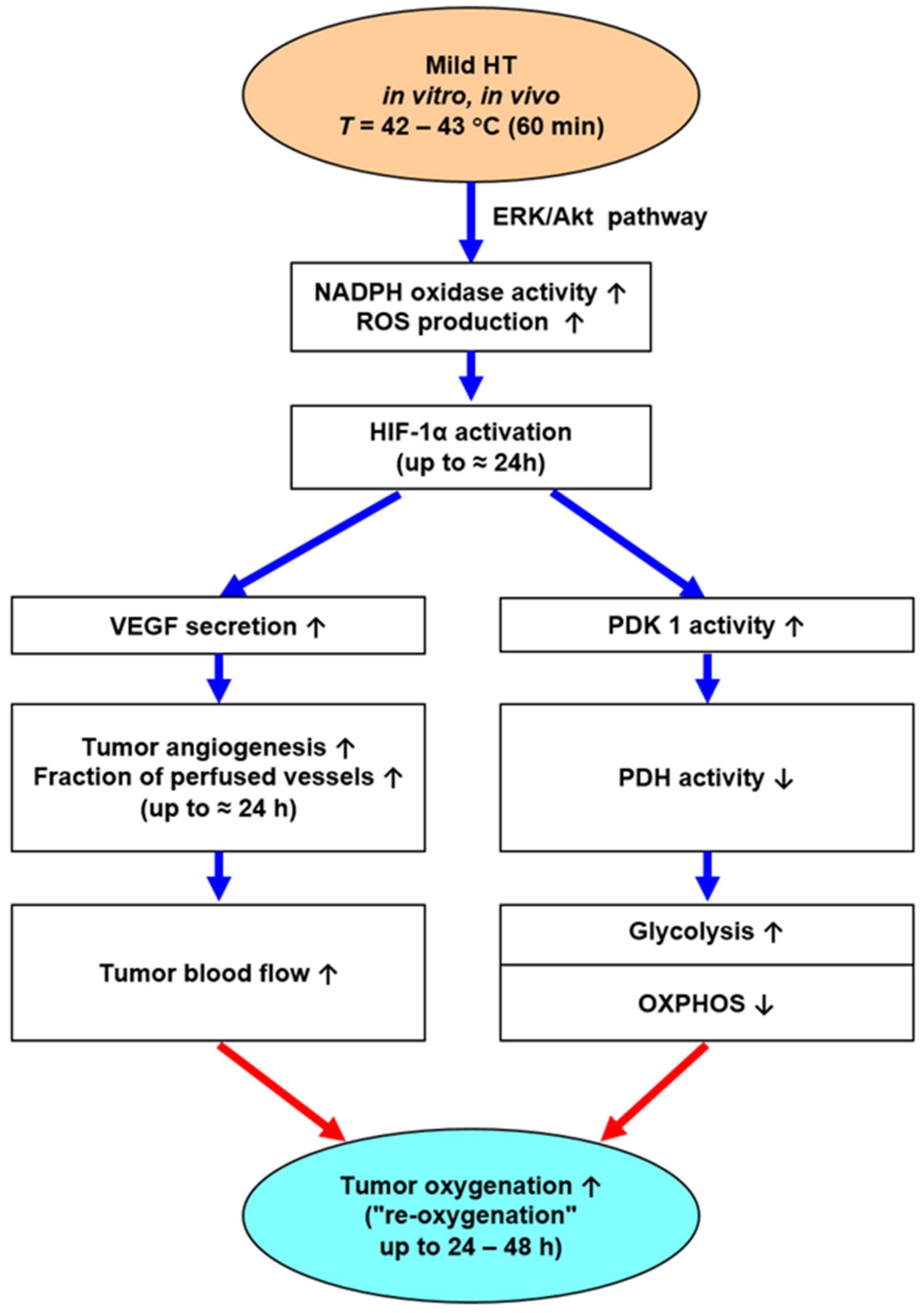
- Besides anaerobic glycolysis (because of tumor hypoxia), cancer cells rely on aerobic glycolysis (due to metabolic reprogramming, a core hallmark of cancer [57]). Both pathways produce high amounts of lactate and protons H+ (“lactic acid”), which are exported into the extracellular space, leading to extracellular acidosis (mean pHe ≈ 6.75). Aerobic glycolysis is stimulated by mHT-induced activation of HIF-1α, leading to an intensified Warburg effect for 24–48 h (schematically shown in Figure 5).
- In addition, tissue heating intensifies ATP hydrolysis with proton production as well as inhibiting the Na+/H+ antiport of the cell membrane [10].
- mHT per se intensifies tissue acidosis due to changes in chemical equilibria of the intra- and extracellular buffer systems: ΔpH/ΔT = −0.016 pH units/K [10].
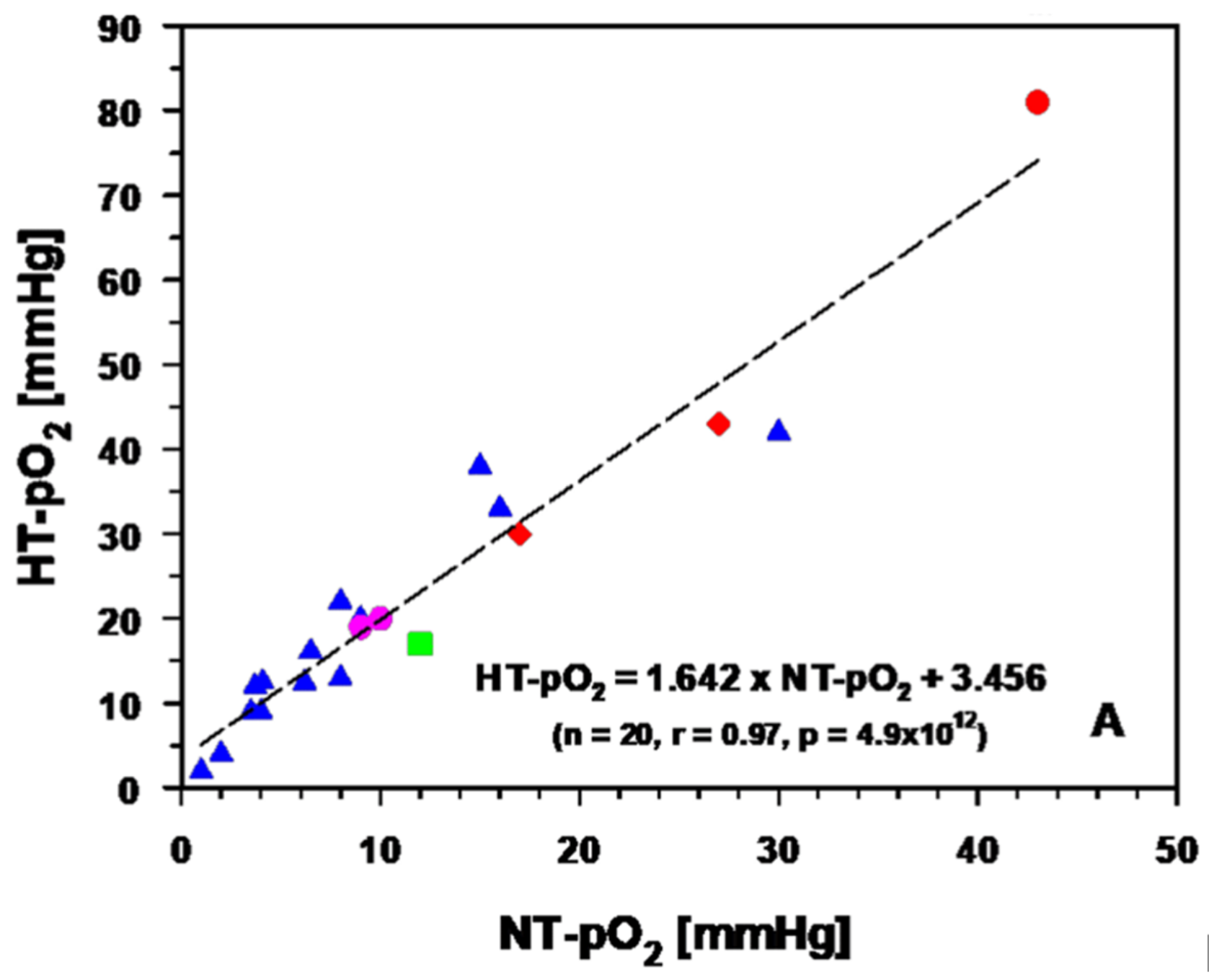
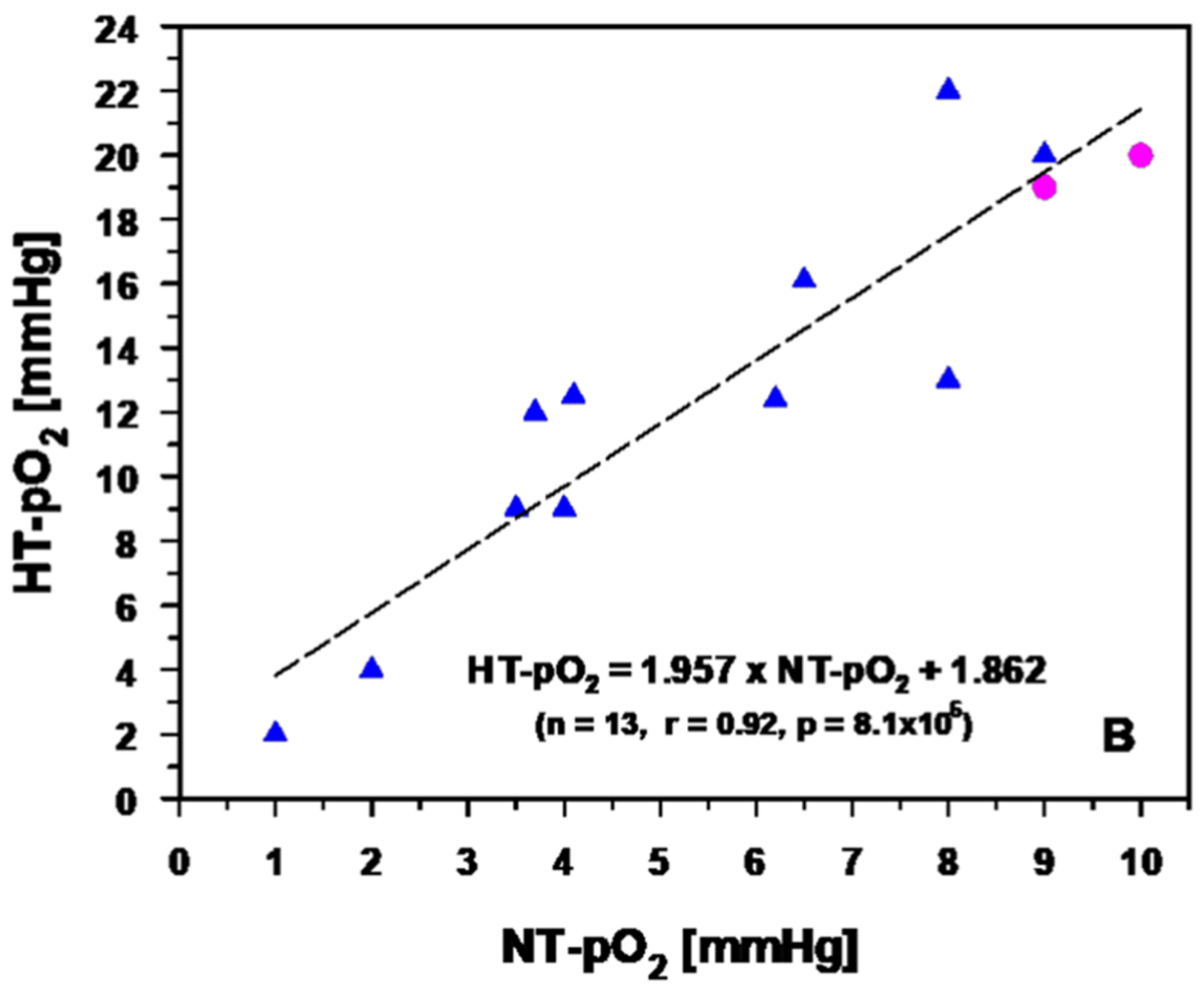
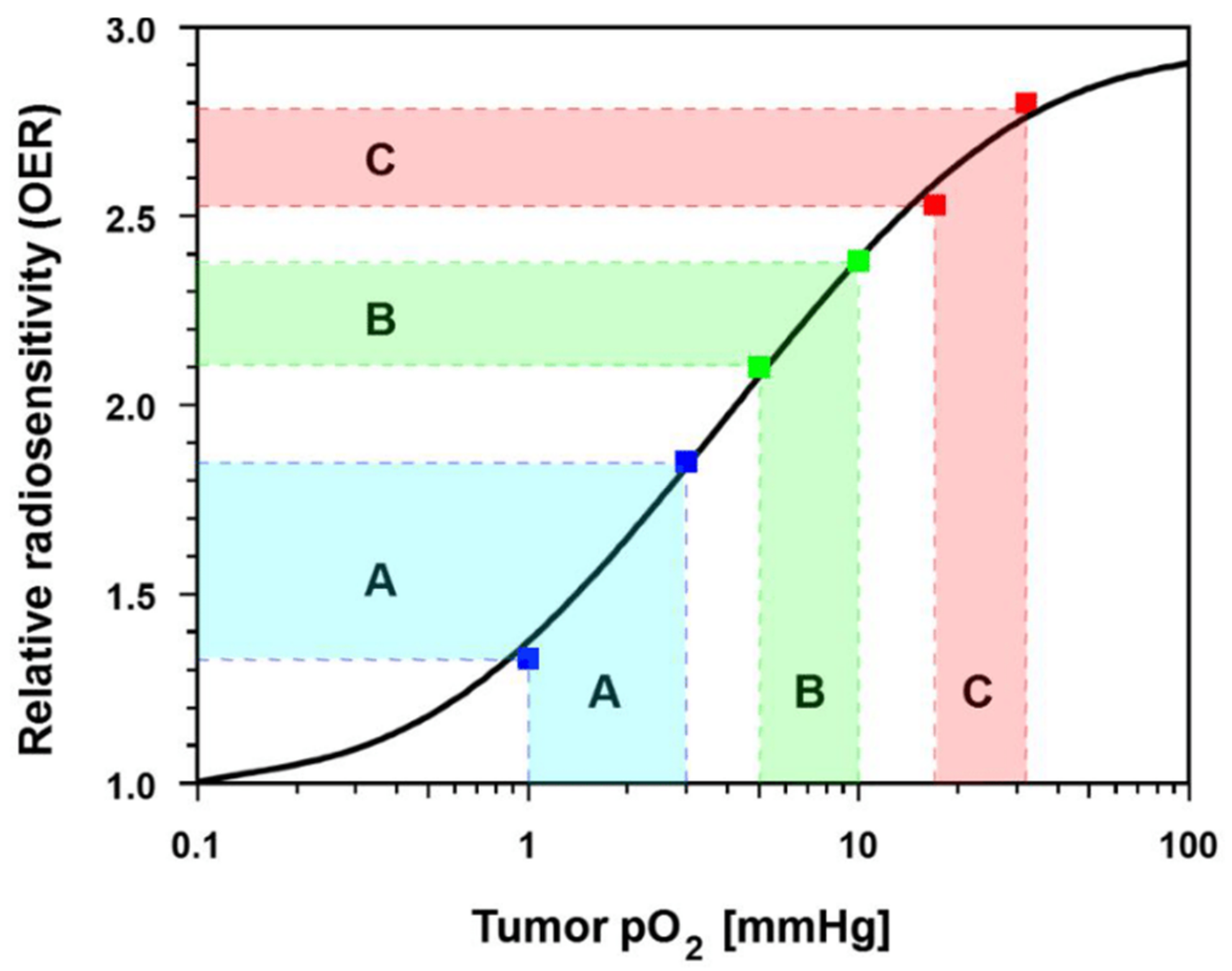
3.2.2. Experimental and Clinical Evidence for Improved Tumor Oxygenation upon Localized Mild Hyperthermia: Updated Data Analysis
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Rhoon, G.C.; Franckena, M.; ten Hagen, T.L.M. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef]
- Crezee, J.; Franken, N.A.P.; Oei, A.L. Hyperthermia-based anti-cancer treatments. Cancers 2021, 13, 1240. [Google Scholar] [CrossRef]
- Kok, H.P.; van Rhoon, G.C.; Herrera, T.D.; Overgaard, J.; Crezee, J. Biological modeling in thermoradiotherapy: Present status and ongoing developments toward routine clinical use. Int. J. Hyperth. 2022, 39, 1126–1140. [Google Scholar] [CrossRef]
- Vaupel, P.; Piazena, H. Strong correlation between specific heat capacity and water content in human tissues suggests preferred heat deposition in malignant tumors upon electromagnetic irradiation. Int. J. Hyperth. 2022, 39, 987–997. [Google Scholar] [CrossRef]
- Hannon, G.; Tansi, F.L.; Hilger, I.; Prina-Mello, A. The effects of localized heat on the hallmarks of cancer. Adv. Therap. 2021, 4, 2000267. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Song, C.W. Physiological mechanisms underlying heat-induced radiosensitization. Int. J. Hyperth. 2004, 20, 163–174. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef]
- Horsman, M.R. Tissue physiology and the response to heat. Int. J. Hyperth. 2006, 22, 197–203. [Google Scholar] [CrossRef]
- Vaupel, P.W.; Kelleher, D.K. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int. J. Hyperth. 2010, 26, 211–223. [Google Scholar] [CrossRef]
- Vaupel, P.W.; Kelleher, D.K. Blood flow and associated pathophysiology of uterine cervix cancers: Characterisation and relevance for localised hyperthermia. Int. J. Hyperth. 2012, 28, 518–527. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lee, C.T.; Ashcraft, K.A. The future of biology in driving the field of hyperthermia. Int. J. Hyperth. 2016, 32, 4–13. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Oleson, J.R.; Kirkpatrick, J.; Secomb, T.W. Accurate three-dimensional thermal dosimetry and assessment of physiologic response are essential for optimizing thermoradiotherapy. Cancers 2022, 14, 1701. [Google Scholar] [CrossRef]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug. Deliv. Rev. 2020, 163–164, 84–97. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fiorentini, G.; Szasz, A.M.; Szigeti, G.; Szasz, A.; Minnaar, C.A. Quo vadis oncological hyperthermia (2020)? Front. Oncol. 2020, 10, 1690. [Google Scholar] [CrossRef]
- Notter, M.; Thomsen, A.R.; Nitsche, M.; Hermann, R.M.; Wolff, H.A.; Habl, G.; Munch, K.; Grosu, A.L.; Vaupel, P. Combined wIRA-hyperthermia and hypofractionated re-irradiation in the treatment of locally recurrent breast cancer: Evaluation of therapeutic outcome based on a novel size classification. Cancers 2020, 12, 606. [Google Scholar] [CrossRef]
- Notter, M.; Stutz, E.; Thomsen, A.R.; Vaupel, P. Radiation-associated angiosarcoma of the breast and chest wall treated with thermography-controlled, contactless wIRA-hyperthermia and hypofractionated re-irradiation. Cancers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating hyperthermia into modern radiation oncology: What evidence is necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef]
- Multhoff, G.; Vaupel, P. Hypoxia compromises anti-cancer immune responses. Adv. Exp. Med. Biol. 2020, 1232, 131–143. [Google Scholar] [CrossRef]
- Muthana, M.; Multhoff, G.; Pockley, A.G. Tumour infiltrating host cells and their significance for hyperthermia. Int. J. Hyperth. 2010, 26, 247–255. [Google Scholar] [CrossRef]
- Finkel, P.; Frey, B.; Mayer, F.; Bosl, K.; Werthmoller, N.; Mackensen, A.; Gaipl, U.S.; Ullrich, E. The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology 2016, 5, e1101206. [Google Scholar] [CrossRef]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef]
- Werthmoller, N.; Frey, B.; Ruckert, M.; Lotter, M.; Fietkau, R.; Gaipl, U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of b16-f10 melanoma cells in vitro and in vivo. Int. J. Hyperth. 2016, 32, 23–30. [Google Scholar] [CrossRef]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP 70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug. Deliv. Rev. 2020, 163–164, 98–124. [Google Scholar] [CrossRef]
- Vaupel, P.; Flood, A.B.; Swartz, H.M. Oxygenation status of malignant tumors vs. normal tissues: Critical evaluation and updated data source based on direct measurements with pO2 microsensors. Appl. Magn. Reson. 2021, 52, 1451–1479. [Google Scholar] [CrossRef]
- Gersing, E.; Kelleher, D.K.; Vaupel, P. Tumour tissue monitoring during photodynamic and hyperthermic treatment using bioimpedance spectroscopy. Physiol. Meas. 2003, 24, 625–637. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar]
- Rich, L.J.; Winslow, T.B.; Alberico, R.A.; Repasky, E.A.; Seshadri, M.; Singh, A.K. Enhanced tumour perfusion following treatment with water-filtered IR-A radiation to the thorax in a patient with head and neck cancer. Int. J. Hyperth. 2016, 32, 539–542. [Google Scholar] [CrossRef]
- Vaupel, P. Pathophysiology of solid tumors: In The Impact of Tumor Biology on Cancer Treatment and Multidisciplinary Strategies; Molls, M., Vaupel, P., Nieder, C., Anscher, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 51–92. [Google Scholar]
- Horsman, M.R.; Vaupel, P. Tumor hypoxia and the response to radiotherapy. In Tumor Hypoxia in the Clinical Setting; Osinsky, S., Friess, H., Vaupel, P., Eds.; Adacemperiodyka: Kyiv, Ukraine, 2011; pp. 69–110. [Google Scholar]
- Vaupel, P. Pathophysiological mechanisms of hyperthermia in cancer therapy. In Biological Basis of Oncologic Thermotherapy; Gautherie, M., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1990; pp. 73–134. [Google Scholar]
- Kelleher, D.K.; Vaupel, P. Vascular effects of localized hyperthermia. In Hyperthermia in Cancer Treatment: A Primer; Baronzio, G.F., Hager, E.D., Eds.; Springer Science: New York, NY, USA, 2006; pp. 99–109. [Google Scholar]
- Kelleher, D.K.; Vaupel, P. No sustained improvement in tumor oxygenation after localized mild hyperthermia. Adv. Exp. Med. Biol. 2010, 662, 393–398. [Google Scholar] [CrossRef]
- Sun, X.; Xing, L.; Ling, C.C.; Li, G.C. The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: Relevance for radiotherapy, vascular targeting and imaging. Int. J. Hyperth. 2010, 26, 224–231. [Google Scholar] [CrossRef]
- Rhee, J.G.; Eddy, H.A.; Hong, J.J.; Suntharalingam, M.; Vaupel, P.W. Divergent changes of flow through individual blood vessels upon localized heating. Int. J. Hyperth. 1996, 12, 757–769. [Google Scholar] [CrossRef]
- Thews, O.; Li, Y.; Kelleher, D.K.; Chance, B.; Vaupel, P. Microcirculatory function, tissue oxygenation, microregional redox status and ATP distribution in tumors upon localized infrared-A -hyperthermia at 42 °C. Adv. Exp. Med. Biol. 2003, 530, 237–247. [Google Scholar] [CrossRef]
- Vaupel, P. Oxygenation of solid tumors. In Drug Resistance in Oncology; Teicher, B.A., Ed.; Marcel Dekker: New York, NY, USA; Basel, Switzerland; Hongkong, China, 1993; pp. 53–85. [Google Scholar]
- Kelleher, D.K.; Nauth, C.; Thews, O.; Krueger, W.; Vaupel, P. Localized hypothermia: Impact on oxygenation, microregional perfusion, metabolic and bioenergetic status of subcutaneous rat tumours. Br. J. Cancer. 1998, 78, 56–61. [Google Scholar] [CrossRef]
- Leonhardt, H.; Stelter, I. Investigations on the influence of thrombocytes and temperature on the whole blood- and plasma-viscosity as comparative measurements between a capillary- and a plate-cone-viscosimeter (author’s transl). Experientia 1975, 31, 1179–1181. [Google Scholar] [CrossRef]
- Buono, M.J.; Krippes, T.; Kolkhorst, F.W.; Williams, A.T.; Cabrales, P. Increases in core temperature counterbalance effects of haemoconcentration on blood viscosity during prolonged exercise in the heat. Exp. Physiol. 2016, 101, 332–342. [Google Scholar] [CrossRef]
- Khnouf, R.; Karasneh, D.; Abdulhay, E.; Abdelhay, A.; Sheng, W.; Fan, Z.H. Microfluidics-based device for the measurement of blood viscosity and its modeling based on shear rate, temperature, and heparin concentration. Biomed. Microdevices 2019, 21, 80. [Google Scholar] [CrossRef]
- Vaupel, P.; Ostheimer, K.; Müller-Klieser, W. Circulatory and metabolic responses of malignant tumors during localized hyperthermia. J. Cancer. Res. Clin. Oncol. 1980, 98, 15–29. [Google Scholar] [CrossRef]
- Kuzman, D.; Znidarcic, T.; Gros, M.; Vrhovec, S.; Svetina, S.; Zeks, B. Effect of pH on red blood cell deformability. Pflügers Arch. 2000, 440, R193–R194. [Google Scholar] [CrossRef]
- Sen, A.; Capitano, M.L.; Spernyak, J.A.; Schueckler, J.T.; Thomas, S.; Singh, A.K.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef]
- Baronzio, G.; Parmar, G.; Baronzio, M. Overview of methods for overcoming hindrance to drug delivery to tumors, with special attention to tumor interstitial fluid. Front. Oncol. 2015, 5, 165. [Google Scholar] [CrossRef]
- Bayer, C.; Shi, K.; Astner, S.T.; Maftei, C.A.; Vaupel, P. Acute versus chronic hypoxia: Why a simplified classification is simply not enough. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 965–968. [Google Scholar] [CrossRef]
- Moon, E.J.; Sonveaux, P.; Porporato, P.E.; Danhier, P.; Gallez, B.; Batinic-Haberle, I.; Nien, Y.C.; Schroeder, T.; Dewhirst, M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 2010, 107, 20477–20482. [Google Scholar] [CrossRef]
- Wan, J.; Wu, W. Hyperthermia induced HIF-1α expression of lung cancer through akt and erk signaling pathways. J. Exp. Clin. Cancer Res. 2016, 35, 119. [Google Scholar] [CrossRef]
- Vaupel, P. Pathophysiology of human tumors. In Tumor Hypoxia in the Clinical Setting; Osinsky, S., Friess, H., Vaupel, P., Eds.; Academperiodyka: Kyiv, Ukraine, 2011; pp. 20–66. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Vaupel, P.; Piazena, H. Hyperhydration of cancers: A characteristic biophysical trait strongly increasing O2, CO2, glucose and lactate diffusivities, and improving thermophysical properties of solid malignancies. Adv. Exp. Med. Biol. 2023, in press. [Google Scholar]
- Grote, J.; Süsskind, R.; Vaupel, P. Oxygen diffusivity in tumor tissue (DS-carcinosarcoma) under temperature conditions within the range of 20–40 °C. Pflügers Arch. 1977, 372, 37–42. [Google Scholar] [CrossRef]
- Bentley, T.B.; Meng, H.; Pittman, R.N. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am. J. Physiol. 1993, 264, H1825–H1830. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Bicher, H.I.; Hetzel, F.W.; Vaupel, P.; Sandhu, T.S. Microcirculation modifications by localized microwave hyperthermia and hematoporphyrin phototherapy. Bibl. Anat. 1981, 20, 628–632. [Google Scholar]
- Vaupel, P. Impact of localized microwave hyperthermia on the pH-distribution in malignant tumors (author’s transl). Strahlentherapie 1982, 158, 168–173. [Google Scholar]
- Vaupel, P.; Müller-Klieser, W.; Otte, J.; Manz, R.; Kallinowski, F. Blood flow, tissue oxygenation, and pH-distribution in malignant tumors upon localized hyperthermia. Basic pathophysiological aspects and the role of various thermal doses. Strahlentherapie 1983, 159, 73–81. [Google Scholar]
- Vaupel, P.; Kallinowski, F. Physiological effects of hyperthermia. Recent Results Cancer Res. 1987, 104, 71–109. [Google Scholar] [CrossRef]
- Bicher, H.I.; Sandhu, T.S.; Vaupel, P.; Hetzel, F.W. Effect of localizes microwave hyperthermia on physiological responses. J. Natl. Cancer Inst. 1980, 61, 217–219. [Google Scholar]
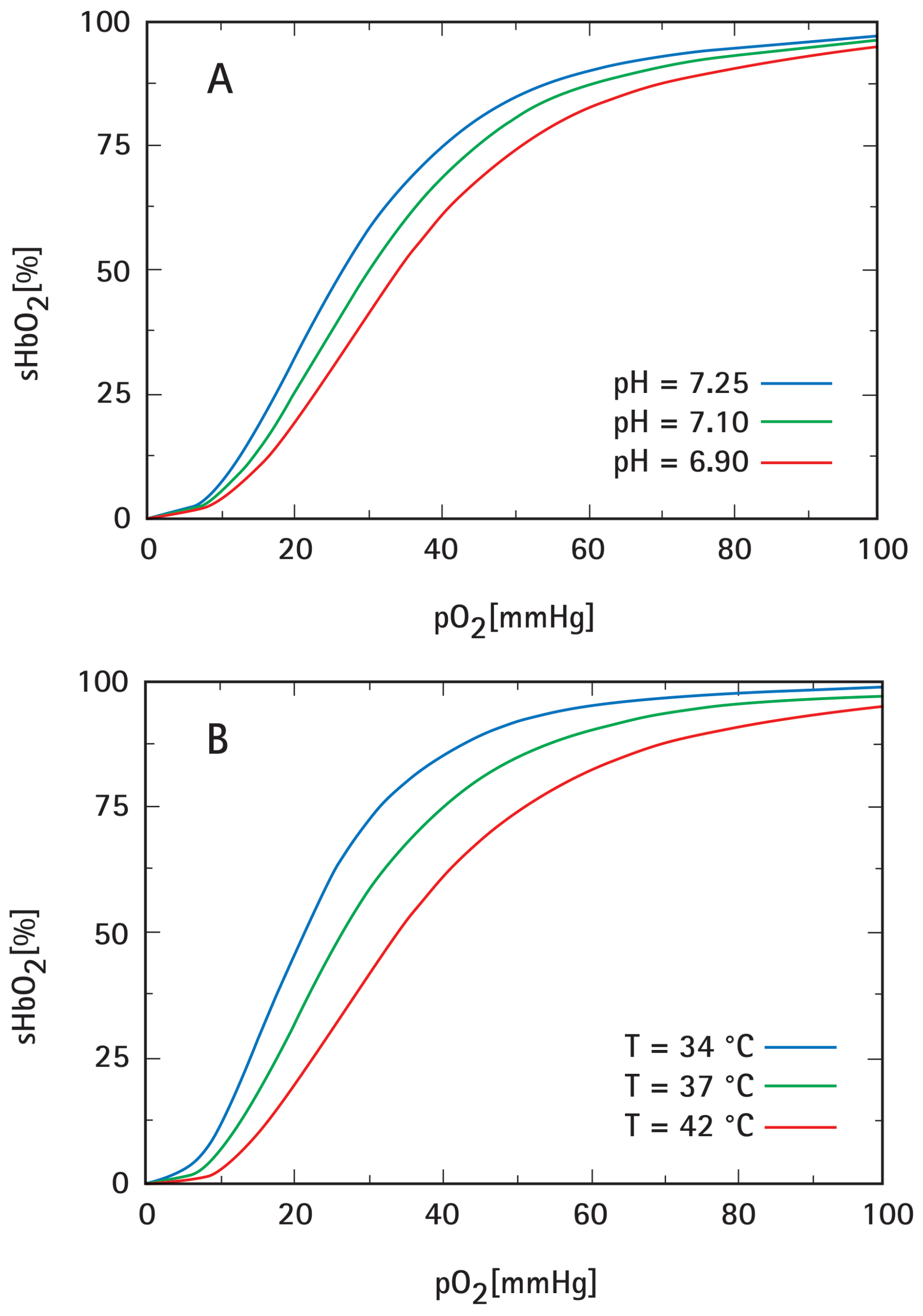
- Severinghaus, J.W. Blood oxygen dissociation line charts: Man. In Biological Data Book, 2nd ed.; Altman, P.L., Dittmar, D.S., Eds.; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 1971; pp. 1871–1872. [Google Scholar]
- Dash, R.K.; Bassingthwaighte, J.B. Blood HbO2 and HbCO2 dissociation curves at varied O2, CO2, pH, 2,3-DPG and temperature levels. Ann. Biomed. Eng. 2010, 38, 1683–1701. [Google Scholar] [CrossRef]
- Woyke, S.; Brugger, H.; Strohle, M.; Haller, T.; Gatterer, H.; Dal Cappello, T.; Strapazzon, G. Effects of carbon dioxide and temperature on the oxygen-hemoglobin dissociation curve of human blood: Implications for avalanche victims. Front. Med. 2021, 8, 808025. [Google Scholar] [CrossRef]
- Gullino, P.M.; Yi, P.N.; Grantham, F.H. Relationship between temperature and blood supply or consumption of oxygen and glucose by rat mammary carcinomas. J. Natl. Cancer Inst. 1978, 60, 835–847. [Google Scholar] [CrossRef]
- Thomsen, A.R.; Saalmann, M.R.; Nicolay, N.H.; Grosu, A.L.; Vaupel, P. Temperature profiles and oxygenation status in human skin and subcutis upon thermography-controlled wIRA-hyperthermia. In Water-Filtered Infrared A (wIRA) Irradiation; Vaupel, P., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 69–80. [Google Scholar]
- Vaupel, P.; Frinak, S.; Mueller-Klieser, W.; Bicher, H.I. Impact of localized hyperthermia on the cellular microenvironment in solid tumors. Natl. Cancer Inst. Monogr. 1982, 61, 207–209. [Google Scholar]
- Tanaka, Y.; Hasegawa, A.; Murata, T. Effect of irradiation and hyperthermia on vascular function in normal and tumor tissue. In Hyperthermic Oncology; Overgaard, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 145–148. [Google Scholar]
- Iwata, K.; Shakil, A.; Hur, W.J.; Makepeace, C.M.; Griffin, R.J.; Song, C.W. Tumour pO2 can be increased markedly by mild hyperthermia. Br. J. Cancer Suppl. 1996, 27, S217–S221. [Google Scholar]
- Shakil, A.; Osborn, J.L.; Song, C.W. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 859–865. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.; Griffin, R.J. Improvement of tumor oxygenation by mild hyperthermia. Radiat. Res. 2001, 155, 515–528. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Thews, O.; Scherz, A.; Salomon, Y.; Vaupel, P. Combined hyperthermia and chlorophyll-based photodynamic therapy: Tumour growth and metabolic microenvironment. Br. J. Cancer 2003, 89, 2333–2339. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. Can mild hyperthermia improve tumour oxygenation? Int. J. Hyperth. 1997, 13, 141–147. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Poulson, J.M.; Gaskin, A.A.; Thrall, D.E.; Page, R.L.; Charles, H.C.; Macfall, J.R.; Brizel, D.M.; Meyer, R.E.; Prescott, D.M.; et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 179–185. [Google Scholar] [CrossRef]
- Thrall, D.E.; Larue, S.M.; Pruitt, A.F.; Case, B.; Dewhirst, M.W. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int. J. Hyperth. 2006, 22, 365–373. [Google Scholar] [CrossRef]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Dodge, R.K.; Charles, H.C.; Samulski, T.V.; Prosnitz, L.R.; Dewhirst, M.W. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996, 56, 5347–5350. [Google Scholar]
- Vujaskovic, Z.; Rosen, E.L.; Blackwell, K.L.; Jones, E.L.; Brizel, D.M.; Prosnitz, L.R.; Samulski, T.V.; Dewhirst, M.W. Ultrasound guided pO2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int. J. Hyperth. 2003, 19, 498–506. [Google Scholar] [CrossRef]
- Jones, E.L.; Prosnitz, L.R.; Dewhirst, M.W.; Marcom, P.K.; Hardenbergh, P.H.; Marks, L.B.; Brizel, D.M.; Vujaskovic, Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin. Cancer. Res. 2004, 10, 4287–4293. [Google Scholar] [CrossRef]
- Vaupel, P.; Thews, G. pO2 distribution in tumor tissue of DS-carcinosarcoma. Oncology 1974, 30, 475–484. [Google Scholar] [CrossRef]
- Notter, M.; Piazena, H.; Vaupel, P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: A retrospective study of 73 patients. Int. J. Hyperth. 2017, 33, 227–236. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaupel, P.; Piazena, H.; Notter, M.; Thomsen, A.R.; Grosu, A.-L.; Scholkmann, F.; Pockley, A.G.; Multhoff, G. From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy. Cancers 2023, 15, 1394. https://doi.org/10.3390/cancers15051394
Vaupel P, Piazena H, Notter M, Thomsen AR, Grosu A-L, Scholkmann F, Pockley AG, Multhoff G. From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy. Cancers. 2023; 15(5):1394. https://doi.org/10.3390/cancers15051394
Chicago/Turabian StyleVaupel, Peter, Helmut Piazena, Markus Notter, Andreas R. Thomsen, Anca-L. Grosu, Felix Scholkmann, Alan Graham Pockley, and Gabriele Multhoff. 2023. "From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy" Cancers 15, no. 5: 1394. https://doi.org/10.3390/cancers15051394
APA StyleVaupel, P., Piazena, H., Notter, M., Thomsen, A. R., Grosu, A.-L., Scholkmann, F., Pockley, A. G., & Multhoff, G. (2023). From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy. Cancers, 15(5), 1394. https://doi.org/10.3390/cancers15051394








