A Triphenylphosphonium-Functionalized Delivery System for an ATM Kinase Inhibitor That Ameliorates Doxorubicin Resistance in Breast Carcinoma Mammospheres
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation and Characterization of KU-Loaded PTPP Nanoparticles
2.3. Cell Culture and Treatments
2.4. Mammosphere Cultures
2.5. MTS Assay
2.6. Quantification of DOX Uptake
2.7. Western Blotting (WB)
2.8. Statistical Analysis
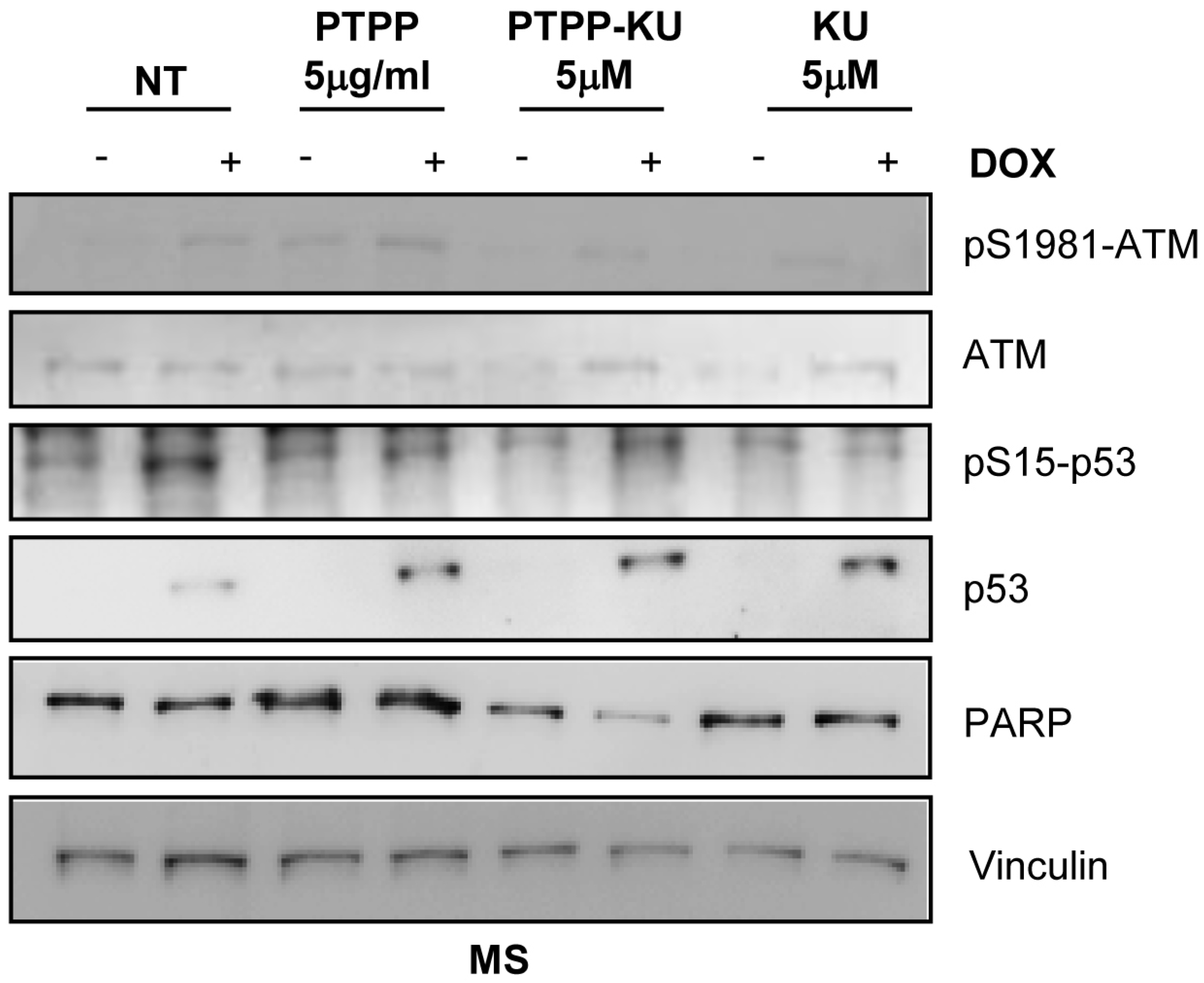
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.-Y.; Guan, Y.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2020, 11, 629266. [Google Scholar] [CrossRef]
- Tu, S.-M.; Guo, C.C.; Chow, D.S.-L.; Zacharias, N.M. Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care. Cancers 2022, 14, 1548. [Google Scholar] [CrossRef]
- Hickson, I.; Zhao, Y.; Richardson, C.J.; Green, S.J.; Martin, N.M.B.; Orr, A.I.; Reaper, P.M.; Jackson, S.P.; Curtin, N.J.; Smith, G.C.M. Identification and Characterization of a Novel and Specific Inhibitor of the Ataxia-Telangiectasia Mutated Kinase ATM. Cancer Res. 2004, 64, 9152–9159. [Google Scholar] [CrossRef]
- Stagni, V.; Orecchia, S.; Mignini, L.; Beji, S.; Antonioni, A.; Caggiano, C.; Barilà, D.; Bielli, P.; Sette, C. DNA Damage Regulates the Functions of the RNA Binding Protein Sam68 through ATM-Dependent Phosphorylation. Cancers 2022, 14, 3847. [Google Scholar] [CrossRef]
- Moolmuang, B.; Ruchirawat, M. The Antiproliferative Effects of Ataxia-Telangiectasia Mutated and ATM- and Rad3-Related Inhibitions and Their Enhancements with the Cytotoxicity of DNA Damaging Agents in Cholangiocarcinoma Cells. J. Pharm. Pharmacol. 2021, 73, 40–51. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.-Q. The ATM Inhibitor KU-55933 Suppresses Cell Proliferation and Induces Apoptosis by Blocking Akt in Cancer Cells with Overactivated Akt. Mol. Cancer Ther. 2010, 9, 113–125. [Google Scholar] [CrossRef]
- GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group; Wellcome Trust Case Control Consortium 2; Zhou, K.; Bellenguez, C.; Spencer, C.C.A.; Bennett, A.J.; Coleman, R.L.; Tavendale, R.; Hawley, S.A.; Donnelly, L.A.; et al. Common Variants near ATM Are Associated with Glycemic Response to Metformin in Type 2 Diabetes. Nat. Genet. 2011, 43, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.R.E.; Zhang, Y.; Tao, J.; Shen, R.; Zhao, X.; Cleary, M.P.; Wang, T.; Yang, D.-Q. ATM Inhibitor KU-55933 Induces Apoptosis and Inhibits Motility by Blocking GLUT1-Mediated Glucose Uptake in Aggressive Cancer Cells with Sustained Activation of Akt. FASEB J. 2021, 35, e21264. [Google Scholar] [CrossRef] [PubMed]
- Stakyte, K.; Rotheneder, M.; Lammens, K.; Bartho, J.D.; Grädler, U.; Fuchß, T.; Pehl, U.; Alt, A.; van de Logt, E.; Hopfner, K.P. Molecular basis of human ATM kinase inhibition. Nat. Struct. Mol. Biol. 2021, 28, 789–798. [Google Scholar] [CrossRef]
- Tang, H.; Shu, M.; Dai, B.; Xu, L.; Dong, B.; Gao, G.; Chen, X. DNA damage response-initiated cytokine secretion in bone marrow stromal cells promotes chemoresistance of myeloma cells. Leuk. Lymphoma 2018, 59, 2220–2226. [Google Scholar] [CrossRef]
- Shaheen, F.S.; Znojek, P.; Fisher, A.; Webster, M.; Plummer, R.; Gaughan, L.; Smith, G.C.; Leung, H.Y.; Curtin, N.J.; Robson, C.N. Targeting the DNA double strand break repair machinery in prostate cancer. PLoS ONE 2011, 6, e20311. [Google Scholar] [CrossRef]
- Ferri, A.; Stagni, V.; Barilà, D. Targeting the DNA Damage Response to Overcome Cancer Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 4910. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Glass, J. The Phenotypic Radiation Resistance of CD44+/CD24−or low Breast Cancer Cells Is Mediated through the Enhanced Activation of ATM Signaling. PLoS ONE 2011, 6, e24080. [Google Scholar] [CrossRef]
- Antonelli, M.; Strappazzon, F.; Arisi, I.; Brandi, R.; D’Onofrio, M.; Sambucci, M.; Manic, G.; Vitale, I.; Barilà, D.; Stagni, V. ATM Kinase Sustains Breast Cancer Stem-like Cells by Promoting ATG4C Expression and Autophagy. Oncotarget 2017, 8, 21692–21709. [Google Scholar] [CrossRef]
- Andugulapati, S.B.; Sundararaman, A.; Lahiry, M.; Rangarajan, A. AMP-Activated Protein Kinase Promotes Breast Cancer Stemness and Drug Resistance. Dis. Models Mech. 2022, 15, dmm049203. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Vega, Y.A.; Kastan, M.B. A New Role for ATM: Regulating Mitochondrial Function and Mitophagy. Autophagy 2012, 8, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Stagni, V.; Cirotti, C.; Barilà, D. Ataxia-Telangiectasia Mutated Kinase in the Control of Oxidative Stress, Mitochondria, and Autophagy in Cancer: A Maestro With a Large Orchestra. Front. Oncol. 2018, 8, 73. [Google Scholar] [CrossRef]
- Stagni, V.; Ferri, A.; Cirotti, C.; Barilà, D. ATM Kinase-Dependent Regulation of Autophagy: A Key Player in Senescence? Front. Cell Dev. Biol. 2020, 8, 599048. [Google Scholar] [CrossRef]
- Cirotti, C.; Rizza, S.; Giglio, P.; Poerio, N.; Allega, M.F.; Claps, G.; Pecorari, C.; Lee, J.H.; Benassi, B.; Barilà, D.; et al. Redox activation of ATM enhances GSNOR translation to sustain mitophagy and tolerance to oxidative stress. EMBO Rep. 2021, 22, e50500. [Google Scholar] [CrossRef]
- Zakikhani, M.; Bazile, M.; Hashemi, S.; Javeshghani, S.; Avizonis, D.; St Pierre, J.; Pollak, M.N. Alterations in Cellular Energy Metabolism Associated with the Antiproliferative Effects of the ATM Inhibitor KU-55933 and with Metformin. PLoS ONE 2012, 7, e49513. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhou, S.; Qin, M.; Tang, J.; Yan, X.; Huang, L.; Huang, M.; Deng, J.; Xiao, D.; Hu, X.; et al. Phenformin and ataxia-telangiectasia mutated inhibitors synergistically co-suppress liver cancer cell growth by damaging mitochondria. FEBS Open Bio 2021, 11, 1440–1451. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Palaniappan, V.V.; Espinosa, J.M. ATM regulates cell fate choice upon p53 activation by modulating mitochondrial turnover and ROS levels. Cell Cycle 2015, 14, 56–63. [Google Scholar] [CrossRef]
- Eaton, J.S.; Lin, Z.P.; Sartorelli, A.C.; Bonawitz, N.D.; Shadel, G.S. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J. Clin. Investig. 2007, 117, 2723–2734. [Google Scholar] [CrossRef]
- Finichiu, P.G.; James, A.M.; Larsen, L.; Smith, R.A.J.; Murphy, M.P. Mitochondrial Accumulation of a Lipophilic Cation Conjugated to an Ionisable Group Depends on Membrane Potential, PH Gradient and PK(a): Implications for the Design of Mitochondrial Probes and Therapies. J. Bioenerg. Biomembr. 2013, 45, 165–173. [Google Scholar] [CrossRef]
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting Mitochondria. Acc. Chem. Res. 2008, 41, 87–97. [Google Scholar] [CrossRef]
- Yamada, Y.; Satrialdi; Hibino, M.; Sasaki, D.; Abe, J.; Harashima, H. Power of Mitochondrial Drug Delivery Systems to Produce Innovative Nanomedicines. Adv. Drug Deliv. Rev. 2020, 154–155, 187–209. [Google Scholar] [CrossRef]
- Milane, L.; Dolare, S.; Jahan, T.; Amiji, M. Mitochondrial Nanomedicine: Subcellular Organelle-Specific Delivery of Molecular Medicines. Nanomedicine 2021, 37, 102422. [Google Scholar] [CrossRef]
- Liew, S.S.; Qin, X.; Zhou, J.; Li, L.; Huang, W.; Yao, S.Q. Smart Design of Nanomaterials for Mitochondria-Targeted Nanotherapeutics. Angew. Chem. Int. Ed. Engl. 2021, 60, 2232–2256. [Google Scholar] [CrossRef] [PubMed]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Triphenylphosphonium Decorated Liposomes and Dendritic Polymers: Prospective Second Generation Drug Delivery Systems for Targeting Mitochondria. Mol. Pharm. 2016, 13, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Boddapati, S.V.; D’Souza, G.M.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-Targeted Nanocarriers: Specific Delivery of Liposomal Ceramide to Mitochondria Enhances Its Cytotoxicity in Vitro and in Vivo. Nano Lett. 2008, 8, 2559–2563. [Google Scholar] [CrossRef]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. WIREs Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef]
- Panagiotaki, K.N.; Sideratou, Z.; Vlahopoulos, S.A.; Paravatou-Petsotas, M.; Zachariadis, M.; Khoury, N.; Zoumpourlis, V.; Tsiourvas, D. A Triphenylphosphonium-Functionalized Mitochondriotropic Nanocarrier for Efficient Co-Delivery of Doxorubicin and Chloroquine and Enhanced Antineoplastic Activity. Pharmaceuticals 2017, 10, 91. [Google Scholar] [CrossRef]
- Stagni, V.; Kaminari, A.; Sideratou, Z.; Sakellis, E.; Vlahopoulos, S.A.; Tsiourvas, D. Targeting Breast Cancer Stem-like Cells Using Chloroquine Encapsulated by a Triphenylphosphonium-Functionalized Hyperbranched Polymer. Int. J. Pharm. 2020, 585, 119465. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.; Guo, F.; Xuan, C. Mitochondrial Membrane Potential and Reactive Oxygen Species in Cancer Stem Cells. Fam. Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Yang, J.; Wu, F.-G. Mitochondria-Targetable Carbon Quantum Dots for Differentiating Cancerous Cells from Normal Cells. Nanoscale 2017, 9, 18368–18378. [Google Scholar] [CrossRef]
- Kaminari, A.; Nikoli, E.; Athanasopoulos, A.; Sakellis, E.; Sideratou, Z.; Tsiourvas, D. Engineering Mitochondriotropic Carbon Dots for Targeting Cancer Cells. Pharmaceuticals 2021, 14, 932. [Google Scholar] [CrossRef]
- Stagni, V.; Manni, I.; Oropallo, V.; Mottolese, M.; Di Benedetto, A.; Piaggio, G.; Falcioni, R.; Giaccari, D.; Di Carlo, S.; Sperati, F.; et al. ATM kinase sustains HER2 tumorigenicity in breast cancer. Nat. Commun. 2015, 16, 6886. [Google Scholar] [CrossRef]
- Singh, B.; Cook, K.R.; Vincent, L.; Hall, C.S.; Berry, J.A.; Multani, A.S.; Lucci, A. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. J. Surg. Res. 2008, 147, 240–246. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Ózsvári, B.; Sotgia, F.; Lisanti, M.P. Dodecyl-TPP targets mitochondria and potently eradicates cancer stem cells (CSCs): Synergy with FDA-approved drugs and natural compounds (vitamin C and berberine). Front. Oncol. 2019, 9, 615. [Google Scholar] [CrossRef]
- Mao, J.; Qiu, L.; Ge, L.; Zhou, J.; Ji, Q.; Yang, Y.; Long, M.; Wang, D.; Teng, L.; Chen, J. Overcoming multidrug resistance by intracellular drug release and inhibiting p-glycoprotein efflux in breast cancer. Biomed. Pharmacother. 2021, 134, 111108. [Google Scholar] [CrossRef]
- Calcagno, A.M.; Salcido, C.D.; Gillet, J.P.; Wu, C.P.; Fostel, J.M.; Mumau, M.D.; Gottesman, M.M.; Varticovski, L.; Ambudkar, S.V. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl. Cancer Inst. 2010, 102, 1637–1652. [Google Scholar] [CrossRef]
- Tsou, S.H.; Hou, M.H.; Hsu, L.C.; Chen, T.M.; Chen, Y.H. Gain-of-function p53 mutant with 21-bp deletion confers susceptibility to multidrug resistance in MCF-7 cells. Int. J. Mol. Med. 2016, 37, 233–242. [Google Scholar] [CrossRef]
- Arfaoui, A.; Douik, H.; Kablouti, G.; Chaaben, A.B.; Handiri, N.; Zid, Z.; Ouni, N.; Zouiouch, F.; Ayari, F.; Mamoughli, T.; et al. Role of p53 Codon72 SNP in breast cancer risk and anthracycline resistance. Anticancer Res. 2015, 35, 1763–1769. [Google Scholar]
- Markandeywar, T.S.; Narang, R.K.; Singh, D.; Rai, V.K. Targeted Delivery of Doxorubicin as a Potential Chemotherapeutic Agent. Curr. Drug Deliv. 2022. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Gao, Z.; Huang, W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm. Sin. B 2021, 11, 55–70. [Google Scholar] [CrossRef]
- Abrahams, C.; Woudberg, N.J.; Lecour, S. Anthracycline-induced cardiotoxicity: Targeting high-density lipoproteins to limit the damage? Lipids Health Dis. 2022, 21, 85. [Google Scholar] [CrossRef]
- Czupiel, P.; Delplace, V.; Shoichet, M. Nanoparticle Delivery of a pH-sensitive Prodrug of Doxorubicin and a Mitochondrial Targeting VES-H8R8 Synergistically Kill Multi-drug Resistant Breast Cancer Cells. Sci. Rep. 2020, 10, 8726. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Chen, G.; Feng, B.; Zhang, T.P.; Ma, E.L.; Wu, Y.D. Microarray Analysis of Gene Expression Profile of Multidrug Resistance in Pancreatic Cancer. Chin. Med. J. 2007, 120, 1743–1752. [Google Scholar] [CrossRef]
- Dinic, J.; Podolski-Renic, A.; Stankovic, T.; Bankovic, J.; Pesic, M. New Approaches with Natural Product Drugs for Overcoming Multidrug Resistance in Cancer. Curr. Pharm. Des. 2015, 21, 5589–5604. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Chang, J.-C.; Chang, H.-S.; Wu, Y.-C.; Cheng, W.-L.; Lin, T.-T.; Chang, H.-J.; Kuo, S.-J.; Chen, S.-T.; Liu, C.-S. Mitochondrial Transplantation Regulates Antitumour Activity, Chemoresistance and Mitochondrial Dynamics in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 30. [Google Scholar] [CrossRef]
- Liang, L.; Peng, Y.; Qiu, L. Mitochondria-Targeted Vitamin E Succinate Delivery for Reversal of Multidrug Resistance. J. Control. Release 2021, 337, 117–131. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.C.; Chen, Y.; Shi, X.B.; Xing, Z.H.; He, Z.J.; Wang, S.T.; Liu, W.J.; Zhang, P.W.; Yu, Z.Z.; et al. AZ32 Reverses ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Front. Oncol. 2021, 11, 680663. [Google Scholar] [CrossRef]
- Kurz, E.U.; Douglas, P.; Lees-Miller, S.P. Doxorubicin Activates ATM-dependent Phosphorylation of Multiple Downstream Targets in Part Through the Generation of Reactive Oxygen Species. J. Biol. Chem. 2004, 279, 53272–53281. [Google Scholar] [CrossRef]
- Stagni, V.; Santini, S.; Barilà, D. A New Player in the Development of TRAIL Based Therapies for Hepatocarcinoma Treatment: ATM Kinase. Cancers 2012, 4, 354–378. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Li, W.; Su, Q.; Huang, Z.; Zhang, X.; Chen, H.; Mo, C.; Huang, B.; Ou, W.; et al. ATM/NEMO Signaling Modulates the Expression of PD-L1 Following Docetaxel Chemotherapy in Prostate Cancer. J. Immunother. Cancer 2021, 9, e001758. [Google Scholar] [CrossRef]
- Zhang, Q.; Green, M.D.; Lang, X.; Lazarus, J.; Parsels, J.D.; Wei, S.; Parsels, L.A.; Shi, J.; Ramnath, N.; Wahl, D.R.; et al. Inhibition of ATM Increases Interferon Signaling and Sensitizes Pancreatic Cancer to Immune Checkpoint Blockade Therapy. Cancer Res. 2019, 79, 3940–3951. [Google Scholar] [CrossRef]
- Fortini, P.; Ferretti, C.; Pascucci, B.; Narciso, L.; Pajalunga, D.; Puggioni, E.M.R.; Castino, R.; Isidoro, C.; Crescenzi, M.; Dogliotti, E. DNA Damage Response by Single-Strand Breaks in Terminally Differentiated Muscle Cells and the Control of Muscle Integrity. Cell Death Differ. 2012, 19, 1741–1749. [Google Scholar] [CrossRef]
- Tomasini, P.P.; Guecheva, T.N.; Leguisamo, N.M.; Péricart, S.; Brunac, A.-C.; Hoffmann, J.S.; Saffi, J. Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer. Cancers 2021, 13, 3130. [Google Scholar] [CrossRef]
- Ke, X.; Yang, C.; Cheng, W.; Yang, Y.Y. Delivery of NF-ΚB ShRNA Using Carbamate-Mannose Modified PEI for Eliminating Cancer Stem Cells. Nanomedicine 2018, 14, 405–414. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-ΚB be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070. [Google Scholar] [CrossRef] [PubMed]
- Abdin, S.M.; Tolba, M.F.; Zaher, D.M.; Omar, H.A. Nuclear Factor-ΚB Signaling Inhibitors Revert Multidrug-Resistance in Breast Cancer Cells. Chem. Biol. Interact. 2021, 340, 109450. [Google Scholar] [CrossRef]
- Vlahopoulos, S.A. Aberrant Control of NF-ΚB in Cancer Permits Transcriptional and Phenotypic Plasticity, to Curtail Dependence on Host Tissue: Molecular Mode. Cancer Biol. Med. 2017, 14, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Vlahopoulos, S.; Adamaki, M.; Khoury, N.; Zoumpourlis, V.; Boldogh, I. Roles of DNA Repair Enzyme OGG1 in Innate Immunity and Its Significance for Lung Cancer. Pharmacol. Ther. 2019, 194, 59–72. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Z.; Master, R.P.; Maharjan, C.K.; Carelock, M.E.; Reccoppa, T.B.A.; Kim, M.-C.; Kolb, R.; Zhang, W. Breast Cancer Stem Cells: Signaling Pathways, Cellular Interactions, and Therapeutic Implications. Cancers 2022, 14, 3287. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagni, V.; Kaminari, A.; Contadini, C.; Barilà, D.; Sessa, R.L.; Sideratou, Z.; Vlahopoulos, S.A.; Tsiourvas, D. A Triphenylphosphonium-Functionalized Delivery System for an ATM Kinase Inhibitor That Ameliorates Doxorubicin Resistance in Breast Carcinoma Mammospheres. Cancers 2023, 15, 1474. https://doi.org/10.3390/cancers15051474
Stagni V, Kaminari A, Contadini C, Barilà D, Sessa RL, Sideratou Z, Vlahopoulos SA, Tsiourvas D. A Triphenylphosphonium-Functionalized Delivery System for an ATM Kinase Inhibitor That Ameliorates Doxorubicin Resistance in Breast Carcinoma Mammospheres. Cancers. 2023; 15(5):1474. https://doi.org/10.3390/cancers15051474
Chicago/Turabian StyleStagni, Venturina, Archontia Kaminari, Claudia Contadini, Daniela Barilà, Rosario Luigi Sessa, Zili Sideratou, Spiros A. Vlahopoulos, and Dimitris Tsiourvas. 2023. "A Triphenylphosphonium-Functionalized Delivery System for an ATM Kinase Inhibitor That Ameliorates Doxorubicin Resistance in Breast Carcinoma Mammospheres" Cancers 15, no. 5: 1474. https://doi.org/10.3390/cancers15051474
APA StyleStagni, V., Kaminari, A., Contadini, C., Barilà, D., Sessa, R. L., Sideratou, Z., Vlahopoulos, S. A., & Tsiourvas, D. (2023). A Triphenylphosphonium-Functionalized Delivery System for an ATM Kinase Inhibitor That Ameliorates Doxorubicin Resistance in Breast Carcinoma Mammospheres. Cancers, 15(5), 1474. https://doi.org/10.3390/cancers15051474








