Tumor Microenvironment and Immune Response in Lip Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of TILs
2.2. Assessment of TLSs
2.3. Assessment of Tumor-Budding (TB)
2.4. Immunohistochemistry
- SMA: Mouse monoclonal, 1A4 (DAKO), dilution 1:50, 60 min incubation
- Ki-67: Mouse monoclonal, MIB1 (DAKO), dilution 1:60, 60 min incubation
- p16: Mouse monoclonal, IHC016 (GenomeMe), dilution 1:100, 60 min incubation
- HIF1α: Mouse monoclonal, ESEE122 (OXFORD), dilution 1:10, overnight incubation
- LDH5: Mouse monoclonal, clone Ab9002 (Abcam, UK), dilution 1:200, overnight incubation
- CD4: Mouse monoclonal, 4B12 (DAKO), dilution 1:40, 60 min incubation
- CD8: Mouse monoclonal, C8/144B (DAKO), ready to use, 60 min incubation
- FOXP3: Mouse monoclonal, 236A/37 (OXFORD), dilution 1:50, overnight incubation
- CD20: Mouse monoclonal, clone L26, (DAKO), dilution 1:200, 60 min incubation
- CD68: Mouse monoclonal, clone KP1 (Immunologic), dilution 1:300, 60 min incubation
- CD31: Mouse monoclonal, clone JC70 (DAKO), dilution 1:20, 30 min incubation
- VEGF: Mouse monoclonal, clone VG1 (Diagnostic Biosystems), dilution 1:30, 60 min incubation
2.5. Assessment of Immunohistochemical Markers
2.6. Statistical Analysis
3. Results
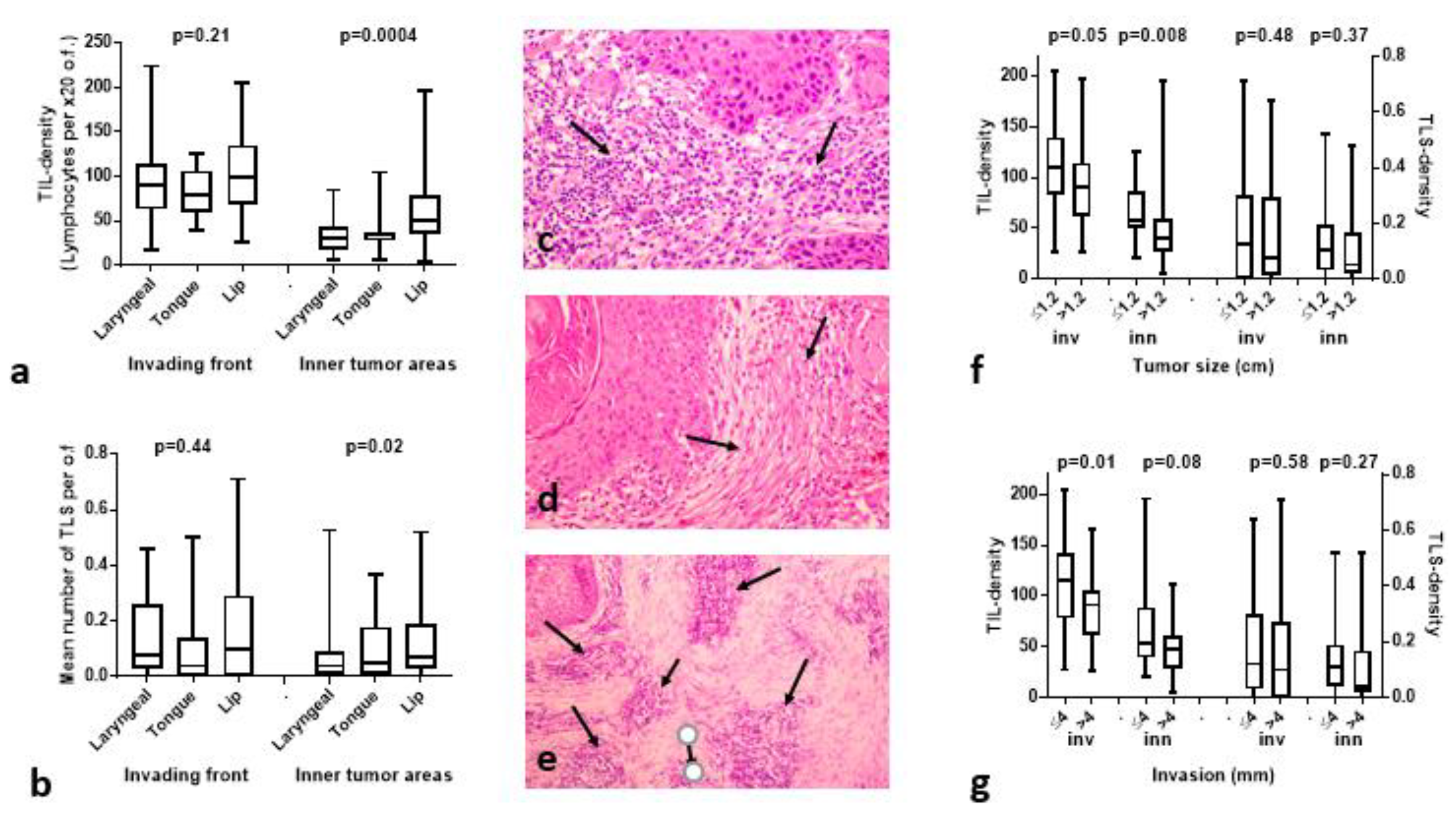
3.1. TIL and TLS Density Is Higher in Lip Cancer Compared to Other HNCs
3.2. Histopathological Variables vs. TILs/TLSs
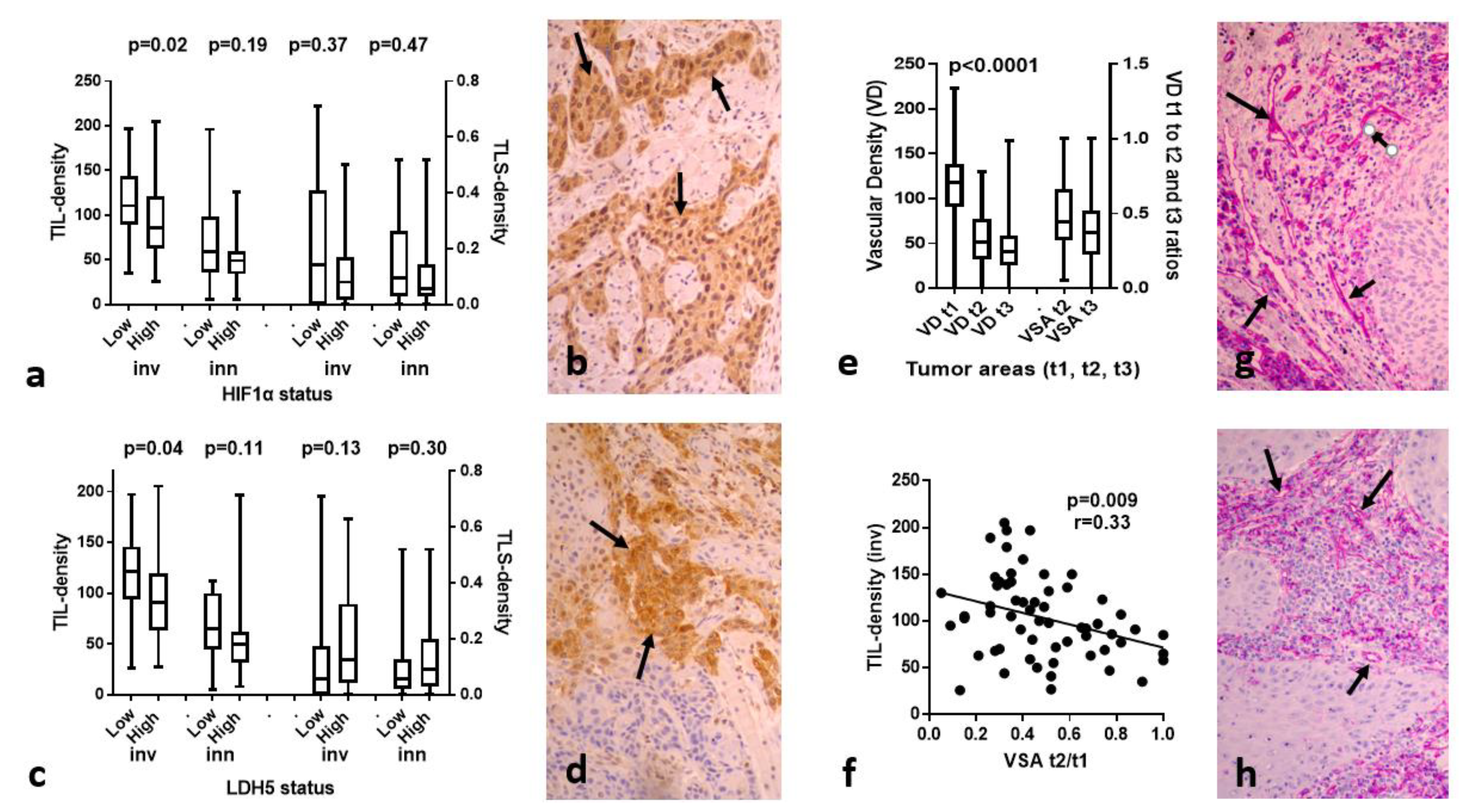
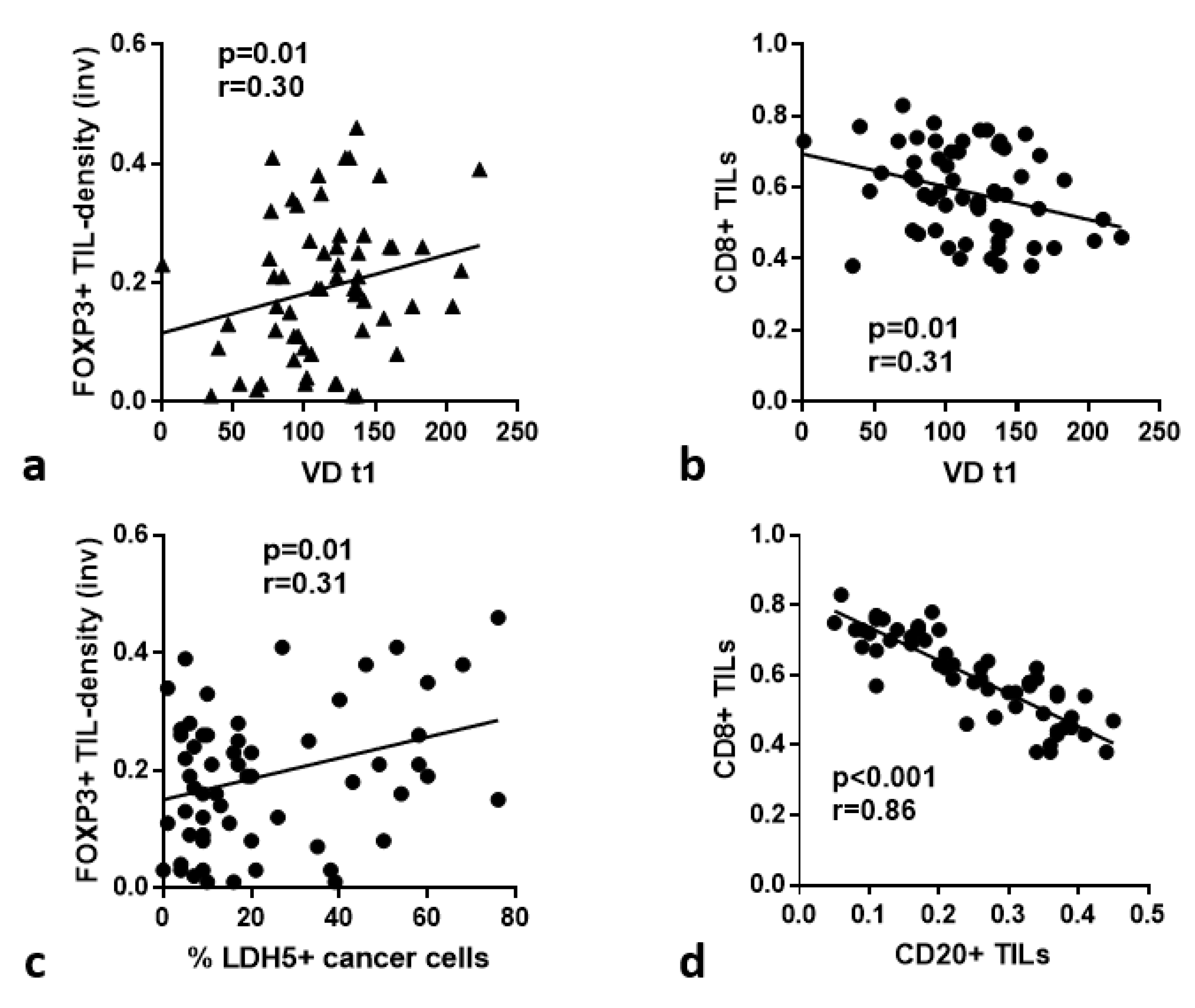
3.3. HIF1α, LDH5 vs. TILs/TLSs
3.4. VD and VSA vs. TILs/TLSs
3.5. p16, MIB1 vs. TILs/TLSs
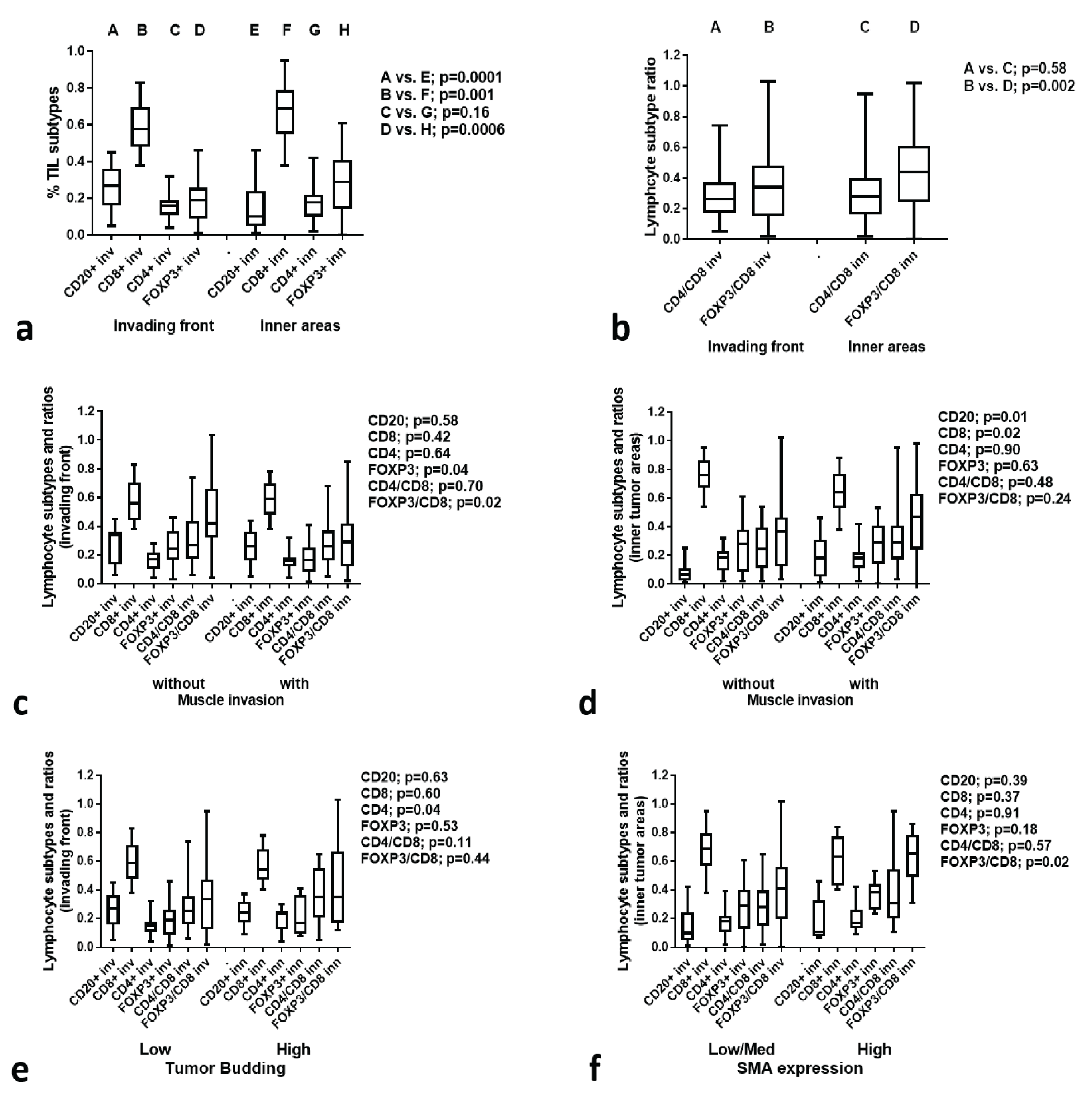
3.6. TIL Subtype Analysis
3.7. TIL Subtypes vs. Histological Variables
3.8. TIL Subtypes vs. Immunohistochemical Variables
3.9. CD68+ Macrophage Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vukadinovic, M.; Jezdic, Z.; Petrovic, M.; Medenica, L.M.; Lens, M. Surgical management of squamous cell carcinoma of the lip: Analysis of a 10-year experience in 223 patients. J. Oral Maxillofac. Surg. 2007, 65, 675–679. [Google Scholar] [CrossRef]
- Casal, D.; Carmo, L.; Melancia, T.; Zagalo, C.; Cid, O.; Rosa-Santos, J. Lip cancer: A 5-year review in a tertiary referral centre. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 2040–2045. [Google Scholar] [CrossRef]
- Salgarelli, A.C.; Sartorelli, F.; Cangiano, A.; Collini, M. Treatment of lower lip cancer: An experience of 48 cases. Int. J. Oral Maxillofac. Surg. 2005, 34, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.A.; Sciubba, J.J.; Jordan, R.C.K. Oral Pathology: Clinical Pathologic Correlations, 6th ed.; Elsevier Saunders: St. Louis, MO, USA, 2011; 388p. [Google Scholar]
- Salgarelli, A.C.; Sartorelli, F.; Cangiano, A.; Pagani, R.; Collini, M. Surgical treatment of lip cancer: Our experience with 106 cases. J. Oral Maxillofac. Surg. 2009, 67, 840–845. [Google Scholar] [CrossRef]
- Pogoda, J.M.; Preston-Martin, S. Solar radiation, lip protection, and lip cancer risk in Los Angeles County women (California, United States). Cancer Causes Control 1996, 7, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Khuder, S.A. Etiologic clues to lip cancer from epidemiologic studies on farmers. Scand. J. Work. Environ. Health 1999, 25, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Czerninski, R.; Zini, A.; Sgan-Cohen, H.D. Lip cancer: Incidence, trends, histology and survival: 1970–2006. Br. J. Dermatol. 2010, 162, 1103–1109. [Google Scholar] [CrossRef]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Bhandari, K.; Wang, D.C.; Li, S.C.; Jiang, B.H.; Guo, Y.X.; Koirala, U.; Du, X.Y. Primary cN0 lip squamous cell carcinoma and elective neck dissection: Systematic review and meta-analysis. Head Neck 2015, 37, 1392–1400. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Bjerkli, I.H.; Jetlund, O.; Karevold, G.; Karlsdóttir, Á.; Jaatun, E.; Uhlin-Hansen, L.; Rikardsen, O.G.; Hadler-Olsen, E.; Steigen, S.E. Characteristics and prognosis of primary treatment-naïve oral cavity squamous cell carcinoma in Norway, a descriptive retrospective study. PLoS ONE 2020, 15, e0227738. [Google Scholar] [CrossRef] [PubMed]
- Duhen, R.; Ballesteros-Merino, C.; Frye, A.K.; Tran, E.; Rajamanickam, V.; Chang, S.C.; Koguchi, Y.; Bifulco, C.B.; Bernard, B.; Leidner, R.S.; et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat. Commun. 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Hudson, J.L.; Peterson, K.A.; Miller, H.L.; McClatchey, K.D. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: Correlations with extent of tumor and prognosis. Otolaryngol. Head Neck Surg. 1986, 95, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemo-radiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.P.; Bhaskaran, M.K.; George, A.L.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in Oral Cancer. J. Pharm. Bioallied Sci. 2019, 11, S107–S111. [Google Scholar] [CrossRef]
- Kujan, O.; van Schaijik, B.; Farah, C.S. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers 2020, 12, 1937. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A. Warburg effect, lactate dehydrogenase, and radio/chemotherapy efficacy. Int. J. Radiat. Biol. 2019, 95, 408–426. [Google Scholar] [CrossRef]
- Everin, T.; Müller, B.; Giese, G.; Uhl, B.; Wolf, B.; Hauschildt, S.; Kreutz, W. pH-dependent LAK cell cytotoxicity. Tumour Biol. 1994, 15, 304–310. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Juneau, P.L.; Heppner, G.H. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int. J. Cancer 1991, 48, 895–899. [Google Scholar] [CrossRef]
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; De Milito, A.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012, 72, 2746–2756. [Google Scholar] [CrossRef] [Green Version]
- Giatromanolaki, A.; Gkegka, A.G.; Pouliliou, S.; Biziota, E.; Kakolyris, S.; Koukourakis, M. Hypoxia and anaerobic metabolism relate with immunologically cold breast cancer and poor prognosis. Breast Cancer Res. Treat. 2022, 194, 13–23. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, I.M.; Balaska, K.; Mitrakas, A.G.; Harris, A.L.; Koukourakis, M.I. Programmed death-1 receptor (PD-1) and PD-ligand-1 (PD-L1) expression in non-small cell lung cancer and the immune-suppressive effect of anaerobic glycolysis. Med. Oncol. 2019, 36, 76. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [Green Version]
- Giatromanolaki, A.; Koukourakis, M.I.; Koutsopoulos, A.; Mendrinos, S.; Sivridis, E. The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. Cancer Biol. Ther. 2012, 13, 1284–1289. [Google Scholar] [CrossRef] [Green Version]
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; O’Byrne, K.; Gatter, K.C.; Harris, A.L. ‘Invading edge vs. inner’ (edvin) patterns of vascularization: An interplay between angiogenic and vascular survival factors defines the clinical behaviour of non-small cell lung cancer. J. Pathol. 2000, 192, 140–149. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Koukourakis, M.I. The pathology of tumor stromatogenesis. Cancer Biol. Ther. 2007, 6, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, J.P.; Sánchez-Canteli, M.; López, F.; Wolf, G.T.; Hernández-Prera, J.C.; Williams, M.D.; Willems, S.M.; Franchi, A.; Coca-Pelaz, A.; Ferlito, A. Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Biomedicines 2021, 9, 486. [Google Scholar] [CrossRef]
- Koukourakis, I.M.; Gkegka, A.G.; Xanthopoulou, E.; Nanos, C.; Giatromanolaki, A.; Koukourakis, M.I. Prognostic and Predictive Relevance of Tumor-Infiltrating Lymphocytes in Squamous Cell Head-Neck Cancer Patients Treated with Radical Radiotherapy/Chemo-Radiotherapy. Curr. Oncol. 2022, 29, 4274–4284. [Google Scholar] [CrossRef]
- De Keukeleire, S.J.; Vermassen, T.; Hilgert, E.; Creytens, D.; Ferdinande, L.; Rottey, S. Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives. Cancers 2021, 13, 1714. [Google Scholar] [CrossRef]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Jacquelot, N.; Tellier, J.; Nutt, S.; Belz, G. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [PubMed] [Green Version]
- Buisseret, L.; Desmedt, C.; Garaud, S.; Fornili, M.; Wang, X.; Van den Eyden, G.; de Wind, A.; Duquenne, S.; Boisson, A.; Naveaux, C.; et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod. Pathol. 2017, 30, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Werner, F.; Wagner, C.; Simon, M.; Glatz, K.; Mertz, K.D.; Läubli, H.; Griss, J.; Wagner, S.N. A Standardized Analysis of Tertiary Lymphoid Structures in Human Melanoma: Disease Progression- and Tumor Site-Associated Changes With Germinal Center Alteration. Front. Immunol. 2021, 12, 675146. [Google Scholar] [CrossRef] [PubMed]
- Rakaee, M.; Kilvaer, T.K.; Jamaly, S.; Berg, T.; Paulsen, E.E.; Berglund, M.; Richardsen, E.; Andersen, S.; Al-Saad, S.; Poehl, M.; et al. Tertiary lymphoid structure score: A promising approach to refine the TNM staging in resected non-small cell lung cancer. Br. J. Cancer 2021, 124, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, A.; Moraru, L.; Lupu, M.; Vasilescu, F.; Dumitrescu, M.; Cioplea, M.; Popp, C.; Dragusin, A.; Caruntu, C.; Zurac, S. Prognostic Potential of Tumor-Infiltrating Immune Cells in Resectable Oral Squamous Cell Carcinoma. Cancers 2021, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Zancope, E.; Costa, N.L.; Junqueira-Kipnis, A.P.; Valadares, M.C.; Silva, T.A.; Leles, C.R.; Mendonça, E.F.; Batista, A.C. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J. Oral. Pathol. Med. 2010, 39, 162–167. [Google Scholar] [CrossRef]
- Green, V.L.; Michno, A.; Stafford, N.D.; Greenman, J. Increased prevalence of tumour infiltrating immune cells in oropharyngeal tumours in comparison to other subsites: Relationship to peripheral immunity. Cancer Immunol. Immunother. 2013, 62, 863–873. [Google Scholar] [CrossRef]
- Noda, Y.; Ishida, M.; Ueno, Y.; Fujisawa, T.; Iwai, H.; Tsuta, K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: A retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer 2022, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.; Fulgêncio, L.B.; DEMendonça, E.F.; Leles, C.R.; Batista, A.C.; DASilva, T.A. T regulatory cell markers in oral squamous cell carcinoma: Relationship with survival and tumor aggressiveness. Oncol. Lett. 2010, 1, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.; Chakrabarti, S.; Deb, A.R. Tumor infiltrating lymphocytes in head and neck squamous cell carcinoma—Evaluation and clinical impact. J. Cancer Res. Ther. 2022, 18, 49–54. [Google Scholar] [PubMed]
- Jiang, L.; Wang, X.; Tan, Y.; Liang, D.; Yang, B. To assess the significance and prognostic value of tumor-infiltrating lymphocytes before and after chemotherapy in head and neck squamous cell carcinoma patients. Rev. Argent. Clínica Psicológica 2020, XXIX, 1103–1111. [Google Scholar]
- Tian, C.; Jing, H.; Wang, C.; Wang, W.; Cui, Y.; Chen, J.; Sha, D. Prognostic role of tumour-infiltrating lymphocytes assessed by H&E-stained section in gastric cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e044163. [Google Scholar]
- Zidlik, V.; Brychtova, S.; Uvirova, M.; Ziak, D.; Dvorackova, J. The changes of angiogenesis and immune cell infiltration in the intra- and peri-tumoral melanoma microenvironment. Int. J. Mol. Sci. 2015, 16, 7876–7889. [Google Scholar] [CrossRef] [Green Version]
- Lang-Schwarz, C.; Melcher, B.; Haumaier, F.; Schneider-Fuchs, A.; Lang-Schwarz, K.; Krugmann, J.; Vieth, M.; Sterlacci, W. Budding, tumor-infiltrating lymphocytes, gland formation: Scoring leads to new prognostic groups in World Health Organization low-grade colorectal cancer with impact on survival. Hum. Pathol. 2019, 89, 81–89. [Google Scholar] [CrossRef]
- Safaa, M.M.; El Khalek, A.; Halim, M.I. Tumor budding in gastric adenocarcinoma; reflections on tumor microenvironment and programmed death ligand 1 (PD-L1) expression. Immunopathol. Persa. 2023, 9, e31365. [Google Scholar]
- Zhang, N.; Wang, D.; Duan, Y.; Ayarick, V.A.; Cao, M.; Wang, Y.; Zhang, G.; Wang, Y. The special immune microenvironment of tumor budding and its impact on prognosis in gastric adenocarcinoma. Pathol. -Res. Pr. 2020, 216, 152926. [Google Scholar] [CrossRef]
- Wang, E.; Shibutani, M.; Nagahara, H.; Fukuoka, T.; Iseki, Y.; Okazaki, Y.; Kashiwagi, S.; Tanaka, H.; Maeda, K.; Hirakawa, K.; et al. Abundant intratumoral fibrosis prevents lymphocyte infiltration into peritoneal metastases of colorectal cancer. PLoS ONE 2021, 16, e0255049. [Google Scholar] [CrossRef] [PubMed]
- Zadka, Ł.; Chabowski, M.; Grybowski, D.; Piotrowska, A.; Dzięgiel, P. Interplay of stromal tumor-infiltrating lymphocytes, normal colonic mucosa, cancer-associated fibroblasts, clinicopathological data and the immunoregulatory molecules of patients diagnosed with colorectal cancer. Cancer Immunol. Immunother. 2021, 70, 2681–2700. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, C.; Yuan, X.; Feng, Z.; Han, Z. Prognostic Value of Tumor-Infiltrating Lymphocytes for Patients With Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2017, 10, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, K.M.; Le, A.; Duong, H.; Wu, Y.; Zhang, Q.; Messadi, D.V. Correlation between VEGF and HIF-1α expression in human oral squamous cell carcinoma. Exp. Mol. Pathol. 2004, 76, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Simopoulos, C.; Polychronidis, A.; Sivridis, E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin. Exp. Metastasis 2005, 22, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Büchler, P.; Reber, H.A.; Büchler, M.; Shrinkante, S.; Büchler, M.W.; Friess, H.; Semenza, G.L.; Hines, O.J. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas 2003, 26, 56–64. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, G.; Bag, S.; Chakraborty, P.; Dey, D.; Roy, S.; Jain, P.; Roy, P.; Soong, R.; Majumder, P.P.; Dutt, S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE 2020, 15, e0242058. [Google Scholar] [CrossRef]
- Cho, Y.A.; Yoon, H.J.; Lee, J.I.; Hong, S.P.; Hong, S.D. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011, 47, 1148–1153. [Google Scholar] [CrossRef]
- Lequerica-Fernández, P.; Suárez-Canto, J.; Rodriguez-Santamarta, T.; Rodrigo, J.P.; Suárez-Sánchez, F.J.; Blanco-Lorenzo, V.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers. Biomedicines 2021, 9, 653. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Sun, J.; Zhang, J.; Lin, L.; Hu, C. The presence of tumour-infiltrating lymphocytes (TILs) and the ratios between different subsets serve as prognostic factors in advanced hypopharyngeal squamous cell carcinoma. BMC Cancer 2020, 20, 731. [Google Scholar] [CrossRef]
- Jang, T.J. Progressive Increase of Regulatory T Cells and Decrease of CD8+ T Cells and CD8+ T Cells/Regulatory T Cells Ratio during Colorectal Cancer Development. Korean J. Pathol. 2013, 47, 443–451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkegka, A.G.; Koukourakis, M.I.; Lambropoulou, M.; Giatromanolaki, A. Tumor Microenvironment and Immune Response in Lip Cancer. Cancers 2023, 15, 1478. https://doi.org/10.3390/cancers15051478
Gkegka AG, Koukourakis MI, Lambropoulou M, Giatromanolaki A. Tumor Microenvironment and Immune Response in Lip Cancer. Cancers. 2023; 15(5):1478. https://doi.org/10.3390/cancers15051478
Chicago/Turabian StyleGkegka, Anastasia G., Michael I. Koukourakis, Maria Lambropoulou, and Alexandra Giatromanolaki. 2023. "Tumor Microenvironment and Immune Response in Lip Cancer" Cancers 15, no. 5: 1478. https://doi.org/10.3390/cancers15051478
APA StyleGkegka, A. G., Koukourakis, M. I., Lambropoulou, M., & Giatromanolaki, A. (2023). Tumor Microenvironment and Immune Response in Lip Cancer. Cancers, 15(5), 1478. https://doi.org/10.3390/cancers15051478







