Multidimensional Oncological Frailty Scale (MOFS): A New Quick-To-Use Tool for Detecting Frailty and Stratifying Risk in Older Patients with Cancer—Development and Validation Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
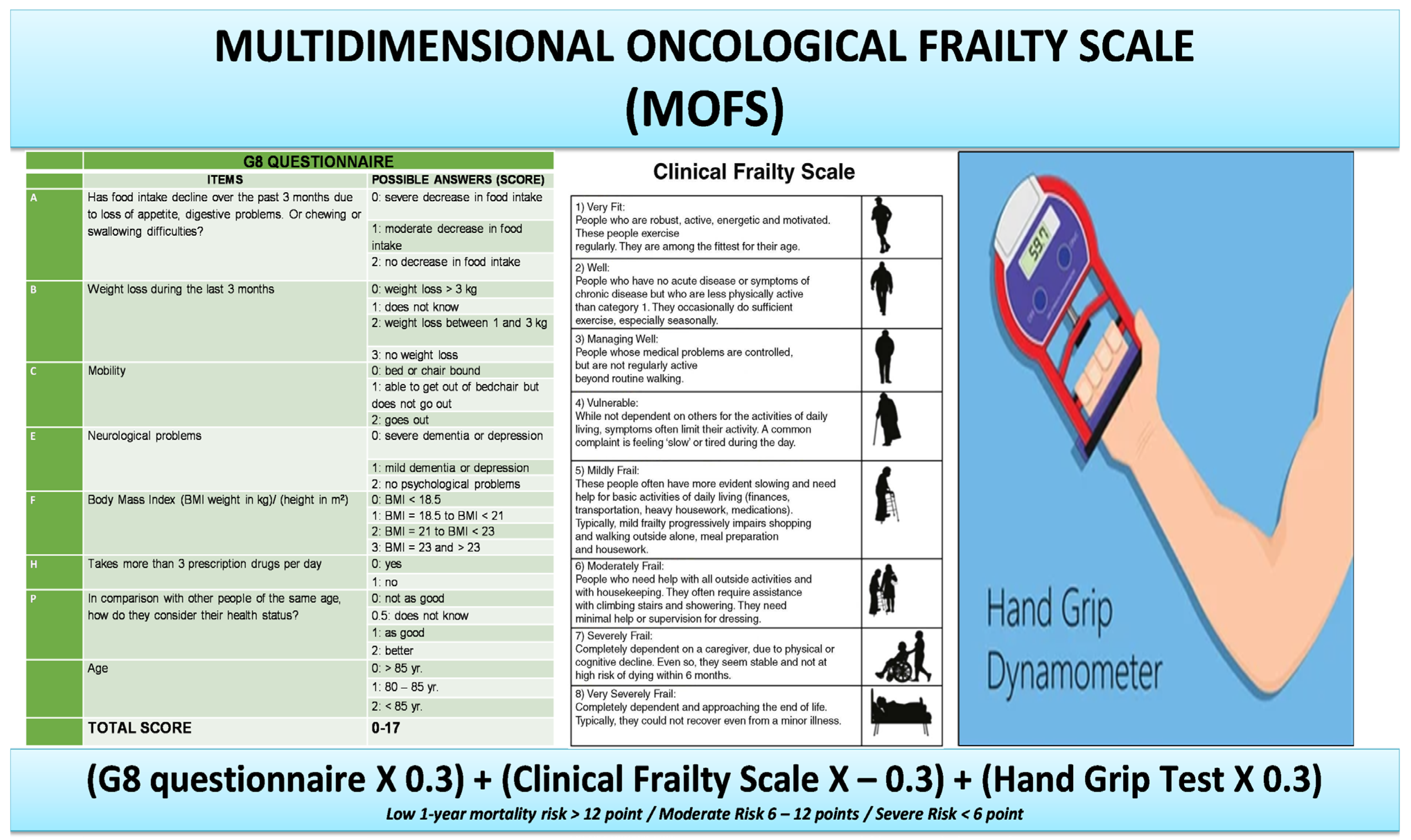
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global Cancer Incidence in Older Adults, 2012 and 2035: A Population-Based Study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarchand, G.R.; Morrison, V.; Klein, M.A.; Watkins, E. Use of Comprehensive Geriatric Assessment in Oncology Patients to Guide Treatment Decisions and Predict Chemotherapy Toxicity. Fed. Pract. 2021, 38, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Velarde, C.; Hurria, A.; Magnuson, A.; Lowenstein, L.; Pandya, C.; O’Donovan, A.; Gorawara-Bhat, R.; Dale, W. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J. Natl. Compr. Cancer Netw. 2015, 13, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- van Winden, M.E.C.; Garcovich, S.; Peris, K.; Colloca, G.; de Jong, E.M.G.J.; Hamaker, M.E.; van de Kerkhof, P.C.M.; Lubeek, S.F.K. Frailty Screening in Dermato-Oncology Practice: A Modified Delphi Study and a Systematic Review of the Literature. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 95–104. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Prins, M.C.; Stauder, R. The Relevance of a Geriatric Assessment for Elderly Patients with a Haematological Malignancy—A Systematic Review. Leuk. Res. 2014, 38, 275–283. [Google Scholar] [CrossRef]
- McCarthy, A.L.; Peel, N.M.; Gillespie, K.M.; Berry, R.; Walpole, E.; Yates, P.; Hubbard, R.E. Validation of a Frailty Index in Older Cancer Patients with Solid Tumours. BMC Cancer 2018, 18, 892. [Google Scholar] [CrossRef] [Green Version]
- Mohile, S.G.; Xian, Y.; Dale, W.; Fisher, S.G.; Rodin, M.; Morrow, G.R.; Neugut, A.; Hall, W. Association of a Cancer Diagnosis with Vulnerability and Frailty in Older Medicare Beneficiaries. J. Natl. Cancer Inst. 2009, 101, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The Prevalence and Outcomes of Frailty in Older Cancer Patients: A Systematic Review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Kristjansson, S.R.; Nesbakken, A.; Jordhøy, M.S.; Skovlund, E.; Audisio, R.A.; Johannessen, H.-O.; Bakka, A.; Wyller, T.B. Comprehensive Geriatric Assessment Can Predict Complications in Elderly Patients after Elective Surgery for Colorectal Cancer: A Prospective Observational Cohort Study. Crit. Rev. Oncol. Hematol. 2010, 76, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Clough-Gorr, K.M.; Thwin, S.S.; Stuck, A.E.; Silliman, R.A. Examining Five- and Ten-Year Survival in Older Women with Breast Cancer Using Cancer-Specific Geriatric Assessment. Eur. J. Cancer 2012, 48, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and Cancer: Implications for Oncology Surgery, Medical Oncology, and Radiation Oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening Older Cancer Patients: First Evaluation of the G-8 Geriatric Screening Tool. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Brunello, A.; Fontana, A.; Zafferri, V.; Panza, F.; Fiduccia, P.; Basso, U.; Copetti, M.; Lonardi, S.; Roma, A.; Falci, C.; et al. Development of an Oncological-Multidimensional Prognostic Index (Onco-MPI) for Mortality Prediction in Older Cancer Patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R.H.; Conroy, S.P.; Kircher, T.; Somme, D.; Saltvedt, I.; et al. Comprehensive Geriatric Assessment for Older Adults Admitted to Hospital. Cochrane Database Syst. Rev. 2017, 9, CD006211. [Google Scholar] [CrossRef] [Green Version]
- Rubenstein, L.Z.; Stuck, A.E.; Siu, A.L.; Wieland, D. Impacts of Geriatric Evaluation and Management Programs on Defined Outcomes: Overview of the Evidence. J. Am. Geriatr. Soc. 1991, 39, 8S–16S, discussion 17S–18S. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.-P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Use of Comprehensive Geriatric Assessment in Older Cancer Patients: Recommendations from the Task Force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef]
- Garcia, M.V.; Agar, M.R.; Soo, W.-K.; To, T.; Phillips, J.L. Screening Tools for Identifying Older Adults with Cancer Who May Benefit from a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021, 7, 616–627. [Google Scholar] [CrossRef]
- van Walree, I.C.; Scheepers, E.; van Huis-Tanja, L.; Emmelot-Vonk, M.H.; Bellera, C.; Soubeyran, P.; Hamaker, M.E. A Systematic Review on the Association of the G8 with Geriatric Assessment, Prognosis and Course of Treatment in Older Patients with Cancer. J. Geriatr. Oncol. 2019, 10, 847–858. [Google Scholar] [CrossRef]
- Cavusoglu, C.; Tahtaci, G.; Dogrul, R.T.; Ileri, I.; Yildirim, F.; Candemir, B.; Kizilarslanoglu, M.C.; Uner, A.; Goker, B. Predictive Ability of the G8 Screening Test to Determine Probable Sarcopenia and Abnormal Comprehensive Geriatric Assessment in Older Patients with Solid Malignancies. BMC Geriatr. 2021, 21, 574. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, H.M.; Giger, A.-K.W.; Lund, C.M.; Ditzel, H.J.; Mohammadnejad, A.; Pfeiffer, P.; Ryg, J.; Jørgensen, T.L.; Ewertz, M. Predictive Value of Geriatric Oncology Screening and Geriatric Assessment in Older Patients with Solid Cancers: Protocol for a Danish Prospective Cohort Study (PROGNOSIS-G8). J. Geriatr. Oncol. 2021, 12, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Paillaud, E.; Brugel, L.; Bertolus, C.; Baron, M.; Bequignon, E.; Caillet, P.; Schouman, T.; Lacau Saint Guily, J.; Périé, S.; Bouvard, E.; et al. Effectiveness of Geriatric Assessment-Driven Interventions on Survival and Functional and Nutritional Status in Older Patients with Head and Neck Cancer: A Randomized Controlled Trial (EGeSOR). Cancers 2022, 14, 3290. [Google Scholar] [CrossRef] [PubMed]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive Geriatric Assessment: A Meta-Analysis of Controlled Trials. Lancet 1993, 342, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and Validation of a Multidimensional Prognostic Index for One-Year Mortality from Comprehensive Geriatric Assessment in Hospitalized Older Patients. Rejuvenation Res. 2008, 11, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A Global Clinical Measure of Fitness and Frailty in Elderly People. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. the Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the Elderly at Risk for Malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M.R.; McLaren, R.; Exton-Smith, A.N. Mattresses for Preventing Pressure Sores in Geriatric Patients. Mon. Bull. Minist. Health Public Health Lab. Serv. 1966, 25, 238–268. [Google Scholar] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2018, 64, 61. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Banna, G.L.; Cantale, O.; Haydock, M.M.; Battisti, N.M.L.; Bambury, K.; Musolino, N.; O’Carroll, E.; Maltese, G.; Garetto, L.; Addeo, A.; et al. International Survey on Frailty Assessment in Patients with Cancer. Oncologist 2022, 27, e796–e803. [Google Scholar] [CrossRef]
- Soto Perez De Celis, E.; Li, D.; Sun, C.-L.; Kim, H.; Twardowski, P.; Fakih, M.; Chung, V.M.; Cristea, M.C.; Lim, D.; Yuan, Y.; et al. Patient-Defined Goals and Preferences among Older Adults with Cancer Starting Chemotherapy (CT). J. Clin. Oncol. 2018, 36, 10009. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Kanesvaran, R.; Koo, K.N.; Chen, W.; Poon, D. An Analysis of the Prognostic Value of Handgrip Strength and Its Incorporation into the Comprehensive Geriatric Assessment (CGA) in Elderly Asian Patients with Cancer. J. Clin. Oncol. 2011, 29, 9093. [Google Scholar] [CrossRef]
- Velghe, A.; De Buyser, S.; Noens, L.; Demuynck, R.; Petrovic, M. Hand Grip Strength as a Screening Tool for Frailty in Older Patients with Haematological Malignancies. Acta Clin. Belg. 2016, 71, 227–230. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Krigers, A.; Demetz, M.; Pinggera, D.; Klingenschmid, J.; Pichler, N.; Thomé, C.; Freyschlag, C.F. The Clinical Frailty Scale as Useful Tool in Patients with Brain Metastases. J. Neuro-Oncol. 2022, 158, 51–57. [Google Scholar] [CrossRef]


| Development Cohort | Whole Cohort n = 163 | Deceased n = 21 | Alive n = 142 | p-Value |
|---|---|---|---|---|
| Age mean years (SD) | 80.16 (5.86) | 83.3 (4.80) | 79.9 (5.88) | 0.14 |
| MOFS mean (SD) | 8.96 (2.49) | 6.38 (2.69) | 9.23 (2.33) | <0.001 |
| G8 median (IQR) | 13 (2.5) | 9 (1.625) | 13 (3) | <0.001 |
| CFS median (IQR) | 3 (2) | 4.5 (2.5) | 3 (2) | 0.006 |
| BMI mean (SD) | 26 (5.5) | 25.56 (8.05) | 26.03 (5.33) | 0.79 |
| BADL median (IQR) | 6 (1) | 6 (2) | 6 (1) | 0.15 |
| IADL median (IQR) | 7 (3) | 6 (4.5) | 7 (3) | 0.12 |
| Hand grip mean Kg (SD) | 17.7 (5.3) | 14.06 (4.82) | 18.15 (5.31) | 0.02 |
| SPSMQ median (IQR) | 1 (2) | 1.5 (2.5) | 1 (2) | 0.17 |
| MNA median (IQR) | 12 (3) | 11 (3.5) | 12 (3) | 0.004 |
| CIRS median (IQR) | 2 (1) | 3 (1) | 2 (1) | 0.61 |
| N° of drugs median (IQR) | 5 (4) | 6 (3) | 5 (4) | 0.49 |
| SPPB median (IQR) | 7 (6) | 3 (6) | 8 (7) | 0.006 |
| ESS median (IQR) | 19 (3) | 17.5 (5.5) | 19 (2) | 0.09 |
| MPI mean (SD) | 0.27 (0.14) | 0.41 (0.20) | 0.26 (0.14) | 0.003 |
| GDS median (IQR) | 4 (4) | 4.5 (4) | 4 (4) | 0.91 |
| ECOG PS median (IQR) | 1 (1) | 2 (0.75) | 1 (1) | 0.02 |
| Validation Cohort | Whole Cohort n = 70 | Deceased n = 15 | Alive n = 55 | p-Value |
|---|---|---|---|---|
| Age mean years (SD) | 78.6 (6.6) | 80.8 (5.6) | 78.2 (6.7) | 0.26 |
| Sex F (%) | 42 (60) | 10 (70) | 21 (38) | 0.03 |
| MOFS mean (SD) | 9.23 (3.45) | 5.56 (3.53) | 9.84 (3.05) | <0.001 |
| G8 median (IQR) | 12 (5.375) | 7.25 (3.750) | 12.75 (4.625) | <0.001 |
| CFS median (IQR) | 3 (3) | 6.5 (1.750) | 3 (2) | <0.001 |
| BADL median (IQR) | 6 (1) | 5 (4.75) | 6 (1) | 0.003 |
| IADL median (IQR) | 8 (3) | 0 (7.25) | 8 (2) | <0.001 |
| Hand grip mean Kg (SD) | 19.4 (7.07) | 14.7 (6.69) | 20.24 (6.86) | 0.02 |
| BMI mean (SD) | 24.9 (4.9) | 23.47 (7.8) | 25.38 (5.6) | 0.30 |
| SPSMQ median (IQR) | 1 (2.75) | 5 (5) | 1 (1) | <0.001 |
| MNA median (IQR) | 11 (3) | 4 (2.5) | 11 (3.5) | <0.001 |
| CIRS median (IQR) | 2 (1) | 3.5 (1.75) | 2 (2) | 0.02 |
| N° of drugs median (IQR) | 5 (5) | 7 (2.75) | 5 (5) | 0.07 |
| SPPB median (IQR) | 6 (5) | 2 (5) | 7 (6) | 0.006 |
| ESS median (IQR) | 19 (3) | 14.5 (4.5) | 19.5 (2) | <0.001 |
| MPI mean (SD) | 0.25 (0.30) | 0.57 (0.18) | 0.25 (0.17) | <0.001 |
| GDS median (IQR) | 3 (4) | 4 (4) | 3 (4) | 0.91 |
| Type of Cancer | n = 70 |
|---|---|
| Colorectal (%) | 17 (24.2) |
| Ovarian (%) | 12 (17.1) |
| Lung (%) | 11 (15.7) |
| Breast (%) | 10 (14.2) |
| Gastric (%) | 5 (7.1) |
| Pancreatic (%) | 4 (5.7) |
| Hematological (%) | 3 (4.3) |
| Genitourinary (%) | 3 (4.3) |
| Esophageal (%) | 2 (2.9) |
| Sarcoma (%) | 1 (1.5) |
| Melanoma (%) | 1 (1.5) |
| GIST (%) | 1 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi, R.; Okoye, C.; Antognoli, R.; Pompilii, I.M.; Taverni, I.; Landi, T.; Ghilli, M.; Roncella, M.; Calsolaro, V.; Monzani, F., on behalf of the Multidisciplinary Breast Center Scientific Committee. Multidimensional Oncological Frailty Scale (MOFS): A New Quick-To-Use Tool for Detecting Frailty and Stratifying Risk in Older Patients with Cancer—Development and Validation Pilot Study. Cancers 2023, 15, 1553. https://doi.org/10.3390/cancers15051553
Franchi R, Okoye C, Antognoli R, Pompilii IM, Taverni I, Landi T, Ghilli M, Roncella M, Calsolaro V, Monzani F on behalf of the Multidisciplinary Breast Center Scientific Committee. Multidimensional Oncological Frailty Scale (MOFS): A New Quick-To-Use Tool for Detecting Frailty and Stratifying Risk in Older Patients with Cancer—Development and Validation Pilot Study. Cancers. 2023; 15(5):1553. https://doi.org/10.3390/cancers15051553
Chicago/Turabian StyleFranchi, Riccardo, Chukwuma Okoye, Rachele Antognoli, Igino Maria Pompilii, Irene Taverni, Tommaso Landi, Matteo Ghilli, Manuela Roncella, Valeria Calsolaro, and Fabio Monzani on behalf of the Multidisciplinary Breast Center Scientific Committee. 2023. "Multidimensional Oncological Frailty Scale (MOFS): A New Quick-To-Use Tool for Detecting Frailty and Stratifying Risk in Older Patients with Cancer—Development and Validation Pilot Study" Cancers 15, no. 5: 1553. https://doi.org/10.3390/cancers15051553





