Circular RNA Expression Signatures Provide Promising Diagnostic and Therapeutic Biomarkers for Chronic Lymphocytic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Estimation
2.2. Omics Datasets Mining
2.3. RNA-seq Processing and Normalization
2.4. Enrichment Analysis
2.5. Drug Sensitivity Prediction
2.6. Statistical Analysis
3. Results
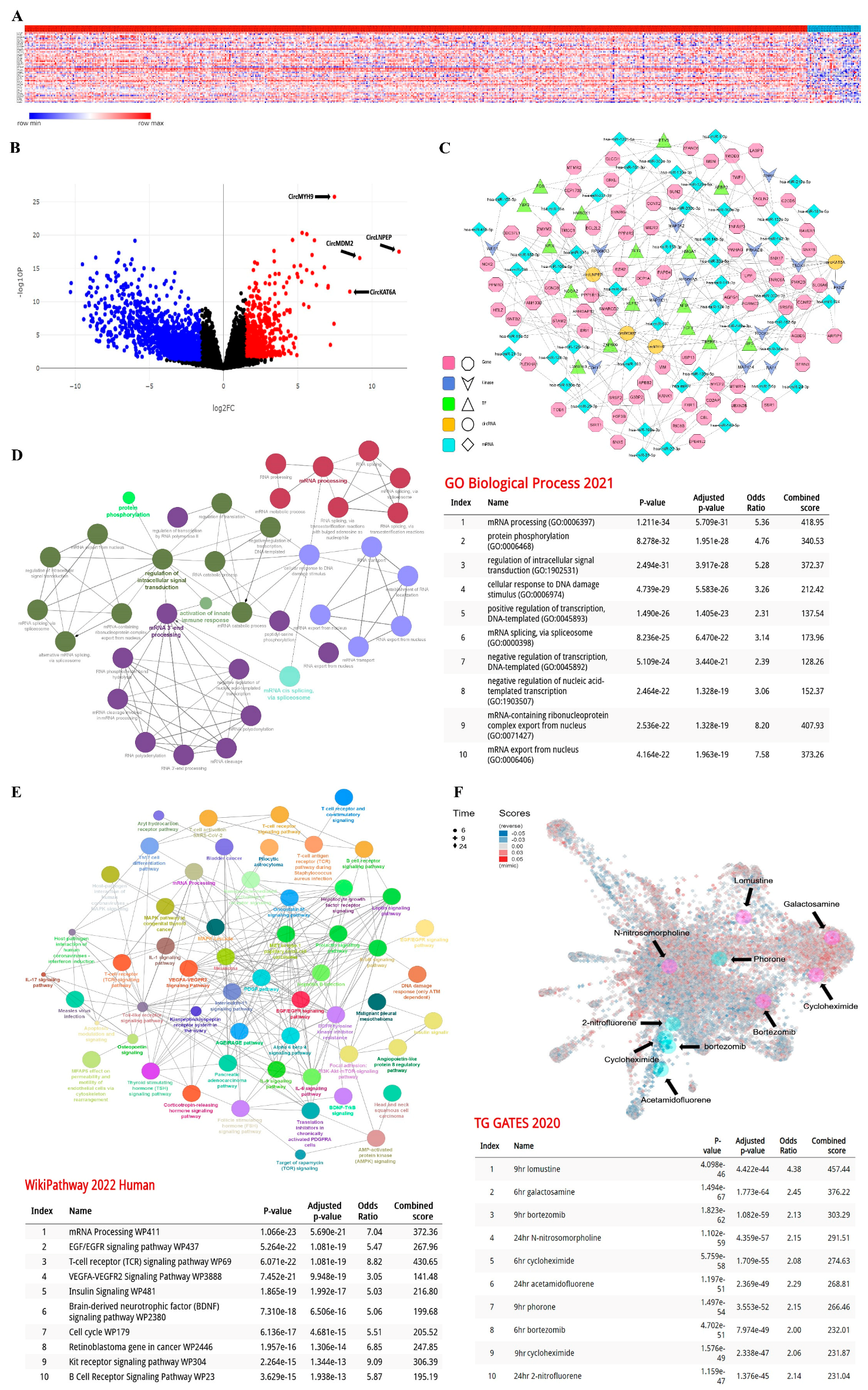
3.1. The Expression Pattern of 52 circRNAs Was Significantly Different in CLL Lymphocytes
3.2. Establishment of the circRNA-Biomarker Panel
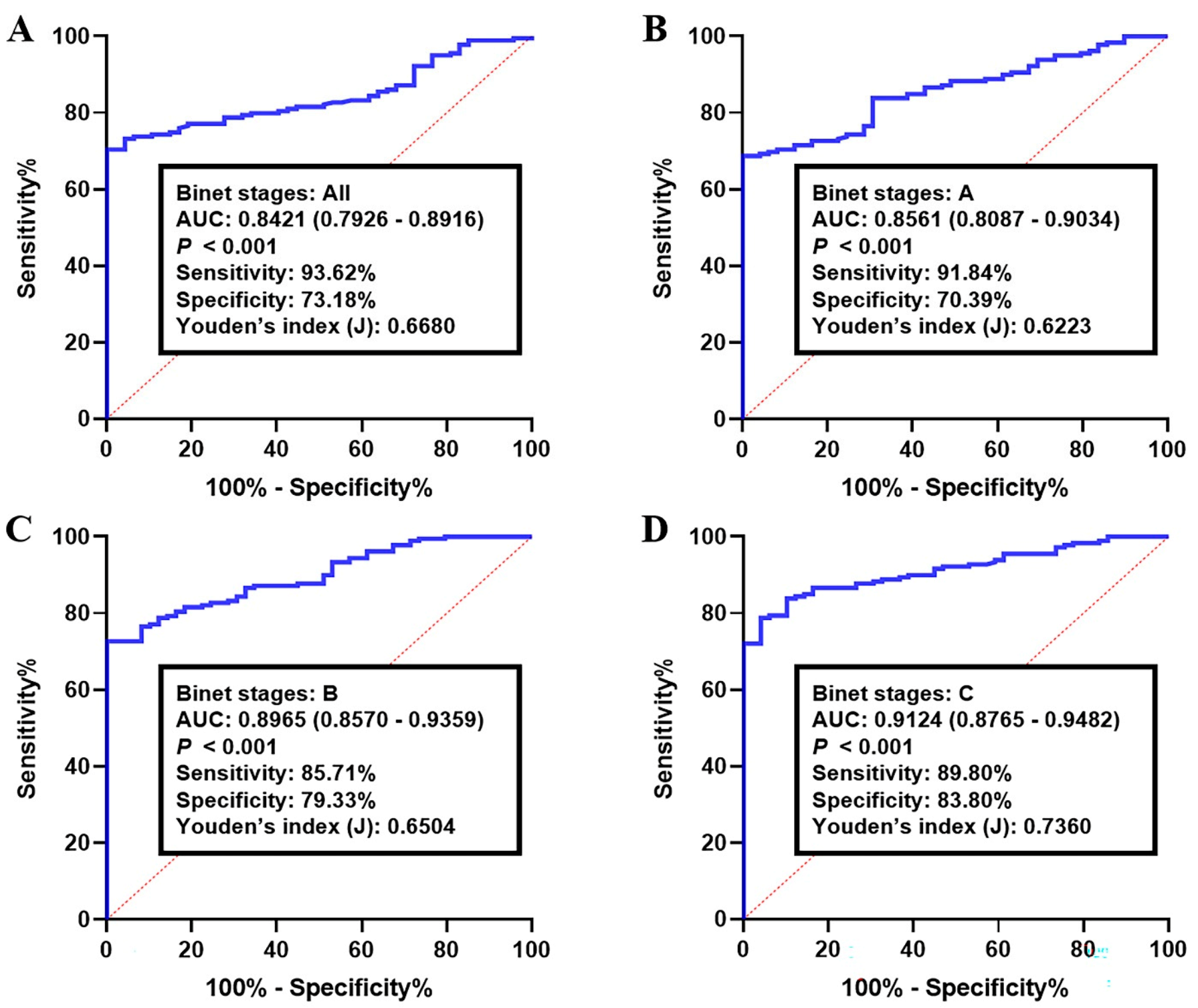
3.3. Validation of the circRNA-Biomarker Panel
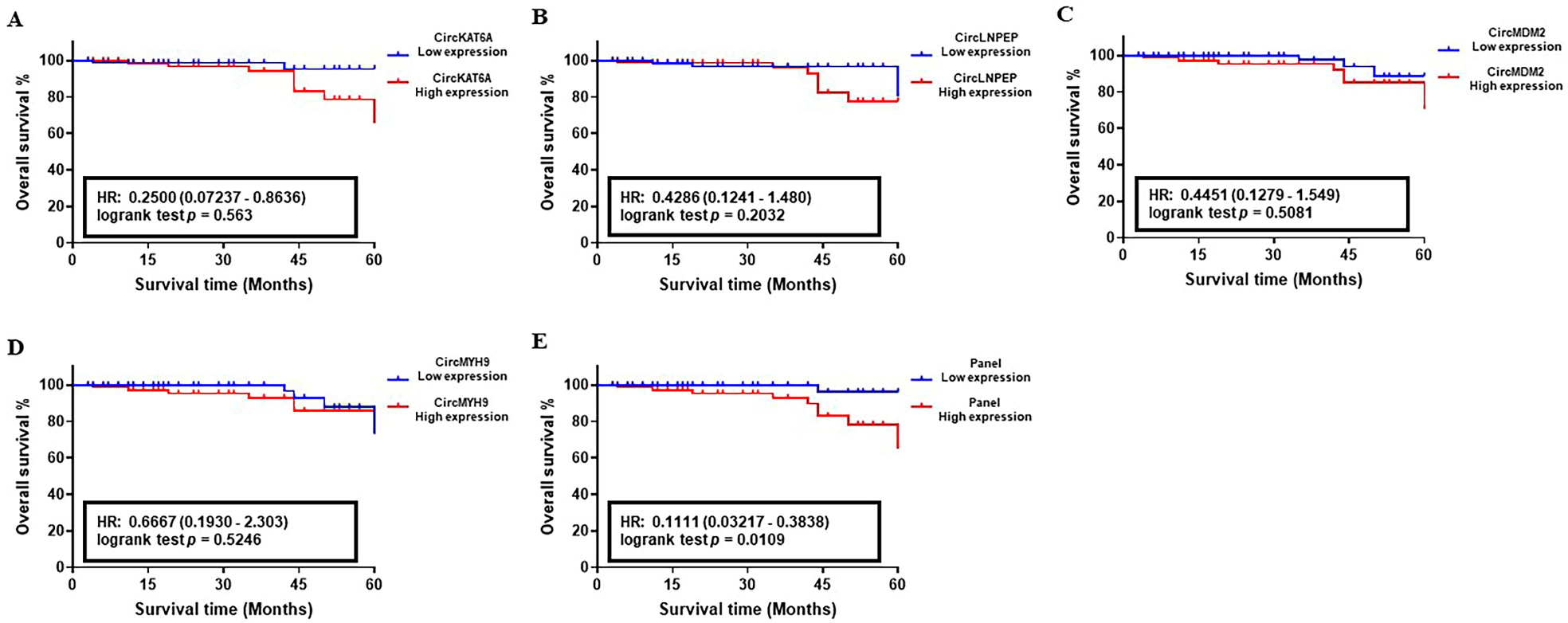
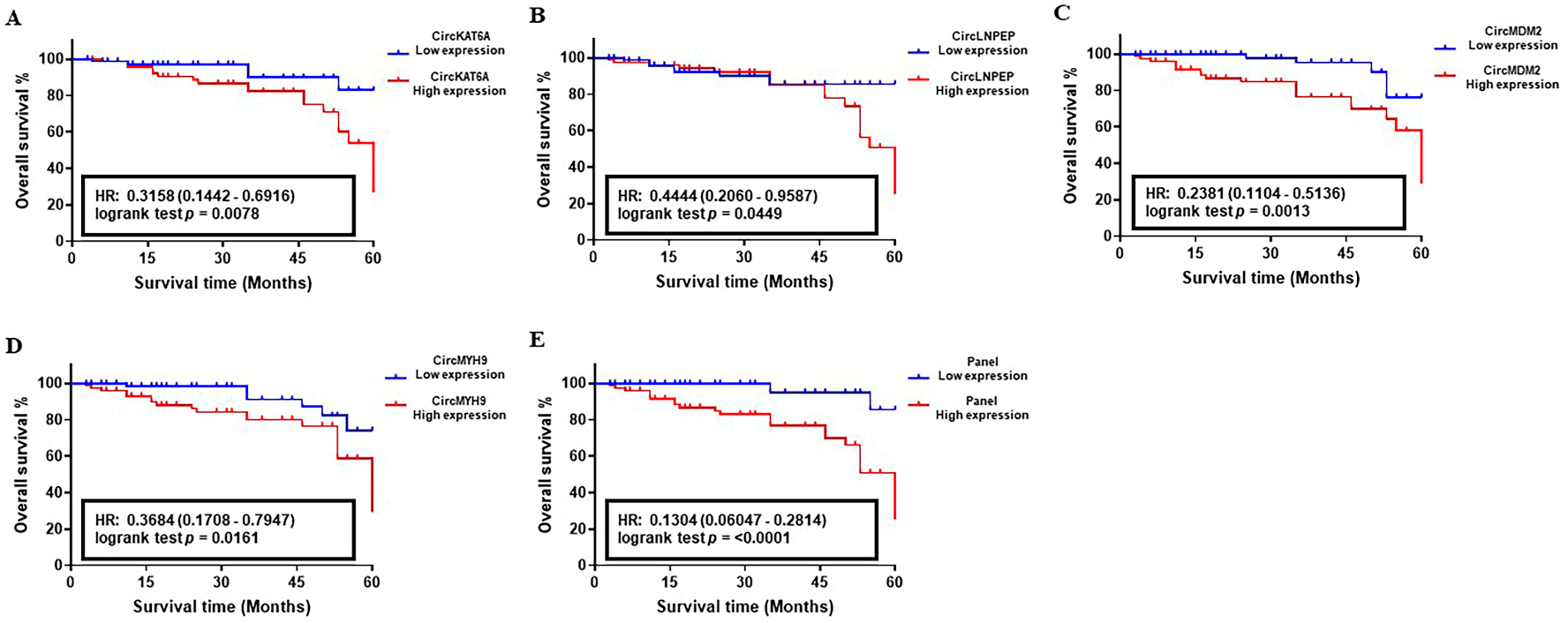
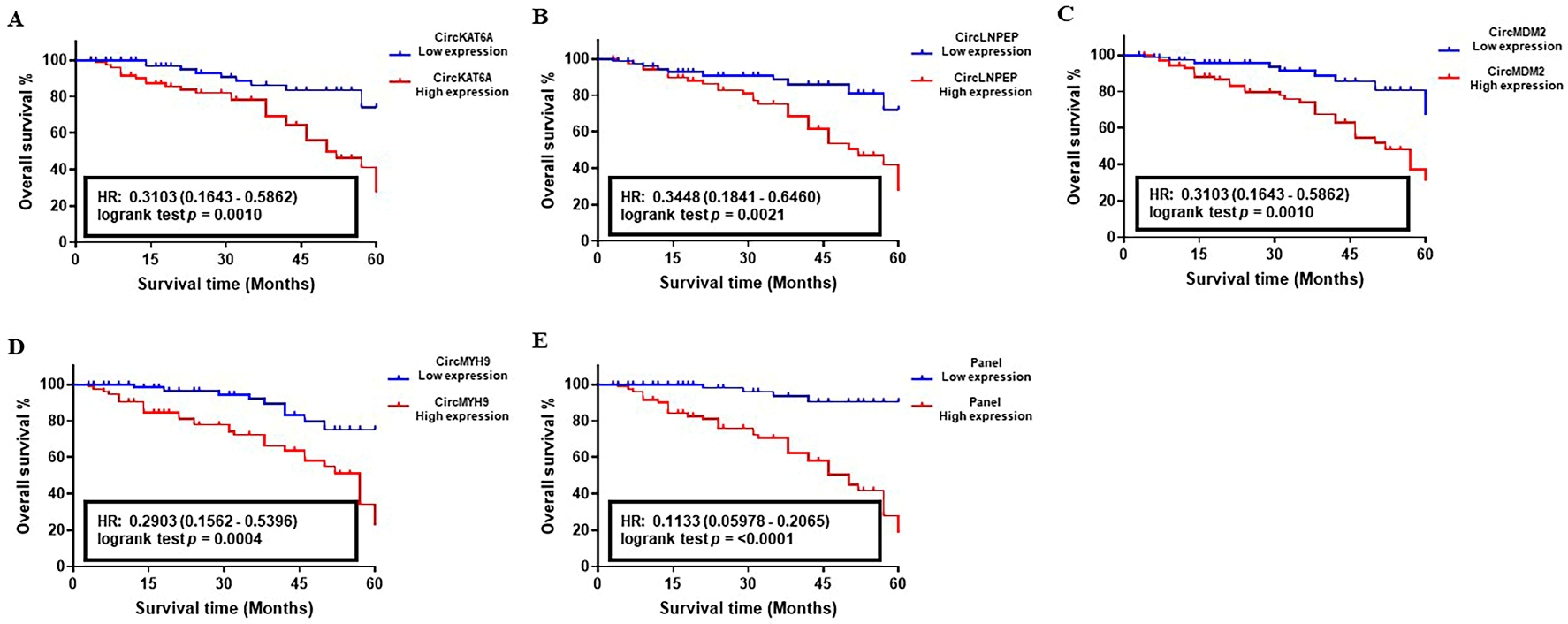
3.4. Prognostic Performance of circRNA-Biomarker Panel in CLL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel Rebecca, L.; Miller Kimberly, D. Jemal Ahmedin. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- NIH. Cancer Stat Facts: Leukemia—Chronic Lymphocytic Leukemia (CLL). Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 12 November 2022).
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2. [Google Scholar]
- Bosch, F.; Montserrat, E. Refining prognostic factors in chronic lymphocytic leukemia. Rev. Clin. Exp. Hematol. 2002, 6, 335–450. [Google Scholar]
- Fais, F.; Ghiotto, F.; Hashimoto, S.; Sellars, B.; Valetto, A.; Allen, S.L.; Schulman, P.; Vinciguerra, V.P.; Rai, K.; Rassenti, L.Z. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Investig. 1998, 102, 1515–1525. [Google Scholar] [CrossRef]
- Crespo, M.; Bosch, F.; Villamor, N.; Bellosillo, B.; Colomer, D.; Rozman, M.; Marcé, S.; López-Guillermo, A.; Campo, E.; Montserrat, E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N. Engl. J. Med. 2003, 348, 1764–1775. [Google Scholar] [CrossRef]
- Rassenti, L.Z.; Huynh, L.; Toy, T.L.; Chen, L.; Keating, M.J.; Gribben, J.G.; Neuberg, D.S.; Flinn, I.W.; Rai, K.R.; Byrd, J.C. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2004, 351, 893–901. [Google Scholar] [CrossRef]
- Binet, J.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical staging of chronic lymphocytic leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Visone, R.; Veronese, A.; Rassenti, L.Z.; Balatti, V.; Pearl, D.K.; Acunzo, M.; Volinia, S.; Taccioli, C.; Kipps, T.J.; Croce, C.M. miR-181b is a biomarker of disease progression in chronic lymphocytic leukemia. Blood J. Am. Soc. Hematol. 2011, 118, 3072–3079. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PloS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Bao, Y.; Yee, M.-C.; Barrett, S.P.; Hogan, G.J.; Olsen, M.N.; Dinneny, J.R.; Brown, P.O.; Salzman, J. Circular RNA is expressed across the eukaryotic tree of life. PloS ONE 2014, 9, e90859. [Google Scholar] [CrossRef]
- Chen, X.; Yang, T.; Wang, W.; Xi, W.; Zhang, T.; Li, Q.; Yang, A.; Wang, T. Circular RNAs in immune responses and immune diseases. Theranostics 2019, 9, 588–607. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Lee, S.K.; Sood, A.K. Circular RNAs in cancer. Mol. Ther.-Nucleic Acids 2019, 16, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y.; Creemers, E.E.; Boon, R.A.; Werfel, S.; Thum, T.; Engelhardt, S.; Dimmeler, S.; Squire, I.; Network, C. Circular RNAs in heart failure. Eur. J. Heart Fail. 2017, 19, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-R.; Jia, Y.-J.; Wang, Q.; Shao, X.-Q.; Lv, R.-J. Circular RNA: A new star in neurological diseases. Int. J. Neurosci. 2017, 127, 726–734. [Google Scholar] [CrossRef]
- Wang, T.; Pan, W.; Hu, J.; Zhang, Z.; Li, G.; Liang, Y. Circular RNAs in metabolic diseases. Circ. RNAs 2018, 1087, 275–285. [Google Scholar]
- Panda, A.C. Circular RNAs act as miRNA sponges. Circ. RNAs 2018, 1087, 67–79. [Google Scholar]
- Wilusz, J.E.; Sharp, P.A. A circuitous route to noncoding RNA. Science 2013, 340, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zheng, G.; Ning, Q.; Zheng, J.; Dong, D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer 2020, 19, 30. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Liu, C.; Liu, Y.; Bai, J.; Li, W. Circular RNA-100290 promotes cell proliferation and inhibits apoptosis in acute myeloid leukemia cells via sponging miR-203. Biochem. Biophys. Res. Commun. 2018, 507, 178–184. [Google Scholar] [CrossRef]
- Wu, D.-M.; Wen, X.; Han, X.-R.; Wang, S.; Wang, Y.-J.; Shen, M.; Fan, S.-H.; Zhang, Z.-F.; Shan, Q.; Li, M.-Q. Role of circular RNA DLEU2 in human acute myeloid leukemia. Mol. Cell. Biol. 2018, 38, e00218–e00259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, X.; Zhang, H.; Wu, Y.; Kang, M.; Fang, Y.; Xue, Y. Mechanism of circADD2 as ceRNA in Childhood Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2021, 9, 639910. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, C.; Li, Y.; Deng, Y.; Lu, W.; Li, J. Upregulation of circ-0000745 in acute lymphoblastic leukemia enhanced cell proliferation by activating ERK pathway. Gene 2020, 751, 144726. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lou, J.; Wang, H.; An, N.; Chen, H.; Zhang, Q.; Du, X. CircBA9. 3 supports the survival of leukaemic cells by up-regulating c-ABL1 or BCR-ABL1 protein levels. Blood Cells Mol. Dis. 2018, 73, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Jian-Jun, C.; Chu-Shu, L.; Guang-Hua, L.; Ming, Z. High circ_100053 predicts a poor outcome for chronic myeloid leukemia and is involved in imatinib resistance. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2021, 30764894. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Nie, S.; Huang, J.; Li, T.; Zhou, J.; Wang, W.; Zhuang, L.; Meng, F. Circular RNA circHIPK3 serves as a prognostic marker to promote chronic myeloid leukemia progression. Neoplasma 2020, 67, 171–177. [Google Scholar] [CrossRef]
- Xia, L.; Wu, L.; Bao, J.; Li, Q.; Chen, X.; Xia, H.; Xia, R. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2018, 503, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, H.; Wang, C.; Liu, W.; Liu, M.; Zhu, Y.; Xu, W.; Jin, H.; Li, J. Mitochondrial genome-derived circRNA mc-COX2 functions as an oncogene in chronic lymphocytic leukemia. Mol. Ther.-Nucleic Acids 2020, 20, 801–811. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, H.; Liu, W.; Zhu, H.; Fu, J.; Yang, C.; Fan, L.; Wang, L.; Liu, Y.; Xu, W. Circ-RPL15: A plasma circular RNA as novel oncogenic driver to promote progression of chronic lymphocytic leukemia. Leukemia 2020, 34, 919–923. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Jungreis, I.; Kellis, M. PhyloCSF: A comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 2011, 27, i275–i282. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Glažar, P.; Papavasileiou, P.; Rajewsky, N. circBase: A database for circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, Z.; Ma, Z.; Li, X.; Wong, K.-C. iCircRBP-DHN: Identification of circRNA-RBP interaction sites using deep hierarchical network. Brief. Bioinform. 2021, 22, bbaa274. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Ochagavia, M.E.; Rabasa, L.C.; Miranda, J.; Fernandez-de-Cossio, J.; Bringas, R. BisoGenet: A new tool for gene network building, visualization and analysis. BMC Bioinform. 2010, 11, 91. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Scardoni, G.; Tosadori, G.; Faizan, M.; Spoto, F.; Fabbri, F.; Laudanna, C. Biological network analysis with CentiScaPe: Centralities and experimental dataset integration. F1000Research 2014, 3, 139. [Google Scholar] [CrossRef]
- Nasri Nasrabadi, P.; Nayeri, Z.; Gharib, E.; Salmanipour, R.; Masoomi, F.; Mahjoubi, F.; Zomorodipour, A. Establishment of a CALU, AURKA, and MCM2 gene panel for discrimination of metastasis from primary colon and lung cancers. PLoS ONE 2020, 15, e0233717. [Google Scholar] [CrossRef]
- Gharib, E.; Nasrinasrabadi, P.; Zali, M.R. Development and validation of a lipogenic genes panel for diagnosis and recurrence of colorectal cancer. PLoS ONE 2020, 15, e0229864. [Google Scholar] [CrossRef] [PubMed]
- Gharib, E.; Anaraki, F.; Baghdar, K.; Ghavidel, P.; Sadeghi, H.; Nasrabadi, P.N.; Peyravian, N.; Aghdaei, H.A.; Zali, M.R.; Mojarad, E.N. Investigating the diagnostic performance of HOTTIP, PVT1, and UCA1 long noncoding RNAs as a predictive panel for the screening of colorectal cancer patients with lymph node metastasis. J. Cell. Biochem. 2019, 120, 14780–14790. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Grever, M.; Kay, N.; Keating, M.J.; O’Brien, S.; Rai, K.R. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood 1996, 87, 4990–4997. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef]
- Boddy, C.S.; Ma, S. Frontline Therapy of CLL: Evolving Treatment Paradigm. Curr. Hematol. Malig. Rep. 2018, 13, 69–77. [Google Scholar] [CrossRef]
- Crombie, J.; Davids, M.S. IGHV mutational status testing in chronic lymphocytic leukemia. Am. J. Hematol. 2017, 92, 1393–1397. [Google Scholar] [CrossRef]
- Arnaiz, E.; Sole, C.; Manterola, L.; Iparraguirre, L.; Otaegui, D.; Lawrie, C.H. CircRNAs and cancer: Biomarkers and master regulators. Semin. Cancer Biol. 2019, 58, 90–99. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Guo, Z.; Li, M.; Li, M.; Liu, S.; Liu, H.; Li, W.; Yin, X.; Tao, J.; et al. Emerging function and potential diagnostic value of circular RNAs in cancer. Mol. Cancer 2018, 17, 123. [Google Scholar] [CrossRef]
- Fontemaggi, G.; Turco, C.; Esposito, G.; Di Agostino, S. New Molecular Mechanisms and Clinical Impact of circRNAs in Human Cancer. Cancers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, Y.; Yang, X.; Mo, Y.; Fan, C.; Xiong, F.; Ren, D.; Ye, X.; Li, C.; Wang, Y.; et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol. Cancer 2018, 17, 79. [Google Scholar] [CrossRef]
- Bonizzato, A.; Gaffo, E.; Te Kronnie, G.; Bortoluzzi, S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. 2016, 6, e483. [Google Scholar] [CrossRef]
- Bhat, A.A.; Younes, S.N.; Raza, S.S.; Zarif, L.; Nisar, S.; Ahmed, I.; Mir, R.; Kumar, S.; Sharawat, S.K.; Hashem, S.; et al. Role of non-coding RNA networks in leukemia progression, metastasis and drug resistance. Mol. Cancer 2020, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, M.; Jiang, L.; Ma, L.; Wan, L.; Chen, Q.; Wei, C.; Wang, Z. CircRNAs in anticancer drug resistance: Recent advances and future potential. Mol. Cancer 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Kong, S.; Wang, F.; Ju, S. CircRNAs: Biogenesis, functions, and role in drug-resistant Tumours. Mol. Cancer 2020, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.N.; Yang, Y.; Schreuder, J.; Nilsson, S.K.; Bilardi, R.; Carotta, S.; McRae, H.M.; Metcalf, D.; Voss, A.K.; Thomas, T. MOZ (KAT6A) is essential for the maintenance of classically defined adult hematopoietic stem cells. Blood J. Am. Soc. Hematol. 2016, 128, 2307–2318. [Google Scholar] [CrossRef]
- Rokudai, S.; Aikawa, Y.; Tagata, Y.; Tsuchida, N.; Taya, Y.; Kitabayashi, I. Monocytic leukemia zinc finger (MOZ) interacts with p53 to induce p21 expression and cell-cycle arrest. J. Biol. Chem. 2009, 284, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.; Phipson, B.; El-Saafin, F.; Vanyai, H.; Downer, N.; Bird, M.; Kueh, A.; May, R.; Smyth, G.; Voss, A. MOZ (MYST3, KAT6A) inhibits senescence via the INK4A-ARF pathway. Oncogene 2015, 34, 5807–5820. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Ayton, P.M.; Carapeti, M.; Kutok, J.L.; Snyder, C.S.; Williams, I.R.; Cross, N.C.; Glass, C.K.; Cleary, M.L.; Gilliland, D.G. MOZ-TIF2-induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2-mediated recruitment of CBP. Cancer Cell 2003, 3, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Borrow, J.; Stanton, V.P.; Andresen, J.M.; Becher, R.; Behm, F.G.; Chaganti, R.S.; Civin, C.I.; Disteche, C.; Dubé, I.; Frischauf, A.M. The translocation t (8; 16)(p11; p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB–binding protein. Nat. Genet. 1996, 14, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, I.; Aikawa, Y.; Nguyen, L.A.; Yokoyama, A.; Ohki, M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ–CBP fusion protein. EMBO J. 2001, 20, 7184–7196. [Google Scholar] [CrossRef]
- Yu, L.; Liang, Y.; Cao, X.; Wang, X.; Gao, H.; Lin, S.-Y.; Schiff, R.; Wang, X.-S.; Li, K. Identification of MYST3 as a novel epigenetic activator of ERα frequently amplified in breast cancer. Oncogene 2017, 36, 2910–2918. [Google Scholar] [CrossRef]
- Lv, D.; Jia, F.; Hou, Y.; Sang, Y.; Alvarez, A.A.; Zhang, W.; Gao, W.-Q.; Hu, B.; Cheng, S.-Y.; Ge, J. Histone Acetyltransferase KAT6A Upregulates PI3K/AKT Signaling through TRIM24 BindingKAT6A Promotes Glioma Tumorigenesis. Cancer Res. 2017, 77, 6190–6201. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Yan, Y.; Wang, H.; Yang, J.; Zheng, Z.; Zha, J.; Bo, P.; Tang, Y.; Guo, X. CircDOCK1 suppresses cell apoptosis via inhibition of miR-196a-5p by targeting BIRC3 in OSCC. Oncol. Rep. 2018, 39, 951–966. [Google Scholar] [CrossRef]
- Paladini, F.; Fiorillo, M.T.; Tedeschi, V.; Mattorre, B.; Sorrentino, R. The multifaceted nature of aminopeptidases ERAP1, ERAP2, and LNPEP: From evolution to disease. Front. Immunol. 2020, 11, 1576. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Zuo, X.-B.; Tang, H.-Y.; Tang, X.-F.; Gao, J.-P.; Sheng, Y.-J.; Yin, X.-Y.; Zhou, F.-S.; Zhang, C. Identification of a missense variant in LNPEP that confers psoriasis risk. J. Investig. Dermatol. 2014, 134, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Fang, Y.-N.; Wu, C.-C.; Chen, M.-C.; Chang, J.-P.; Lin, Y.-S.; Pan, K.-L.; Ho, W.-C.; Chang, T.-H.; Huang, Y.-K. Differential gene expression profile of renin-angiotensin system in the left atrium in mitral regurgitation patients. Dis. Markers 2018, 2018, 6924608. [Google Scholar] [CrossRef]
- Ma, Q.; Che, L.; Wang, W.; Li, G.; Zhang, Y.; Chen, Y.; Gu, Z.; Song, X.; Chang, L. LNPEP as a Prognostic-Related Biomarker in Ovarian Cancer Correlating with Immune Infiltrates. Research Square 2022. [Google Scholar] [CrossRef]
- Ouyang, X.; Yao, L.; Liu, G.; Liu, S.; Gong, L.; Xiao, Y. Loss of androgen receptor promotes HCC invasion and metastasis via activating circ-LNPEP/miR-532–3p/RAB9A signal under hypoxia. Biochem. Biophys. Res. Commun. 2021, 557, 26–32. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Guo, H.; Chu, D.; Zhang, R.; Guo, R. CircLNPEP promotes the progression of ovarian cancer through regulating miR-876-3p/WNT5A axis. Cell Cycle 2021, 20, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Muys, B.R.; Grammatikakis, I.; De, S.; Abdelmohsen, K.; Li, X.L.; Zhu, Y.; Daulatabad, S.V.; Tsitsipatis, D.; Meltzer, P.S. A circular RNA from the MDM2 locus controls cell cycle progression by suppressing p53 levels. Mol. Cell. Biol. 2020, 40, e00419–e00473. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Sun, D.; Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Li, H. Circular RNA circMDM2 accelerates the glycolysis of oral squamous cell carcinoma by targeting miR-532-3p/HK2. J. Cell. Mol. Med. 2020, 24, 7531–7537. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Liu, Z.; Lin, C.; Meng, F.; Xu, L.; Zhang, X.; Zhang, C.; Zhang, P.; Gong, S. CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol. Cancer 2021, 20, 114. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Zhang, Y.; Yang, J. Myosin heavy chain 9: Oncogene or tumor suppressor gene? Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 888. [Google Scholar] [CrossRef]
- Xia, J.; Wu, C.; Tang, Y.; Tang, J.; Zhu, D.; Zhang, F.; Xu, Z.; Sun, D.; Tan, Z.; Zhuo, H. CircMYH9 increases KPNA2 mRNA stability to promote hepatocellular carcinoma progression in an EIF4A3-dependent manner. Am. J. Cancer Res. 2022, 12, 4361. [Google Scholar]
- Zhang, W.; Liu, Q.; Luo, L.; Song, J.; Han, K.; Liu, R.; Gong, Y.; Guo, X. Use Chou’s 5-steps rule to study how Baicalin suppresses the malignant phenotypes and induces the apoptosis of colorectal cancer cells. Arch. Biochem. Biophys. 2021, 705, 108919. [Google Scholar] [CrossRef] [PubMed]
- Pigneux, A.; Harousseau, J.-L.; Witz, F.; Sauvezie, M.; Bene, M.-C.; Luquet, I.; Hunault-Berger, M.; Recher, C.; Lioure, B.; Himberlin, C. Addition of lomustine to idarubicin and cytarabine improves the outcome of elderly patients with de novo acute myeloid leukemia: A report from the GOELAMS. J. Clin. Oncol. 2010, 28, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, D.T.; Karri, S.; Wiernik, P.H.; Dutcher, J.P. Mitoxantrone, vinblastine and CCNU: Long-term follow-up of patients treated for advanced and poor-prognosis Hodgkin’s disease. Leuk. Lymphoma 2006, 47, 641–656. [Google Scholar] [CrossRef]
- Blaxill, J.; Buzzacott, P.; Finlay, J. Prognostic indicators for naïve canine non-indolent T-cell lymphoma treated with combination lomustine, vincristine, procarbazine and prednisolone chemotherapy. Vet. Comp. Oncol. 2022, 20, 215–226. [Google Scholar] [CrossRef]
- Fahey, C.E.; Milner, R.J.; Barabas, K.; Lurie, D.; Kow, K.; Parfitt, S.; Lyles, S.; Clemente, M. Evaluation of the University of Florida lomustine, vincristine, procarbazine, and prednisone chemotherapy protocol for the treatment of relapsed lymphoma in dogs: 33 cases (2003–2009). J. Am. Vet. Med. Assoc. 2011, 239, 209–215. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Mauldin, G.; Milner, R.; LaDue, T.; Mauldin, G.; Bartges, J. Efficacy and toxicity of BOPP and LOPP chemotherapy for the treatment of relapsed canine lymphoma. Vet. Comp. Oncol. 2006, 4, 21–32. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood J. Am. Soc. Hematol. 2008, 111, 5446–5456. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Ferrarini, M. Cellular origin (s) of chronic lymphocytic leukemia: Cautionary notes and additional considerations and possibilities. Blood J. Am. Soc. Hematol. 2011, 117, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Malek, S. Clinical utility of prognostic markers in chronic lymphocytic leukemia. ASCO Educ. Book 2010, 2010, 263–267. [Google Scholar]
- Van Bockstaele, F.; Verhasselt, B.; Philippe, J. Prognostic markers in chronic lymphocytic leukemia: A comprehensive review. Blood Rev. 2009, 23, 25–47. [Google Scholar] [CrossRef]
- Malek, S. Molecular Biomarkers in Chronic Lymphocytic Leukemia. In Advances in Chronic Lymphocytic Leukemia. Advances in Experimental Medicine and Biology; Malek, S., Ed.; Springer: New York, NY, USA; Volume 792. [CrossRef]
- Wang, X.; Liotta, L. Clinical bioinformatics: A new emerging science. J. Clin. Bioinform. 2011, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, D.; Xu, M.; Rong, R.; Tang, Q.; Wang, X.; Zhu, T. Analysis of transcriptional factors and regulation networks in patients with acute renal allograft rejection. J. Proteome Res. 2011, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Z.; Qian, M.; Bai, C.; Wang, X. Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: A preliminary study. J. Cell. Mol. Med. 2012, 16, 1286–1297. [Google Scholar] [CrossRef]
- Cun, Y.; Fröhlich, H. Prognostic gene signatures for patient stratification in breast cancer-accuracy, stability and interpretability of gene selection approaches using prior knowledge on protein-protein interactions. BMC Bioinform. 2012, 13, 69. [Google Scholar] [CrossRef]
- Chiorazzi, N. Implications of new prognostic markers in chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 76–87. [Google Scholar] [CrossRef]
- Patel, K.; Pagel, J.M. Current and future treatment strategies in chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]


| Variable | Training Set (%) | Validation Set I (%) | Validation Set II (%) | p Value |
|---|---|---|---|---|
| Healthy count (%) | ||||
| Sex | - | |||
| Male | 12 (30.77) | 12 (30.77) | 12 (30.77) | |
| Female | 17 (69.23) | 17 (69.23) | 17 (69.23) | |
| Age (year) | - | |||
| Mean + SD | 50 ± 9 | 50 ± 9 | 50 ± 9 | |
| Chronic Lymphocytic Leukemia count (%) | ||||
| Sex | 0.416 | |||
| Male | 31 (31.00) | 121 (55.00) | 163 (64.94) | |
| Female | 69 (69.00) | 99 (45.00) | 88 (35.06) | |
| Age (year) | 0.762 | |||
| Mean + SD | 69 ± 3 | 71 ± 2 | 70 ± 2 | |
| Binet stage | 0.109 | |||
| A | 23 (23.00) | 33 (15.00) | 34 (13.55) | |
| B | 56 (56.00) | 67 (30.45) | 133 (52.99) | |
| C | 21 (21.00) | 120 (54.55) | 84 (33.46) | |
| RNA | Sensitivity | Specificity | Youden’s Index J | AUC | p Value | 95% CI |
|---|---|---|---|---|---|---|
| CircKAT6A | 66.67 | 76.32 | 0.4299 | 0.7828 | <0.0001 | 0.6993–0.8664 |
| CircLNPEP | 62.67 | 71.05 | 0.3372 | 0.7498 | <0.0001 | 0.6587–0.8410 |
| CircMDM2 | 65.33 | 78.95 | 0.4428 | 0.8025 | <0.0001 | 0.7207–0.8842 |
| CircMYH9 | 58.67 | 73.68 | 0.3235 | 0.7504 | <0.0001 | 0.6600–0.8407 |
| Variable | Coefficient | Std. Error | Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|---|
| CircKAT6A | 0.7690 | 0.1042 | 0.9260 | 0.1089–2.252 | <0.0001 |
| CircLNPEP | 0.1786 | 0.0781 | 0.8365 | 0.09177–1.043 | <0.0001 |
| CircMDM2 | 0.2246 | 0.1213 | 1.445 | 1.028–3.811 | <0.0001 |
| CircMYH9 | 0.9938 | 0.1745 | 2.701 | 0.3728–3.241 | <0.0001 |
| Constant | 0.7262 |
| Training Set (n = 100) | Validation Set I (n = 220) | Validation Set II (n = 251) | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Gender | Female Male | 1.823 (1.305–3.081) | 0.819 | 1.059 (0.456–2.474) | 0.782 | 1.808 (0.817–2.88) | 0.502 |
| Age | ≤65 65 < x < 70 ≥70 | 2.267 (1.583–4.111) | 0.232 | 2.262 (1.954–4.453) | 0.291 | 2.132 (1.447–3.302) | 0.122 |
| Binet stage | A B C | 2.119 (1.934–4.482) | 0.071 | 2.829 (1.287–6.015) | <0.01 | 3.492 (2.338–5.963) | <0.01 |
| Training Set (n = 100) | Validation Set I (n = 180) | Validation Set II (n = 203) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CircKAT6A High vs. Low | 3.156 (2.225–4.306) | <0.01 | 2.374 (1.996–3.852) | 0.025 | 3.711 (3.205–4.928) | <0.01 | 3.356 (2.341–4.070) | <0.01 | 4.816 (3.611–6.048) | <0.001 | 4.525 (3.091–5.121) | <0.001 |
| CircLNPEP High vs. Low | 4.113 (2.346–5.288) | <0.001 | 3.222 (2.271–3.578) | <0.01 | 4.869 (3.559–5.251) | <0.001 | 3.848 (3.192–4.645) | <0.01 | 5.138 (4.592–6.09) | <0.001 | 4.279 (3.956–4.739) | <0.001 |
| CircMDM2 High vs. Low | 2.309 (1.884–4.093) | 0.033 | 1.937 (1.509–3.583) | 0.048 | 3.919 (2.704–5.191) | <0.01 | 2.974 (2.117–4.632) | <0.01 | 4.257 (3.816–5.534) | <0.001 | 3.746 (3.164–4.816) | <0.01 |
| HMGCS2 High vs. Low | 2.124 (1.394–4.922) | 0.039 | 2.053 (1.197 –3.872) | 0.042 | 3.983 (2.888–5.307) | <0.001 | 3.136 (2.255–4.333) | <0.01 | 4.249 (3.483–5.525) | <0.001 | 3.958 (3.417–4.783) | <0.001 |
| CircMYH9 High vs. Low | 3.259 (1.847–4.303) | <0.01 | 2.775 (1.362–3.983) | 0.011 | 4.202 (2.581–5.236) | <0.001 | 3.371 (2.016–4.176) | <0.01 | 4.794 (3.503–5.596) | <0.001 | 4.015 (3.139–5.131) | <0.001 |
| Binet Risk High vs. Low | 1.317 (1.052–2.338) | 0.09 | 2.933 (2.350–4.175) | <0.01 | 3.686 (2.802–4.974) | <0.01 | ||||||
| Panel High vs. Low | 6.083 (5.152–8.023) | <0.001 | 4.193 (3.122–5.243) | <0.001 | 7. 517 (6.117–8.886) | <0.001 | 5.432 (4.716–6.915) | <0.001 | 8.294 (7.503–9.167) | <0.001 | 6.992 (5.9365–8.049) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharib, E.; Nasrabadi, P.N.; Robichaud, G.A. Circular RNA Expression Signatures Provide Promising Diagnostic and Therapeutic Biomarkers for Chronic Lymphocytic Leukemia. Cancers 2023, 15, 1554. https://doi.org/10.3390/cancers15051554
Gharib E, Nasrabadi PN, Robichaud GA. Circular RNA Expression Signatures Provide Promising Diagnostic and Therapeutic Biomarkers for Chronic Lymphocytic Leukemia. Cancers. 2023; 15(5):1554. https://doi.org/10.3390/cancers15051554
Chicago/Turabian StyleGharib, Ehsan, Parinaz Nasri Nasrabadi, and Gilles A. Robichaud. 2023. "Circular RNA Expression Signatures Provide Promising Diagnostic and Therapeutic Biomarkers for Chronic Lymphocytic Leukemia" Cancers 15, no. 5: 1554. https://doi.org/10.3390/cancers15051554
APA StyleGharib, E., Nasrabadi, P. N., & Robichaud, G. A. (2023). Circular RNA Expression Signatures Provide Promising Diagnostic and Therapeutic Biomarkers for Chronic Lymphocytic Leukemia. Cancers, 15(5), 1554. https://doi.org/10.3390/cancers15051554







