The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
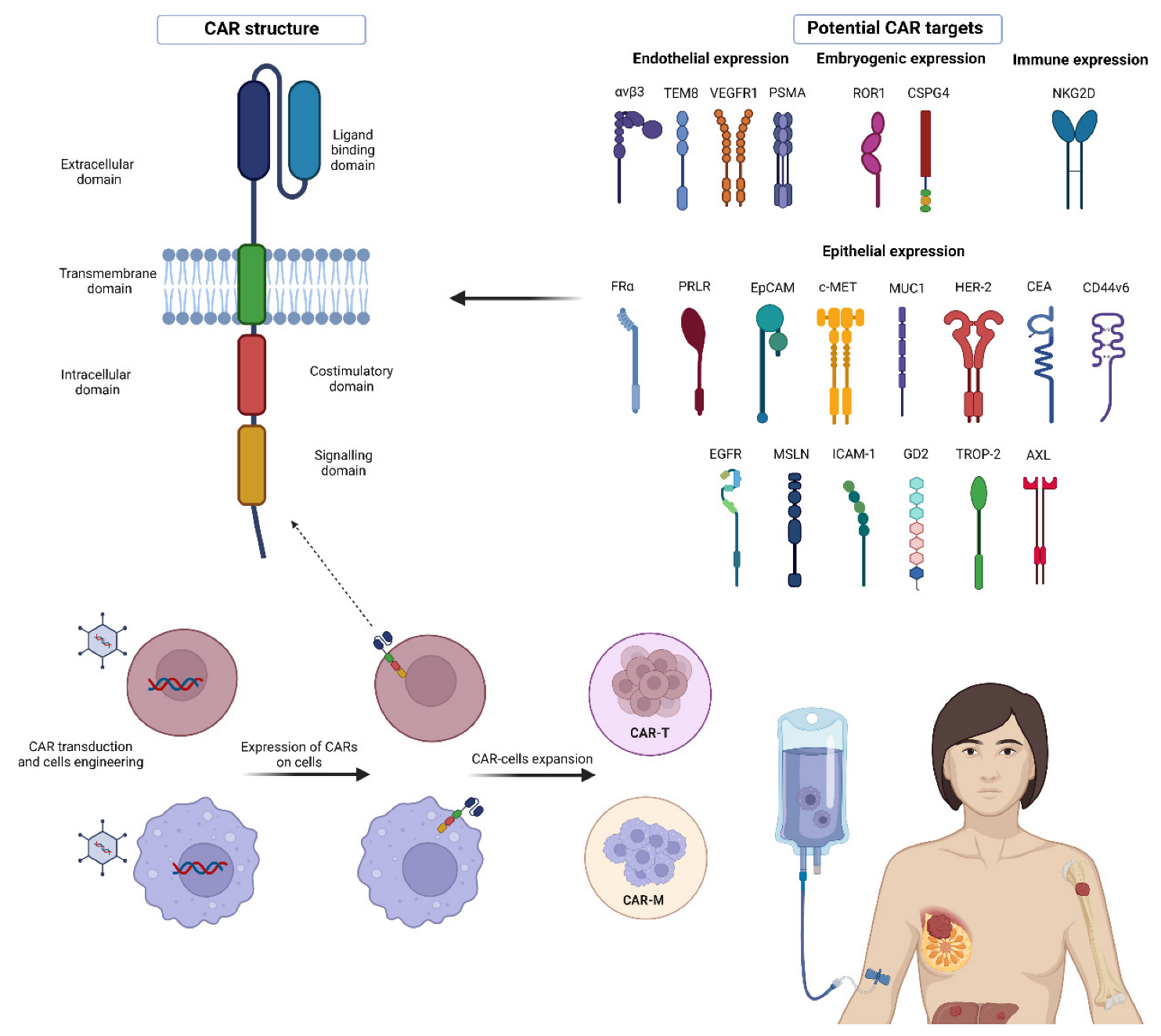
2. CAR Molecule Structure
3. The CAR-T Cell Generations
4. Targets for CAR-T Cell Therapy in BC
4.1. Integrins
4.2. Mesothelin
4.3. TEM8
4.4. MUC1
4.5. ROR1
4.6. Natural Killer Group 2, Member D Ligand (NKG2DL)
4.7. Chondroitin Sulfate Proteoglycan 4 (CSPG4)
4.8. EpCAM
4.9. Intercellular Adhesion Molecule-1 (ICAM-1)
4.10. HER-2
4.11. VEGF
4.12. c-MET
4.13. AXL
4.14. Disialoganglioside GD2
4.15. PRLR
4.16. CEA
4.17. CD44v6
4.18. Trophoblast Cell-Surface Antigen 2 (TROP2)
4.19. Epidermal Growth Factor Receptor (EGFR)
4.20. Prostate-Specific Membrane Antigen (PSMA)
4.21. Folate Receptor Alpha
5. Overcoming the CAR-T- Related Problems in Solid Tumors: Macrophage-Based Cell Therapeutics
6. CAR-M in Solid Tumors and BC
7. Clinical Applications of CAR-M Strategy against BC and Other Solid Tumors
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Daily, K.; Douglas, E.; Romitti, P.A.; Thomas, A. Epidemiology of De Novo Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 302–308. [Google Scholar] [CrossRef]
- Cao, L.; Niu, Y. Triple Negative Breast Cancer: Special Histological Types and Emerging Therapeutic Methods. Cancer Biol. Med. 2020, 17, 293–306. [Google Scholar] [CrossRef]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of Metastatic Spread in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, B.; Tommasi, C.; Cursio, O.E.; Musolino, A.; Migliori, E.; De Silva, P.; Senevirathne, T.H.; Schena, M.; Scartozzi, M.; Farci, D.; et al. A Review of Immune Checkpoint Blockade in Breast Cancer. Semin. Oncol. 2021, 48, 208–225. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Jiang, Z.; Wang, X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl. Oncol. 2018, 11, 311–329. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet. Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-Positive Breast Cancer: State of the Art and Future Perspectives. J. Hematol. Oncol. 2019, 12, 111. [Google Scholar] [CrossRef]
- Dieci, M.V.; Criscitiello, C.; Goubar, A.; Viale, G.; Conte, P.; Guarneri, V.; Ficarra, G.; Mathieu, M.C.; Delaloge, S.; Curigliano, G.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes on Residual Disease after Primary Chemotherapy for Triple-Negative Breast Cancer: A Retrospective Multicenter Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Gao, H.; Cohen, E.N.; Anfossi, S.; Giordano, A.; Sanda, T.; Fouad, T.M.; De Giorgi, U.; Giuliano, M.; Woodward, W.A.; et al. Circulating Tumor Cells (CTC) Are Associated with Defects in Adaptive Immunity in Patients with Inflammatory Breast Cancer. J. Cancer 2016, 7, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Gao, H.; Cohen, E.N.; Anfossi, S.; Giordano, A.; Tin, S.; Fouad, T.M.; De Giorgi, U.; Giuliano, M.; Woodward, W.A.; et al. Circulating Tumor Cells (CTCs) Are Associated with Abnormalities in Peripheral Blood Dendritic Cells in Patients with Inflammatory Breast Cancer. Oncotarget 2017, 8, 35656–35668. [Google Scholar] [CrossRef]
- De Giorgi, U.; Mego, M.; Scarpi, E.; Giordano, A.; Giuliano, M.; Valero, V.; Alvarez, R.H.; Ueno, N.T.; Cristofanilli, M.; Reuben, J.M. Association between Circulating Tumor Cells and Peripheral Blood Monocytes in Metastatic Breast Cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866065. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment Landscape of Triple-Negative Breast Cancer—Expanded Options, Evolving Needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Körner, C.; Ben-Baruch, A. Tumor-Stroma-Inflammation Networks Promote Pro-Metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Hutchinson, K.E.; Yost, S.E.; Chang, C.W.; Johnson, R.M.; Carr, A.R.; McAdam, P.R.; Halligan, D.L.; Chang, C.C.; Schmolze, D.; Liang, J.; et al. Comprehensive Profiling of Poor-Risk Paired Primary and Recurrent Triple-Negative Breast Cancers Reveals Immune Phenotype Shifts. Clin. Cancer Res. 2020, 26, 657–668. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Sammut, S.J.; Ross, E.M.; Bashford-Rogers, R.; Greenstein, E.; Markus, H.; Morganella, S.; Teng, Y.; Maruvka, Y.; Pereira, B.; et al. The Genomic and Immune Landscapes of Lethal Metastatic Breast Cancer. Cell Rep. 2019, 27, 2690–2708.e10. [Google Scholar] [CrossRef] [PubMed]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Yasojima, H.; Iwasa, T.; Kanbayashi, C.; Oshitanai, R.; Tsuneizumi, M.; Watanabe, K.-i.; Matsui, A.; et al. Comparison of Immune Microenvironments between Primary Tumors and Brain Metastases in Patients with Breast Cancer. Oncotarget 2017, 8, 103671–103681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-Associated Macrophages in Breast Cancer: Innocent Bystander or Important Player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Acquired Resistance to Cancer Immunotherapy: Role of Tumor-Mediated Immunosuppression. Semin. Cancer Biol. 2020, 65, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Retecki, K.; Seweryn, M.; Graczyk-Jarzynka, A.; Bajor, M. The Immune Landscape of Breast Cancer: Strategies for Overcoming Immunotherapy Resistance. Cancers 2021, 13, 6012. [Google Scholar] [CrossRef]
- Gianni, C.; Palleschi, M.; Schepisi, G.; Casadei, C.; Bleve, S.; Merloni, F.; Sirico, M.; Sarti, S.; Cecconetto, L.; Di Menna, G.; et al. Circulating Inflammatory Cells in Patients with Metastatic Breast Cancer: Implications for Treatment. Front. Oncol. 2022, 12, 882896. [Google Scholar] [CrossRef]
- Sivaganesh, V.; Promi, N.; Maher, S.; Peethambaran, B. Emerging Immunotherapies against Novel Molecular Targets in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 2433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Huang, K.H.; Fang, X.; Li, Y.; Wang, F.; An, L.; Chen, Q.; Zhang, Y.; Shi, A.; et al. Targeting Epidermal Growth Factor-Overexpressing Triple-Negative Breast Cancer by Natural Killer Cells Expressing a Specific Chimeric Antigen Receptor. Cell Prolif. 2020, 53, e12858. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.T.; Green, J.A.; Friedland, J.S. Analysis of Matrix Metalloproteinase Secretion by Macrophages. Methods Mol. Biol. 2009, 531, 253–265. [Google Scholar] [CrossRef]
- Sloas, C.; Gill, S.; Klichinsky, M. Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors. Front. Immunol. 2021, 12, 783305. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.; Brentjens, R.J.; Gunset, G.; Rivière, I.; Sadelain, M. Human T-Lymphocyte Cytotoxicity and Proliferation Directed by a Single Chimeric TCRzeta/CD28 Receptor. Nat. Biotechnol. 2002, 20, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mei, Q.; Chen, L.; Zhou, J. Chimeric Antigen Receptor (CAR)-T-Cell Therapy in Non-Small-Cell Lung Cancer (NSCLC): Current Status and Future Perspectives. Cancer Immunol. Immunother. 2021, 70, 619–631. [Google Scholar] [CrossRef]
- MacKay, M.; Afshinnekoo, E.; Rub, J.; Hassan, C.; Khunte, M.; Baskaran, N.; Owens, B.; Liu, L.; Roboz, G.J.; Guzman, M.L.; et al. The Therapeutic Landscape for Cells Engineered with Chimeric Antigen Receptors. Nat. Biotechnol. 2020, 38, 233–244. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, J.Y. Recent Advances in Allogeneic CAR-T Cells. Biomolecules 2020, 10, 263. [Google Scholar] [CrossRef]
- Tokarew, N.; Ogonek, J.; Endres, S.; von Bergwelt-Baildon, M.; Kobold, S. Teaching an Old Dog New Tricks: Next-Generation CAR T Cells. Br. J. Cancer 2019, 120, 26–37. [Google Scholar] [CrossRef]
- Firor, A.E.; Jares, A.; Ma, Y. From Humble Beginnings to Success in the Clinic: Chimeric Antigen Receptor-Modified T-Cells and Implications for Immunotherapy. Exp. Biol. Med. 2015, 240, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Ruella, M.; June, C.H. Emerging Cellular Therapies for Cancer. Annu. Rev. Immunol. 2019, 37, 145–171. [Google Scholar] [CrossRef]
- D’Aloia, M.M.; Zizzari, I.G.; Sacchetti, B.; Pierelli, L.; Alimandi, M. CAR-T Cells: The Long and Winding Road to Solid Tumors. Cell Death Dis. 2018, 9, 282. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The Fourth Generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Kopecky, C.; Hombach, A.A.; Abken, H. IL-12 Release by Engineered T Cells Expressing Chimeric Antigen Receptors Can Effectively Muster an Antigen-Independent Macrophage Response on Tumor Cells That Have Shut down Tumor Antigen Expression. Cancer Res. 2011, 71, 5697–5706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef] [PubMed]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A Novel Chimeric Antigen Receptor Containing a JAK-STAT Signaling Domain Mediates Superior Antitumor Effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Chen, X. Integrin Alpha(v)Beta(3)-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef]
- Wallstabe, L.; Mades, A.; Frenz, S.; Einsele, H.; Rader, C.; Hudecek, M. CAR T Cells Targeting αvβ3 Integrin Are Effective against Advanced Cancer in Preclinical Models. Adv. Cell Gene Ther. 2018, 1, e11. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Rivera, A.; Tao, L.; Zhang, X. Genetically Modified T Cells Targeting Neovasculature Efficiently Destroy Tumor Blood Vessels, Shrink Established Solid Tumors and Increase Nanoparticle Delivery. Int. J. Cancer 2013, 133, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, J.C.; Campbell, T.N.; Pierce, P.R.; Watkins, J.D.; Huse, W.D.; Bodkin, D.J.; Cheresh, D.A. Targeted Antiangiogenic Therapy for Cancer Using Vitaxin: A Humanized Monoclonal Antibody to the Integrin Alphavbeta3. Clin. Cancer Res. 2000, 6, 3056–3061. [Google Scholar] [PubMed]
- Hersey, P.; Sosman, J.; O’Day, S.; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; et al. A Randomized Phase 2 Study of Etaracizumab, a Monoclonal Antibody against Integrin αvβ3, ± Dacarbazine in Patients with Stage IV Metastatic Melanoma. Cancer 2010, 116, 1526–1534. [Google Scholar] [CrossRef]
- Carson-Walter, E.B.; Watkins, D.N.; Nanda, A.; Vogelstein, B.; Kinzler, K.W.; St. Croix, B. Cell Surface Tumor Endothelial Markers Are Conserved in Mice and Humans. Cancer Res. 2001, 61, 6649–6655. [Google Scholar]
- Chaudhary, A.; Hilton, M.B.; Seaman, S.; Haines, D.C.; Stevenson, S.; Lemotte, P.K.; Tschantz, W.R.; Zhang, X.M.; Saha, S.; Fleming, T.; et al. TEM8/ANTXR1 Blockade Inhibits Pathological Angiogenesis and Potentiates Tumoricidal Responses against Multiple Cancer Types. Cancer Cell 2012, 21, 212–226. [Google Scholar] [CrossRef]
- Davies, G.; Rmali, K.A.; Watkins, G.; Mansel, R.E.; Mason, M.D.; Jiang, W.G. Elevated Levels of Tumour Endothelial Marker-8 in Human Breast Cancer and Its Clinical Significance. Int. J. Oncol. 2006, 29, 1311–1317. [Google Scholar] [CrossRef]
- Gutwein, L.G.; Al-Quran, S.Z.; Fernando, S.; Fletcher, B.S.; Copeland, E.M.; Grobmyer, S.R. Tumor Endothelial Marker 8 Expression in Triple-Negative Breast Cancer. Anticancer Res. 2011, 31, 3417–3422. [Google Scholar]
- Byrd, T.T.; Fousek, K.; Pignata, A.; Szot, C.; Samaha, H.; Seaman, S.; Dobrolecki, L.; Salsman, V.S.; Oo, H.Z.; Bielamowicz, K.; et al. TEM8/ANTXR1-Specific CAR T Cells as a Targeted Therapy for Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 489–500. [Google Scholar] [CrossRef]
- Feneyrolles, C.; Spenlinhauer, A.; Guiet, L.; Fauvel, B.; Daydé-Cazals, B.; Warnault, P.; Chevé, G.; Yasri, A. Axl Kinase as a Key Target for Oncology: Focus on Small Molecule Inhibitors. Mol. Cancer Ther. 2014, 13, 2141–2148. [Google Scholar] [CrossRef]
- Wei, J.; Sun, H.; Zhang, A.; Wu, X.; Li, Y.; Liu, J.; Duan, Y.; Xiao, F.; Wang, H.; Lv, M.; et al. A Novel AXL Chimeric Antigen Receptor Endows T Cells with Anti-Tumor Effects against Triple Negative Breast Cancers. Cell. Immunol. 2018, 331, 49–58. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. Biomed Res. Int. 2020, 2020, 4795171. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, K.M.; Cheung, A.; Mele, S.; Chiaruttini, G.; Crescioli, S.; Griffin, M.; Nakamura, M.; Spicer, J.F.; Tsoka, S.; Lacy, K.E.; et al. Chondroitin Sulfate Proteoglycan 4 and Its Potential as an Antibody Immunotherapy Target across Different Tumor Types. Front. Immunol. 2018, 8, 1911. [Google Scholar] [CrossRef] [PubMed]
- Geldres, C.; Savoldo, B.; Hoyos, V.; Caruana, I.; Zhang, M.; Yvon, E.; Del Vecchio, M.; Creighton, C.J.; Ittmann, M.; Ferrone, S.; et al. T Lymphocytes Redirected against the Chondroitin Sulfate Proteoglycan-4 Control the Growth of Multiple Solid Tumors Both In Vitro and In Vivo. Clin. Cancer Res. 2014, 20, 962–971. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The Emergence of Trophoblast Cell-Surface Antigen 2 (TROP-2) as a Novel Cancer Target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, L.; Zhang, M.; Huang, X.; Qian, P.; Tang, Q.; Zhu, J.; Feng, Z. The Killing Effect of Novel Bi-Specific Trop2/PD-L1 CAR-T Cell Targeted Gastric Cancer. Am. J. Cancer Res. 2019, 9, 1846–1856. [Google Scholar]
- Seitz, C.M.; Schroeder, S.; Knopf, P.; Krahl, A.C.; Hau, J.; Schleicher, S.; Martella, M.; Quintanilla-Martinez, L.; Kneilling, M.; Pichler, B.; et al. GD2-Targeted Chimeric Antigen Receptor T Cells Prevent Metastasis Formation by Elimination of Breast Cancer Stem-like Cells. Oncoimmunology 2019, 9, 1683345. [Google Scholar] [CrossRef]
- Guo, P.; Huang, J.; Wang, L.; Jia, D.; Yang, J.; Dillon, D.A.; Zurakowski, D.; Mao, H.; Moses, M.A.; Auguste, D.T.; et al. ICAM-1 as a Molecular Target for Triple Negative Breast Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14710–14715. [Google Scholar] [CrossRef] [PubMed]
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 Initiates CTC Cluster Formation and Trans-Endothelial Migration in Lung Metastasis of Breast Cancer. Nat. Commun. 2021, 12, 4867. [Google Scholar] [CrossRef]
- Park, S.; Shevlin, E.; Vedvyas, Y.; Zaman, M.; Park, S.; Hsu, Y.M.S.; Min, I.M.; Jin, M.M. Micromolar Affinity CAR T Cells to ICAM-1 Achieves Rapid Tumor Elimination While Avoiding Systemic Toxicity. Sci. Rep. 2017, 7, 14366. [Google Scholar] [CrossRef]
- Yang, Y.; Vedvyas, Y.; McCloskey, J.E.; Min, I.M.; Jin, M.M. Abstract 2322: ICAM-1 Targeting CAR T Cell Therapy for Triple Negative Breast Cancer. Cancer Res. 2019, 79 (Suppl. S13), 2322. [Google Scholar] [CrossRef]
- Tozbikian, G.; Brogi, E.; Kadota, K.; Catalano, J.; Akram, M.; Patil, S.; Ho, A.Y.; Reis-Filho, J.S.; Weigelt, B.; Norton, L.; et al. Mesothelin Expression in Triple Negative Breast Carcinomas Correlates Significantly with Basal-like Phenotype, Distant Metastases and Decreased Survival. PLoS ONE 2014, 9, e114900. [Google Scholar] [CrossRef]
- Parinyanitikul, N.; Blumenschein, G.R.; Wu, Y.; Lei, X.; Chavez-Macgregor, M.; Smart, M.; Gonzalez-Angulo, A.M. Mesothelin Expression and Survival Outcomes in Triple Receptor Negative Breast Cancer. Clin. Breast Cancer 2013, 13, 378–384. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdanifar, M.; Das Roy, L.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019, 10, 1149. [Google Scholar] [CrossRef]
- Yolken, R.H.; Peterson, J.A.; Vonderfecht, S.L.; Fouts, E.T.; Midthun, K.; Newburg, D.S. Human Milk Mucin Inhibits Rotavirus Replication and Prevents Experimental Gastroenteritis. J. Clin. Investig. 1992, 90, 1984–1991. [Google Scholar] [CrossRef]
- Schroten, H.; Hanisch, F.G.; Plogmann, R.; Hacker, J.; Uhlenbruck, G.; Nobis-Bosch, R.; Wahn, V. Inhibition of Adhesion of S-Fimbriated Escherichia coli to Buccal Epithelial Cells by Human Milk Fat Globule Membrane Components: A Novel Aspect of the Protective Function of Mucins in the Nonimmunoglobulin Fraction. Infect. Immun. 1992, 60, 2893–2899. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Cui, B.; Chuang, H.Y.; Yu, J.; Wang-Rodriguez, J.; Tang, L.; Chen, G.; Basak, G.W.; Kipps, T.J. ROR1 Is Expressed in Human Breast Cancer and Associated with Enhanced Tumor-Cell Growth. PLoS ONE 2012, 7, e31127. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in Physiological Processes and Diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A Perspective on Anti-EGFR Therapies Targeting Triple-Negative Breast Cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar]
- Rubin, I.; Yarden, Y. The Basic Biology of HER2. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Correia, M.P.; Cerwenka, A. The NKG2D/NKG2DL Axis in the Crosstalk between Lymphoid and Myeloid Cells in Health and Disease. Front. Immunol. 2018, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, S. The Carcinoembryonic Antigen (CEA) Family: Structures, Suggested Functions and Expression in Normal and Malignant Tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, K.; Hackert, T.; Zöller, M. CD44/CD44v6 a Reliable Companion in Cancer-Initiating Cell Maintenance and Tumor Progression. Front. Cell Dev. Biol. 2018, 6, 97. [Google Scholar] [CrossRef]
- Kasimir-Bauer, S.; Keup, C.; Hoffmann, O.; Hauch, S.; Kimmig, R.; Bittner, A.K. Circulating Tumor Cells Expressing the Prostate Specific Membrane Antigen (PSMA) Indicate Worse Outcome in Primary, Non-Metastatic Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 1658. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the Folate Receptor GP38 in Normal and Malignant Cell Lines and Tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- O’Shannessy, D.J.; Somers, E.B.; Albone, E.; Cheng, X.; Park, Y.C.; Tomkowicz, B.E.; Hamuro, Y.; Kohl, T.O.; Forsyth, T.M.; Smale, R.; et al. Characterization of the Human Folate Receptor Alpha via Novel Antibody-Based Probes. Oncotarget 2011, 2, 1227–1243. [Google Scholar] [CrossRef]
- Lanitis, E.; Poussin, M.; Klattenhoff, A.W.; Song, D.; Sandaltzopoulos, R.; June, C.H.; Powell, D.J. Chimeric Antigen Receptor T Cells with Dissociated Signaling Domains Exhibit Focused Antitumor Activity with Reduced Potential for Toxicity in Vivo. Cancer Immunol. Res. 2013, 1, 43–53. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.S. An Overview of the C-MET Signaling Pathway. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. S1), S7–S19. [Google Scholar] [CrossRef]
- Faiella, A.; Riccardi, F.; Cartenì, G.; Chiurazzi, M.; Onofrio, L. The Emerging Role of C-Met in Carcinogenesis and Clinical Implications as a Possible Therapeutic Target. J. Oncol. 2022, 2022, 5179182. [Google Scholar] [CrossRef]
- Klampatsa, A.; Dimou, V.; Albelda, S.M. Mesothelin-Targeted CAR-T Cell Therapy for Solid Tumors. Expert Opin. Biol. Ther. 2021, 21, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Wang, L.C.; Selven, B.; Zhang, H.; Conejo-Garcia, J.; Borghaei, H.; Kalos, M.; Vondeheide, R.H.; Albelda, S.M.; June, C.H.; et al. Mesothelin, a Novel Immunotherapy Target for Triple Negative Breast Cancer. Breast Cancer Res. Treat. 2012, 133, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zi, Z.; Jin, Y.; Li, G.; Shao, K.; Cai, Q.; Ma, X.; Wei, F. CRISPR/Cas9-Mediated PD-1 Disruption Enhances Human Mesothelin-Targeted CAR T Cell Effector Functions. Cancer Immunol. Immunother. 2019, 68, 365–377. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- St. Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montegomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes Expressed in Human Tumor Endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef]
- Vargas, M.; Karamsetty, R.; Leppla, S.H.; Chaudry, G.J. Broad Expression Analysis of Human ANTXR1/TEM8 Transcripts Reveals Differential Expression and Novel Splizce Variants. PLoS ONE 2012, 7, e43174. [Google Scholar] [CrossRef]
- Jing, X.; Liang, H.; Hao, C.; Yang, X.; Cui, X. Overexpression of MUC1 Predicts Poor Prognosis in Patients with Breast Cancer. Oncol. Rep. 2019, 41, 801–810. [Google Scholar] [CrossRef]
- Dalziel, M.; Whitehouse, C.; McFarlane, I.; Brockhausen, I.; Gschmeissner, S.; Schwientek, T.; Clausen, H.; Burchell, J.M.; Taylor-Papadimitriou, J. The Relative Activities of the C2GnT1 and ST3Gal-I Glycosyltransferases Determine O-Glycan Structure and Expression of a Tumor-Associated Epitope on MUC1. J. Biol. Chem. 2001, 276, 11007–11015. [Google Scholar] [CrossRef] [PubMed]
- Das Roy, L.; Dillon, L.M.; Zhou, R.; Moore, L.J.; Livasy, C.; El-Khoury, J.M.; Puri, R.; Mukherjee, P. A Tumor Specific Antibody to Aid Breast Cancer Screening in Women with Dense Breast Tissue. Genes Cancer 2017, 8, 536–549. [Google Scholar] [CrossRef]
- Wallstabe, L.; Göttlich, C.; Nelke, L.C.; Kühnemundt, J.; Schwarz, T.; Nerreter, T.; Einsele, H.; Walles, H.; Dandekar, G.; Nietzer, S.L.; et al. ROR1-CAR T Cells Are Effective against Lung and Breast Cancer in Advanced Microphysiologic 3D Tumor Models. JCI Insight 2019, 4, e126345. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Furlan, S.N.; Jaeger-Ruckstuhl, C.A.; Sarvothama, M.; Berger, C.; Smythe, K.S.; Garrison, S.M.; Specht, J.M.; Lee, S.M.; Amezquita, R.A.; et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy When Combined with Checkpoint Blockade. Cancer Cell 2021, 39, 193–208.e10. [Google Scholar] [CrossRef]
- Nasiri, F.; Kazemi, M.; Mirarefin, S.M.J.; Mahboubi Kancha, M.; Ahmadi Najafabadi, M.; Salem, F.; Dashti Shokoohi, S.; Evazi Bakhshi, S.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P. CAR-T Cell Therapy in Triple-Negative Breast Cancer: Hunting the Invisible Devil. Front. Immunol. 2022, 13, 1018786. [Google Scholar] [CrossRef]
- Morisaki, T.; Onishi, H.; Katano, M. Cancer Immunotherapy Using NKG2D and DNAM-1 Systems. Anticancer Res. 2012, 32, 2241–2247. [Google Scholar]
- Zhang, T.; Lemoi, B.A.; Sentman, C.L. Chimeric NK-Receptor-Bearing T Cells Mediate Antitumor Immunotherapy. Blood 2005, 106, 1544–1551. [Google Scholar] [CrossRef]
- Sallman, D.A.; Brayer, J.; Sagatys, E.M.; Lonez, C.; Breman, E.; Agaugué, S.; Verma, B.; Gilham, D.E.; Lehmann, F.F.; Davila, M.L. NKG2D-Based Chimeric Antigen Receptor Therapy Induced Remission in a Relapsed/Refractory Acute Myeloid Leukemia Patient. Haematologica 2018, 103, e424–e426. [Google Scholar] [CrossRef]
- Murad, J.M.; Baumeister, S.H.; Werner, L.; Daley, H.; Trébéden-Negre, H.; Reder, J.; Sentman, C.L.; Gilham, D.; Lehmann, F.; Snykers, S.; et al. Manufacturing Development and Clinical Production of NKG2D Chimeric Antigen Receptor–Expressing T Cells for Autologous Adoptive Cell Therapy. Cytotherapy 2018, 20, 952–963. [Google Scholar] [CrossRef]
- Han, Y.; Xie, W.; Song, D.-G.; Powell, D.J. Control of Triple-Negative Breast Cancer Using Ex Vivo Self-Enriched, Costimulated NKG2D CAR T Cells. J. Hematol. Oncol. 2018, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Beard, R.E.; Zheng, Z.; Lagisetty, K.H.; Burns, W.R.; Tran, E.; Hewitt, S.M.; Abate-Daga, D.; Rosati, S.F.; Fine, H.A.; Ferrone, S.; et al. Multiple Chimeric Antigen Receptors Successfully Target Chondroitin Sulfate Proteoglycan 4 in Several Different Cancer Histologies and Cancer Stem Cells. J. Immunother. Cancer 2014, 2, 25. [Google Scholar] [CrossRef]
- Junttila, T.T.; Li, J.; Johnston, J.; Hristopoulos, M.; Clark, R.; Ellerman, D.; Wang, B.E.; Li, Y.; Mathieu, M.; Li, G.; et al. Antitumor Efficacy of a Bispecific Antibody That Targets HER2 and Activates T Cells. Cancer Res. 2014, 74, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, I.R.; Vicario, R.; Morancho, B.; Morales, C.B.; Arenas, E.J.; Herter, S.; Freimoser-Grundschober, A.; Somandin, J.; Sam, J.; Ast, O.; et al. P95HER2-T Cell Bispecific Antibody for Breast Cancer Treatment. Sci. Transl. Med. 2018, 10, eaat1445. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Rojo, F.; Ocaña, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Ramon y Cajal, S.; Arribas, J.; et al. Expression of P95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Wu, Y.; Hooper, A.T.; Zhong, Z.; Witte, L.; Bohlen, P.; Rafii, S.; Hicklin, D.J. The Vascular Endothelial Growth Factor Receptor (VEGFR-1) Supports Growth and Survival of Human Breast Carcinoma. Int. J. Cancer 2006, 119, 1519–1529. [Google Scholar] [CrossRef]
- Tang, P.; Li, L.; Zhou, Y.; Shen, C.C.; Kang, Y.H.; Yao, Y.Q.; Yi, C.; Gou, L.T.; Yang, J.L. The Preparation of VEGFR1/CD3 Bispecific Antibody and Its Specific Cytotoxicity against VEGFR1-Positive Breast Cancer Cells. Biotechnol. Appl. Biochem. 2014, 61, 376–384. [Google Scholar] [CrossRef]
- Ghoussoub, R.A.; Dillon, D.A.; D’Aquila, T.; Rimm, E.B.; Fearon, E.R.; Rimm, D.L. Expression of C-Met Is a Strong Independent Prognostic Factor in Breast Carcinoma. Cancer 1998, 82, 1513–1520. [Google Scholar] [CrossRef]
- Jin, H.; Yang, R.; Zheng, Z.; Romero, M.; Ross, J.; Bou-Reslan, H.; Carano, R.A.D.; Kasman, I.; Mai, E.; Young, J.; et al. MetMAb, the One-Armed 5D5 Anti-c-Met Antibody, Inhibits Orthotopic Pancreatic Tumor Growth and Improves Survival. Cancer Res. 2008, 68, 4360–4368. [Google Scholar] [CrossRef]
- Martens, T.; Schmidt, N.O.; Eckerich, C.; Filibrandt, R.; Merchant, M.; Schwall, R.; Westphal, M.; Lamszus, K. A Novel One-Armed Anti-c-Met Antibody Inhibits Glioblastoma Growth In Vivo. Clin. Cancer Res. 2006, 12 Pt 1, 6144–6152. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.; Ma, X.; Maun, H.R.; Zheng, Z.; Peng, J.; Romero, M.; Huang, A.; Yang, N.Y.; Nishimura, M.; Greve, J.; et al. Monovalent Antibody Design and Mechanism of Action of Onartuzumab, a MET Antagonist with Anti-Tumor Activity as a Therapeutic Agent. Proc. Natl. Acad. Sci. USA 2013, 110, E2987–E2996. [Google Scholar] [CrossRef]
- Pérol, M. Negative Results of METLung Study: An Opportunity to Better Understand the Role of MET Pathway in Advanced NSCLC. Transl. Lung Cancer Res. 2014, 3, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Stawicki, S.; Couto, S.; Eastham-Anderson, J.; Kallop, D.; Weimer, R.; Wu, Y.; Pei, L. An Anti-Axl Monoclonal Antibody Attenuates Xenograft Tumor Growth and Enhances the Effect of Multiple Anticancer Therapies. Oncogene 2010, 29, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.C.; Rini, B.I. Treatment of Renal Cell Carcinoma: Current Status and Future Directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a Selective Small Molecule Inhibitor of Axl Kinase, Blocks Tumor Spread and Prolongs Survival in Models of Metastatic Breast Cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Voeller, J.; Sondel, P.M. Advances in Anti-GD2 Immunotherapy for Treatment of High-Risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2019, 41, 163–169. [Google Scholar] [CrossRef]
- Al-Chalabi, M.; Bass, A.N.; Alsalman, I. Physiology, Prolactin; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Zhou, Y.; Zong, H.; Han, L.; Xie, Y.; Jiang, H.; Gilly, J.; Zhang, B.; Lu, H.; Chen, J.; Sun, R.; et al. A Novel Bispecific Antibody Targeting CD3 and Prolactin Receptor (PRLR) against PRLR-Expression Breast Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 87. [Google Scholar] [CrossRef]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L. Molecular Mechanisms of Prolactin and Its Receptor. Endocr. Rev. 2012, 33, 504–525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Jiang, D.-Q.; Cui, L.-Z.; He, Z.; Wang, C.; Zhang, Z.-W.; Zhu, H.-L.; Ding, Y.-M.; Li, L.-F.; et al. Antitumor Activity of EGFR-Specific CAR T Cells against Non-Small-Cell Lung Cancer Cells in Vitro and in Mice. Cell Death Dis. 2018, 9, 177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Ding, Y.; Fang, Y.; Wang, P.; Chu, W.; Jin, Z.; Yang, X.; Wang, J.; Lou, J.; et al. Phase I Clinical Trial of EGFR-Specific CAR-T Cells Generated by the PiggyBac Transposon System in Advanced Relapsed/Refractory Non-Small Cell Lung Cancer Patients. J. Cancer Res. Clin. Oncol. 2021, 147, 3725–3734. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Huang, K.H.; Li, Y.; Fang, X.; An, L.; Wang, F.; Chen, Q.; Zhang, Y.; Shi, A.; et al. EGFR-Specific CAR-T Cells Trigger Cell Lysis in EGFR-Positive TNBC. Aging 2019, 11, 11054–11072. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, Z.; Liu, J.; Chen, Y.; Ding, J.; Xia, N.; Luo, W.; Liu, W. EGFR-Targeted CAR-T Cells Are Potent and Specific in Suppressing Triple-Negative Breast Cancer Both in Vitro and in Vivo. Clin. Transl. Immunol. 2020, 9, e01135. [Google Scholar] [CrossRef]
- Yoon, D.H.; Osborn, M.J.; Tolar, J.; Kim, C.J. Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-in CAR-T. Int. J. Mol. Sci. 2018, 19, 340. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef]
- Long, K.B.; Young, R.M.; Boesteanu, A.C.; Davis, M.M.; Melenhorst, J.J.; Lacey, S.F.; DeGaramo, D.A.; Levine, B.L.; Fraietta, J.A. CAR T Cell Therapy of Non-Hematopoietic Malignancies: Detours on the Road to Clinical Success. Front. Immunol. 2018, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, A.; Maus, M.V. Making CAR T Cells a Solid Option for Solid Tumors. Front. Immunol. 2018, 9, 2593. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human Natural Killer Cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.C.; Sutton, C.E.; Ladell, K.; Grant, E.J.; McLaren, J.E.; Roche, F.; Dash, P.; Apiwattanakul, N.; Awad, W.; Miners, K.L.; et al. A Population of Proinflammatory T Cells Coexpresses αβ and γδ T Cell Receptors in Mice and Humans. J. Exp. Med. 2020, 217, e20190834. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on γδ T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T Cells in Cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Fan, Y.; Moustaki, A.; Abdelsamed, H.A.; Dash, P.; Dogra, P.; Carter, R.; Awad, W.; Neale, G.; Thomas, P.G.; et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 2017, 170, 142–157.e19. [Google Scholar] [CrossRef]
- Das, R.K.; Vernau, L.; Grupp, S.A.; Barrett, D.M. Naïve T-Cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019, 9, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Maus, M.V. Wishing on a CAR: Understanding the Scope of Intrinsic T-Cell Deficits in Patients with Cancer. Cancer Discov. 2019, 9, 466–468. [Google Scholar] [CrossRef]
- Abdin, S.M.; Paasch, D.; Morgan, M.; Lachmann, N. CARs and beyond: Tailoring Macrophage-Based Cell Therapeutics to Combat Solid Malignancies. J. Immunother. Cancer 2021, 9, 2741. [Google Scholar] [CrossRef]
- Franken, L.; Schiwon, M.; Kurts, C. Macrophages: Sentinels and Regulators of the Immune System. Cell. Microbiol. 2016, 18, 475–487. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Erreni, M.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011, 4, 141–154. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Takeya, M.; Komohara, Y. Role of Tumor-Associated Macrophages in Human Malignancies: Friend or Foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.X.; Weissman, I.L. Phagocytosis Checkpoints as New Targets for Cancer Immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, K.; Weissman, I.L. Macrophages Are Critical Effectors of Antibody Therapies for Cancer. MAbs 2015, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Antibodies, Fc Receptors and Cancer. Curr. Opin. Immunol. 2007, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.A.; Williamson, A.P.; Steinbach, A.M.; Roberts, E.W.; Kern, N.; Headley, M.B.; Vale, R.D. Chimeric Antigen Receptors That Trigger Phagocytosis. Elife 2018, 7, e36688. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human Chimeric Antigen Receptor Macrophages for Cancer Immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-Macrophage: A New Immunotherapy Candidate against Solid Tumors. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef]
- Roth, T.L.; Puig-Saus, C.; Yu, R.; Shifrut, E.; Carnevale, J.; Li, P.J.; Hiatt, J.; Saco, J.; Krystofinski, P.; Li, H.; et al. Reprogramming Human T Cell Function and Specificity with Non-Viral Genome Targeting. Nature 2018, 559, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent Stem Cell-Derived CAR-Macrophage Cells with Antigen-Dependent Anti-Cancer Cell Functions. J. Hematol. Oncol. 2020, 13, 153. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Su, H.F.; Liu, Q.; Shen, J.; Dai, H.; Zheng, W.; Lu, Y.; Zhang, W.; Bei, Y.; et al. Chimeric Antigen Receptor Macrophage Therapy for Breast Tumours Mediated by Targeting the Tumour Extracellular Matrix. Br. J. Cancer 2019, 121, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M. Macrophages Enter CAR Immunotherapy. Nat. Methods 2020, 17, 561. [Google Scholar] [CrossRef]
- Pierini, S.; Gabbasov, R.; Gabitova, L.; Ohtani, Y.; Klichinsky, M. CAR Macrophages (CAR- M) Elicit a Systemic Anti-Tumor Immune Response and Synergize with PD1 Blockade in Immunocompetent Mouse Models of HER2+ Solid Tumors. BMJ Spec. J. 2020, 8. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus Nab-Paclitaxel as First-Line Treatment for Unresectable, Locally Advanced or Metastatic Triple-Negative Breast Cancer (IMpassion130): Updated Efficacy Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Pfeifer, R. Evaluation of SSEA-4 as a CAR T Cell Therapeutic Target for the Treatment of Chemoresistant Triple Negative Breast Cancers. Ph.D. Thesis, Eberhard Karls Universität, Tübingen, Germany, 2018. [Google Scholar]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Rahbarizadeh, F. Addressing the Obstacles of CAR T Cell Migration in Solid Tumors: Wishing a Heavy Traffic. Crit. Rev. Biotechnol. 2022, 42, 1079–1098. [Google Scholar] [CrossRef]
| Target | Expression in Healthy Tissue | Role | Drug Agent | Trial | Status |
|---|---|---|---|---|---|
| αvβ3-integrin | Platelets, macrophages, dendritic cells, activated endothelial cells [41,42] | Cell proliferation, adhesion, metastatization, angiogenesis [41] | only preclinical data [41,42,43,44,45,46] | ||
| TEM8 | Endothelium [47,48] | Endothelial cell development [47,48] | L2CAR-T | only preclinical data [48,49,50,51] | |
| AXL | Bone marrow stroma and myeloid cells [52] | Tumor expansion, metastasization and survival [52] | AXL-CAR-Tcells | only preclinical data [53,54] | |
| CSPG4 | Oligodendrocyte progenitor cells [55] | neuronal network regulation and epidermal stem cells homeostasis [55] | CSPG4- CAR-T cells | only preclinical data [56] | |
| TROP-2 | Epithelial tissue [57] | Invasiveness [57] | only preclinical data [58] | ||
| GD2 | Neuroectoderm [59] | Cell signal transduction modulation [59] | Her2, GD2, and CD44v6 | NCT04430595 | ongoing |
| ICAM-1 | Endothelial cells and immune cells [60] | Migration, invasiveness [61] | Only preclinical data [62,63] | ||
| Mesothelin | Mesothelial cells [64,65] | Cell adhesion [64,65] | Anti-mesothelin CAR-T cells | NCT01355965 NCT02580747 NCT02414269 NCT02792114 | completed unknown ongoing ongoing |
| MUC1 | Epithelial tissue [66] | Production of mucin [67,68,69] | huMNC2-CAR44 CAR T-TnMUC1 TILs/CAR-TILs targeting multiple antigens * | NCT04020575 NCT04025216 NCT04842812 | ongoing ongoing ongoing |
| ROR1 | Embryogenic tissue [70] | Cell survival and differentiation in embryogenesis [70] | ROR1-CAR-T TILs/CAR-TILs targeting multiple antigens * | NCT02706392 NCT04842812 | ongoing ongoing |
| EpCAM | Epithelial tissue [71] | Cell adhesion [71] | EpCAM CAR-T | NCT02915445 | ongoing |
| EGFR | Epithelial tissue [72] | Cell survival, proliferation [72] | EGFR/B7H3 CAR-T, TGFβR-KO CAR-EGFR T Cells | NCT05341492, NCT04976218 | ongoing ongoing |
| HER-2 | Epithelial tissue (in particular breast, skin, and gastrointestinal, respiratory, reproductive, urinary tracts) [73] | Cell proliferation, differentiation, and survival [73] | CCT303-406 CAR-T BPX-603 CAR-T HER-2 CAR-T HER-2 CAR-T + CAdVEC ** TILs/CAR-TILs targeting multiple antigens * | NCT04511871 NCT04650451 NCT03696030 NCT03740256 NCT04842812 | ongoing ongoing ongoing ongoing ongoing |
| NKG2D | Innate and adaptive immune cells [74] | Cytotoxicity and secretion of cytokines [74] | NKG2DL CAR-T cells | NCT04107142 | unknown |
| CEA | Epithelia (in particular enteric tissue) and embryogenic tissue [75] | Cell migration, proliferation, and survival [75] | CEA CAR-T cells | NCT04348643 | ongoing |
| CD44v6 | Epithelial tissue and hematopoietic cells [76] | Cell survival, proliferation, migration [76] | 4SCAR-CD44v6 T-cell | NCT04427449 | ongoing |
| PSMA | Endothelium, prostate [77] | Angiogenesis and immune modulation [77] | bi-4SCAR PSMA/CD70 Tcells, bi-4SCAR GD2/PSMA T cells, 4SCAR-PSMA T cells | NCT05437341 NCT05437315 NCT04429451 | ongoing ongoing ongoing |
| FRα | Epithelial tissue (mammary ducts, lungs, kidneys the choroid plexus) [78] | Cell growth and survival [79] | only preclinical data [80] | ||
| c-MET | Epithelial tissue [81] | Cell differentiation, proliferation, migration, angiogenesis, and epithelial-mesenchymal transition [82] | cMet RNA CAR- T cells | NCT01837602 | completed |
| Target | Drug Agent | Trial | Status |
|---|---|---|---|
| HER-2 | CT-0508 | NCT04660929 | Recruiting |
| Mesothelin | Intraperitoneal MCY-M11 and cyclophosphamide | NCT03608618 | Terminated (no results posted yet) |
| HER-2 | HER-2 CAR-M | NCT05007379 | Not yet recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepisi, G.; Gianni, C.; Palleschi, M.; Bleve, S.; Casadei, C.; Lolli, C.; Ridolfi, L.; Martinelli, G.; De Giorgi, U. The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer. Cancers 2023, 15, 1597. https://doi.org/10.3390/cancers15051597
Schepisi G, Gianni C, Palleschi M, Bleve S, Casadei C, Lolli C, Ridolfi L, Martinelli G, De Giorgi U. The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer. Cancers. 2023; 15(5):1597. https://doi.org/10.3390/cancers15051597
Chicago/Turabian StyleSchepisi, Giuseppe, Caterina Gianni, Michela Palleschi, Sara Bleve, Chiara Casadei, Cristian Lolli, Laura Ridolfi, Giovanni Martinelli, and Ugo De Giorgi. 2023. "The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer" Cancers 15, no. 5: 1597. https://doi.org/10.3390/cancers15051597
APA StyleSchepisi, G., Gianni, C., Palleschi, M., Bleve, S., Casadei, C., Lolli, C., Ridolfi, L., Martinelli, G., & De Giorgi, U. (2023). The New Frontier of Immunotherapy: Chimeric Antigen Receptor T (CAR-T) Cell and Macrophage (CAR-M) Therapy against Breast Cancer. Cancers, 15(5), 1597. https://doi.org/10.3390/cancers15051597







