Energy Metabolism, Metabolite, and Inflammatory Profiles in Human Ex Vivo Adipose Tissue Are Influenced by Obesity Status, Metabolic Dysfunction, and Treatment Regimes in Patients with Oesophageal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Patient Recruitment
2.2. Clinical Data Collation and Assessment
2.3. Seahorse Analysis of Metabolic Profiles from Adipose Tissue Explants and Generation of Adipose Conditioned Media (ACM)
2.4. Multiplex ELISA
2.5. Metabolomic and Lipidomic Screening
2.6. Statistical Analysis
3. Results
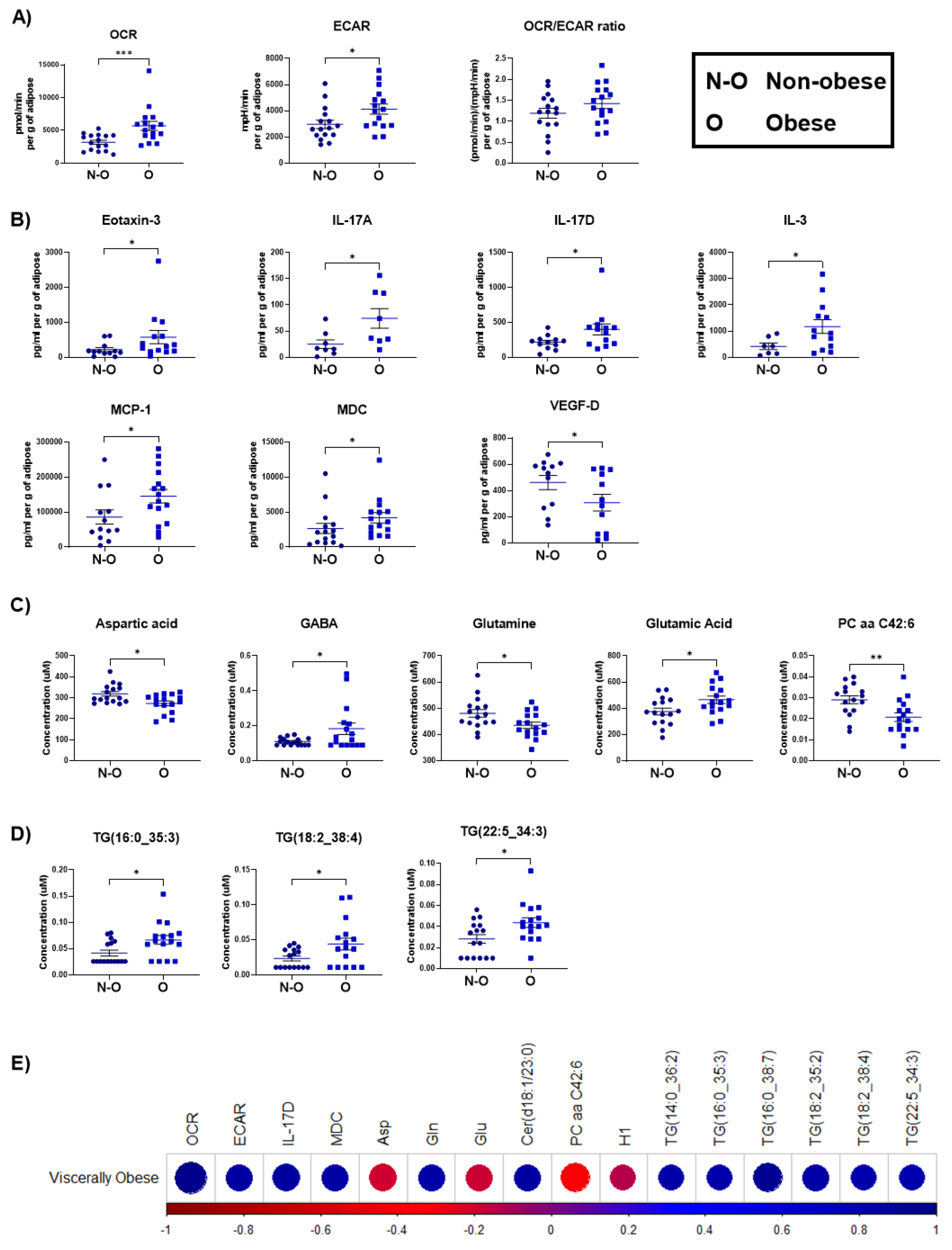
3.1. Increased Oxidative Phosphorylation Metabolism and Elevated Secreted Pro-Inflammatory Mediators Were Observed in Adipose Tissue Explants from Viscerally Obese Patients
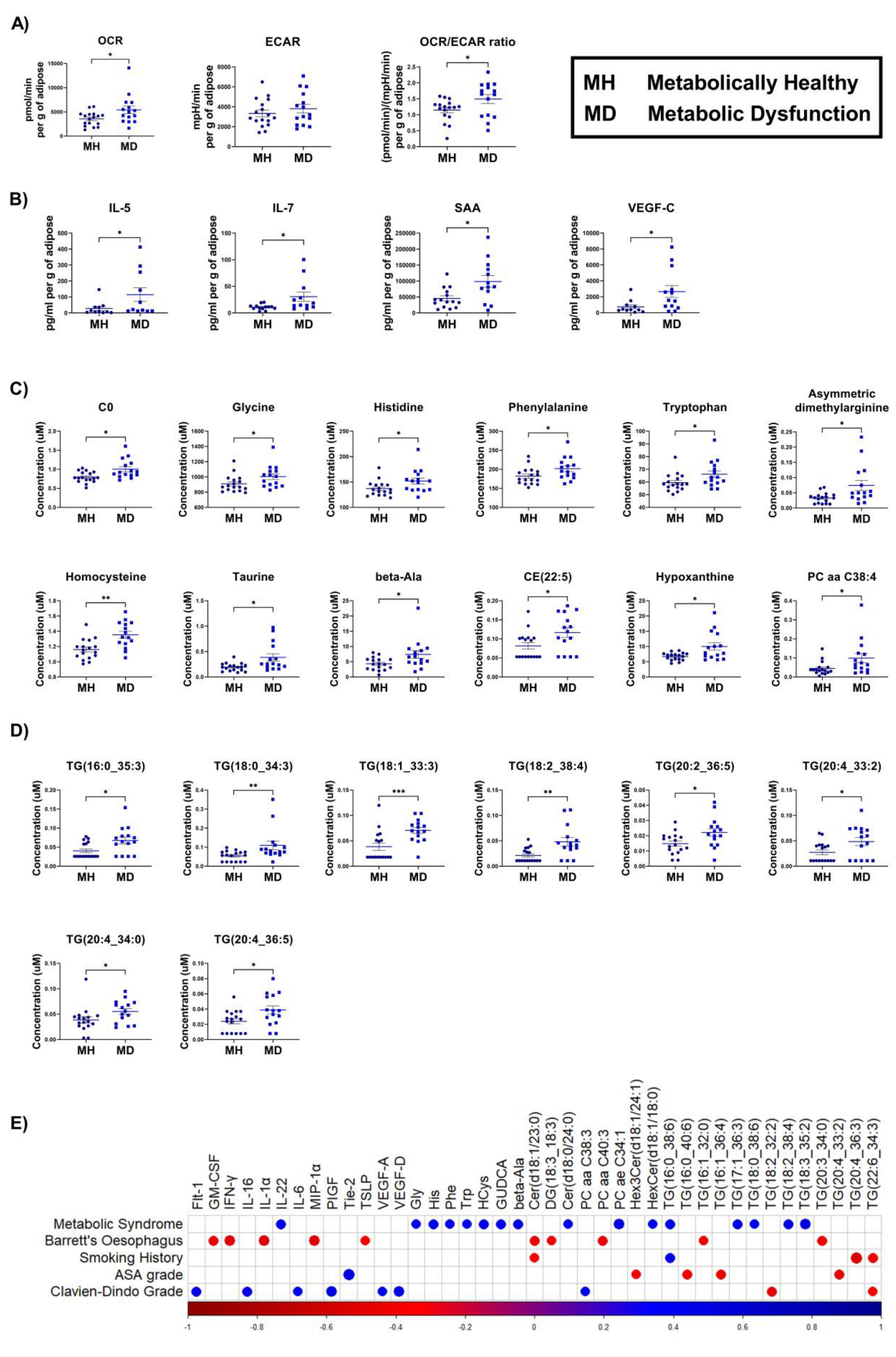
3.2. Adipose Explants Derived from OAC Patients with Metabolic Dysfunction Show Increased Oxidative Phosphorylation Associated Metabolism and Secreted Pro-Inflammatory Mediators
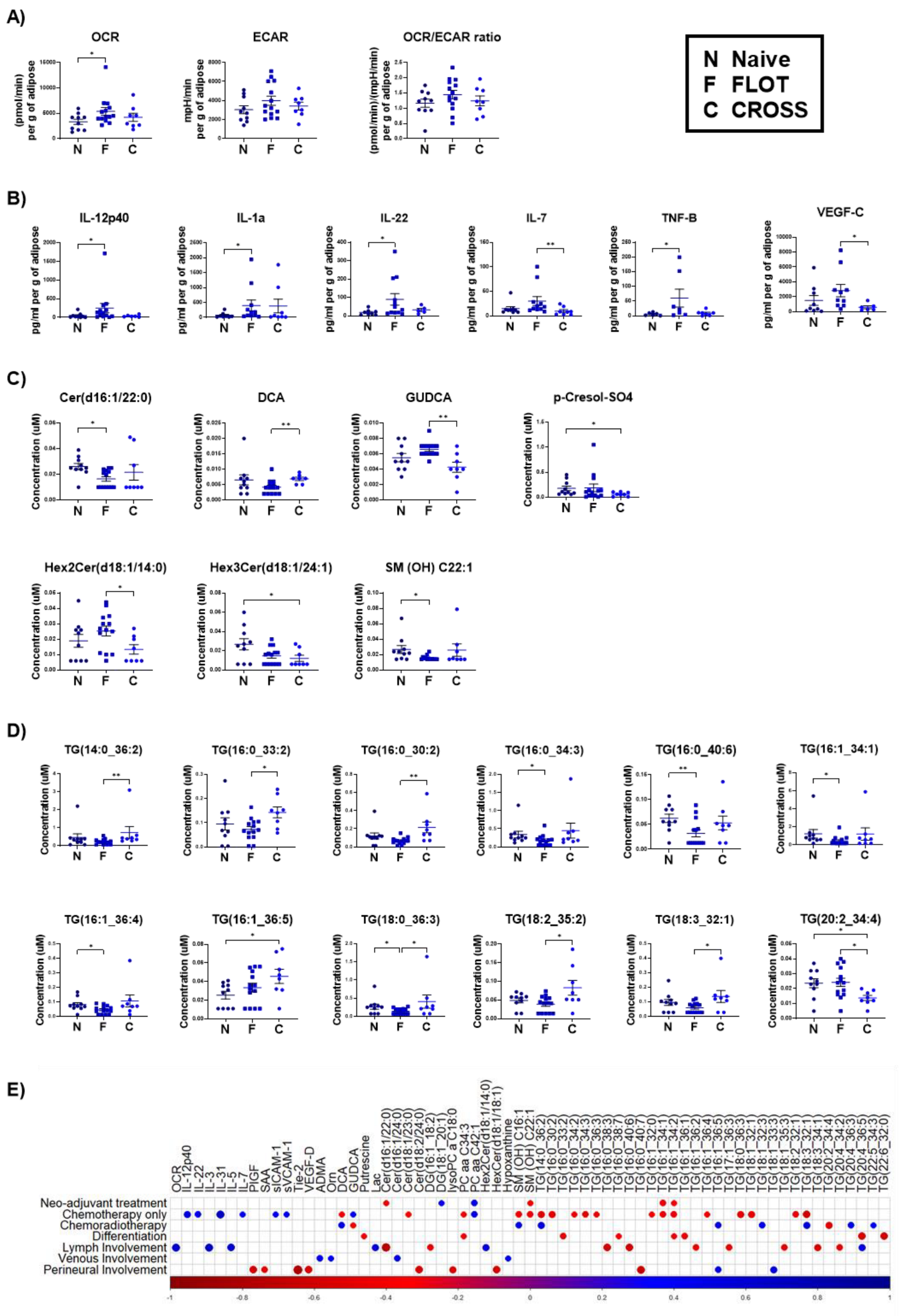
3.3. Adipose Explants from Patients Receiving the FLOT Chemotherapy Regimen Showed Increased Oxidative Phosphorylation and Pro-Inflammatory Mediators and Decreased Triacylglycerides
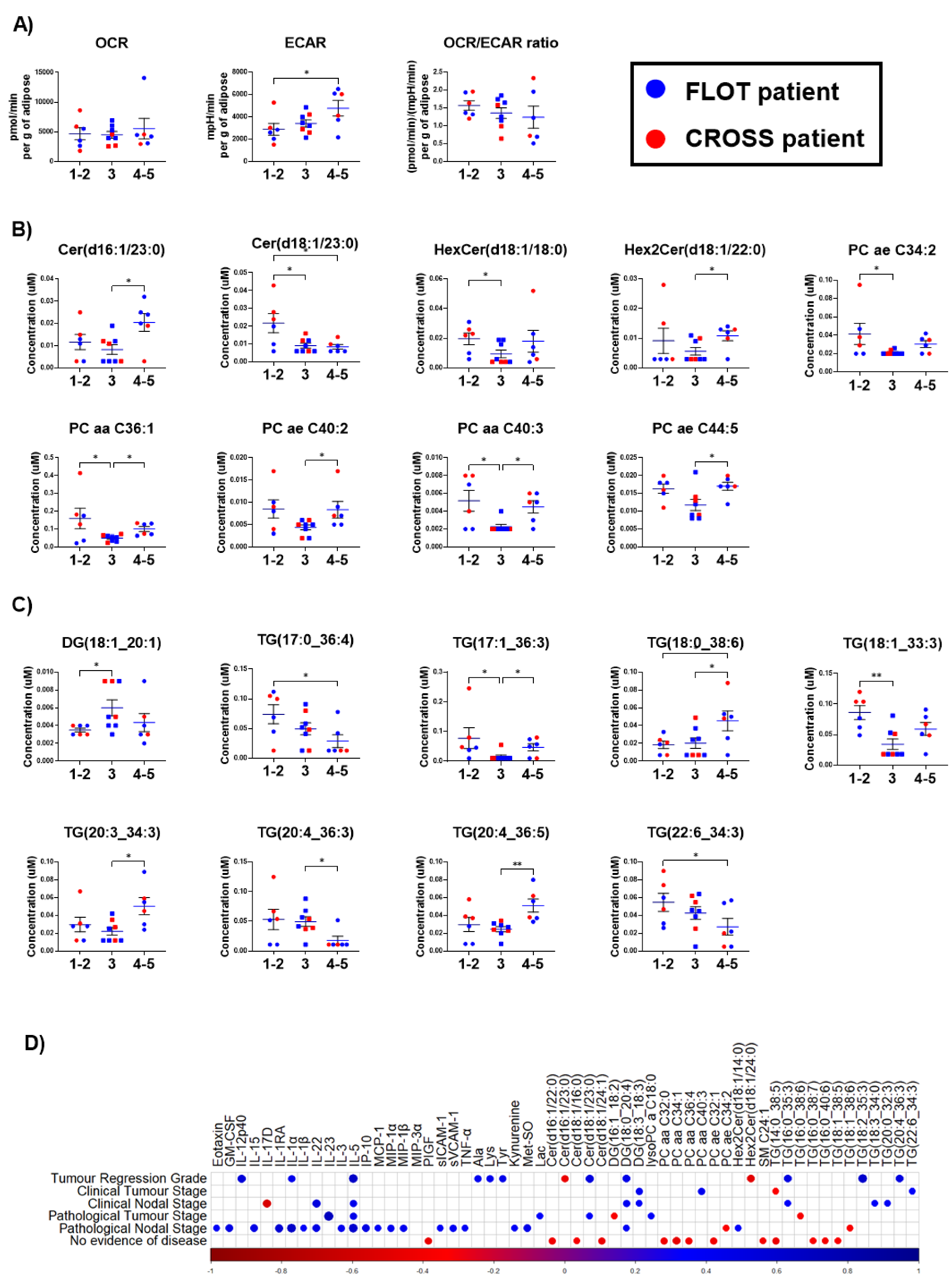
3.4. Increased ECAR and Altered Metabolites Are Observed in Adipose Explants from OAC Patients with Increasing Tumour Regression Grades
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y. Epidemiology of Esophageal Cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef]
- De Vegt, F.; Gommers, J.J.J.; Groenewoud, H.; Siersema, P.D.; Verbeek, A.L.M.; Peters, Y.; Broeders, M.J.M. Trends and Projections in the Incidence of Oesophageal Cancer in the Netherlands: An Age-Period-Cohort Analysis from 1989 to 2041. Int. J. Cancer 2022, 150, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Roy, R.; et al. Neo-AEGIS (Neoadjuvant Trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary Results of Phase III RCT of CROSS versus Perioperative Chemotherapy (Modified MAGIC or FLOT Protocol). (NCT01726452). J. Clin. Oncol. 2021, 39, 4004. [Google Scholar] [CrossRef]
- Noble, F.; Lloyd, M.A.; Turkington, R.; Griffiths, E.; O’Donovan, M.; O’Neill, J.R.; Mercer, S.; Parsons, S.L.; Fitzgerald, R.C.; Underwood, T.J.; et al. Multicentre Cohort Study to Define and Validate Pathological Assessment of Response to Neoadjuvant Therapy in Oesophagogastric Adenocarcinoma. Br. J. Surg. 2017, 104, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohoe, C.L.; O’Farrell, N.J.; Grant, T.; King, S.; Clarke, L.; Muldoon, C.; Reynolds, J.V. Classification of Pathologic Response to Neoadjuvant Therapy in Esophageal and Junctional Cancer: Assessment of Existing Measures and Proposal of a Novel 3-Point Standard. Ann. Surg. 2013, 258, 784–792. [Google Scholar] [CrossRef]
- Ryan, A.M.; Rowley, S.P.; Fitzgerald, A.P.; Ravi, N.; Reynolds, J.V. Adenocarcinoma of the Oesophagus and Gastric Cardia: Male Preponderance in Association with Obesity. Eur. J. Cancer 2006, 42, 1151–1158. [Google Scholar] [CrossRef]
- Renehan, A.G.; Roberts, D.L.; Dive, C. Obesity and Cancer: Pathophysiological and Biological Mechanisms. Arch. Physiol. Biochem. 2008, 114, 71–83. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; Lian, J.; Han, D.; An, P.; Zhou, T.; Liu, S.; Wang, F.; Min, J. Quantitative Association between Body Mass Index and the Risk of Cancer: A Global Meta-Analysis of Prospective Cohort Studies. Int. J. Cancer 2018, 143, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and Cancer—Mechanisms Underlying Tumour Progression and Recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Dannenberg, A.J. The Obese Adipose Tissue Microenvironment in Cancer Development and Progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Davern, M.; Bracken-Clarke, D.; Donlon, N.E.; Sheppard, A.D.; Butler, C.; Donohoe, C.; Ravi, N. Visceral Adipose Tissue Secretome from Early and Late-Stage Oesophageal Cancer Patients Differentially Affects Effector and Regulatory T Cells. J. Cancer Res. Clin. Oncol. 2022, in press. [CrossRef] [PubMed]
- Mylod, E.; O’connell, F.; Donlon, N.E.; Butler, C.; Reynolds, J.V.; Lysaght, J.; Conroy, M.J. The Omentum in Obesity-Associated Cancer: A Hindrance to Effective Natural Killer Cell Migration towards Tumour Which Can Be Overcome by CX3CR1 Antagonism. Cancers 2021, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.; Mylod, E.; Fitzgerald, V.; Donlon, N.E.; Murphy, D.M.; Foley, E.K.; Bhardwaj, A.; Reynolds, J.V.; Doherty, D.G.; Lysaght, J.; et al. Tissue Distribution of Γδ T Cell Subsets in Oesophageal Adenocarcinoma. Clin. Immunol. 2021, 229, 108797. [Google Scholar] [CrossRef]
- Mongan, A.M.; Lynam-Lennon, N.; Doyle, S.L.; Casey, R.; Carr, E.; Cannon, A.; Conroy, M.J.; Pidgeon, G.P.; Brennan, L.; Lysaght, J.; et al. Visceral Adipose Tissue Modulates Radiosensitivity in Oesophageal Adenocarcinoma. Int. J. Med. Sci. 2019, 16, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Heeran, A.B.; McCready, J.; Dunne, M.R.; Donlon, N.E.; Nugent, T.S.; Bhardwaj, A.; Mitchelson, K.A.J.; Buckley, A.M.; Ravi, N.; Roche, H.M.; et al. Opposing Immune-Metabolic Signature in Visceral Versus Subcutaneous Adipose Tissue in Patients with Adenocarcinoma of the Oesophagus and the Oesophagogastric Junction. Metabolites 2021, 11, 768. [Google Scholar] [CrossRef]
- Lashinger, L.M.; Rossi, E.L.; Hursting, S.D. Obesity and Resistance to Cancer Chemotherapy: Interacting Roles of Inflammation and Metabolic Dysregulation. Clin. Pharmacol. Ther. 2014, 96, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.H.; Gogineni, K.; Subhedar, P.D.; Lin, J.Y.; McCullough, L.E. Obesity and Cancer Treatment Efficacy: Existing Challenges and Opportunities. Cancer 2019, 125, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Keuper, M.; Meile, T.; Zdichavsky, M.; Fritsche, A.; Häring, H.U.; de Angelis, M.H.; Staiger, H.; Franko, A. Increased Mitochondrial Respiration of Adipocytes from Metabolically Unhealthy Obese Compared to Healthy Obese Individuals. Sci. Rep. 2020, 10, 12407. [Google Scholar] [CrossRef]
- Buckley, A.M.; Dunne, M.R.; Morrissey, M.E.; Kennedy, S.A.; Nolan, A.; Davern, M.; Foley, E.K.; Clarke, N.; Lysaght, J.; Ravi, N.; et al. Real-Time Metabolic Profiling of Oesophageal Tumours Reveals an Altered Metabolic Phenotype to Different Oxygen Tensions and to Treatment with Pyrazinib. Sci. Rep. 2020, 10, 12105. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Bennett, A.M.; Donohoe, C.L.; Mongan, A.M.; Howard, J.M.; Lithander, F.E.; Pidgeon, G.P.; Reynolds, J.V.; Lysaght, J. Establishing Computed Tomography–Defined Visceral Fat Area Thresholds for Use in Obesity-Related Cancer Research. Nutr. Res. 2013, 33, 171–179. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, (R software version 3.6.2); R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Harrell, F.E., Jr.; Dupont, C. Hmisc: Harrell Miscellaneous, Version 4.8-0; CRAN Repository: Vienna, Austria, 2020. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.92; CRAN Repository: Vienna, Austria, 2017. [Google Scholar]
- Zhang, J.; Wu, H.; Wang, R. Metabolic Syndrome and Esophageal Cancer Risk: A Systematic Review and Meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 8. [Google Scholar] [CrossRef]
- Ashton, T.M.; Gillies McKenna, W.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [Green Version]
- Lynam-Lennon, N.; Maher, S.G.; Maguire, A.; Phelan, J.; Muldoon, C.; Reynolds, J.V.; O’Sullivan, J. Altered Mitochondrial Function and Energy Metabolism is Associated with a Radioresistant Phenotype in Oesophageal Adenocarcinoma. PLoS ONE 2014, 9, e100738. [Google Scholar] [CrossRef]
- Choi, M.J.; Jung, S.B.; Lee, S.E.; Kang, S.G.; Lee, J.H.; Ryu, M.J.; Chung, H.K.; Chang, J.Y.; Kim, Y.K.; Hong, H.J.; et al. An Adipocyte-Specific Defect in Oxidative Phosphorylation Increases Systemic Energy Expenditure and Protects against Diet-Induced Obesity in Mouse Models. Diabetologia 2020, 63, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Vellinga, T.T.; De Boer, V.C.J.; Fatrai, S.; Van Schelven, S.; Trumpi, K.; Verheem, A.; Snoeren, N.; Emmink, B.L.; Koster, J.; Rinkes, I.H.M.B.; et al. SIRT1/PGC1a-Dependent Increase in Oxidative Phosphorylation Supports Chemotherapy Resistance of Colon Cancer. Clin. Cancer Res. 2015, 21, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, X.; Parmentier, J.-H.; Tucci, J.; Pei, H.; Cortez-Toledo, O.; Dieli-Conwright, C.M.; Oberley, M.J.; Neely, M.; Orgel, E.; Louie, S.G.; et al. Adipocytes Sequester and Metabolize the Chemotherapeutic Daunorubicin. Mol. Cancer Res. 2017, 15, 1704–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizi, B.S.; Caneba, C.; Nowicka, A.; Nabiyar, A.W.; Liu, X.; Chen, K.; Klopp, A.; Nagrath, D. Nitric Oxide Mediates Metabolic Coupling of Omentum-Derived Adipose Stroma to Ovarian and Endometrial Cancer Cells. Cancer Res. 2015, 75, 456–471. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, F.; O’Sullivan, J. Help or Hindrance: The Obesity Paradox in Cancer Treatment Response. Cancer Lett. 2021, 522, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, C.Y.; Deng, W.M. The Role of Pro-Inflammatory Cytokines in Lipid Metabolism of Metabolic Diseases. Int. Rev. Immunol. 2019, 38, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Zaibi, M.S.; Alomar, S.Y.; De Vuono, S.; Ricci, M.A.; Alaeddin, A.; Siepi, D.; Boni, M.; Vaudo, G.; Trayhurn, P.; et al. Circulating Levels of the Adipokines Monocyte Chemotactic Protein-4 (MCP-4), Macrophage Inflammatory Protein-1β (MIP-1β), and Eotaxin-3 in Severe Obesity and Following Bariatric Surgery. Horm. Metab. Res. 2016, 48, 847–853. [Google Scholar] [CrossRef]
- Donlon, N.E.; Sheppard, A.; Davern, M.; O’connell, F.; Phelan, J.J.; Power, R.; Nugent, T.; Dinneen, K.; Aird, J.; Greene, J.; et al. Linking Circulating Serum Proteins with Clinical Outcomes in Esophageal Adenocarcinoma—An Emerging Role for Chemokines. Cancers 2020, 12, 3356. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Lai, W.; Zeng, Y.; Liu, L.; Li, S.; Jin, S.; Zhang, Y.; Luo, X.; Xu, H.; Lin, X.; et al. CCL26 Participates in the PRL-3-Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Mol. Cancer Ther. 2018, 17, 276–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Mantovani, A.; Gray, P.W. Human Macrophage–Derived Chemokine (MDC), a Novel Chemoattractant for Monocytes, Monocyte-Derived Dendritic Cells, and Natural Killer Cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Imamichi, T.; Weiss, J.M.; Sato, M.; Li, L.; Matsukawa, A.; Wang, J.M. Induction of Monocyte Chemoattractant Proteins in Macrophages via the Production of Granulocyte/Macrophage Colony-Stimulating Factor by Breast Cancer Cells. Front. Immunol. 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef] [Green Version]
- Carolan, E.; Tobin, L.M.; Mangan, B.A.; Corrigan, M.; Gaoatswe, G.; Byrne, G.; Geoghegan, J.; Cody, D.; O’Connell, J.; Winter, D.C.; et al. Altered Distribution and Increased IL-17 Production by Mucosal-Associated Invariant T Cells in Adult and Childhood Obesity. J. Immunol. 2015, 194, 5775–5780. [Google Scholar] [CrossRef]
- Punt, S.; Langenhoff, J.M.; Putter, H.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. The Correlations between IL-17 vs. Th17 Cells and Cancer Patient Survival: A Systematic Review. Oncoimmunology 2015, 4, e984547. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Lotti, F.; Jarrar, A.M.; Pai, R.K.; Hitomi, M.; Lathia, J.; Mace, A.; Gantt, G.A.; Sukhdeo, K.; DeVecchio, J.; Vasanji, A.; et al. Chemotherapy Activates Cancer-Associated Fibroblasts to Maintain Colorectal Cancer-Initiating Cells by IL-17A. J. Exp. Med. 2013, 210, 2851–2872. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Park, H.J.; Lee, I.J.; Kim, W.W.; Ha, S.J.; Suh, Y.G.; Seong, J. Inhibition of IL-17A Suppresses Enhanced-Tumor Growth in Low Dose Pre-Irradiated Tumor Beds. PLoS ONE 2014, 9, e106423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Involvement of Serum Vascular Endothelial Growth Factor Family Members in the Development of Obesity in Mice and Humans. J. Nutr. Biochem. 2010, 21, 774–780. [Google Scholar] [CrossRef]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.I.; Kubo, H.; Achen, M.G. VEGF-D Promotes the Metastatic Spread of Tumor Cells via the Lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, P.; Reichman, H.; Zab-Bar, I.; Itan, M.; Pasmanik-Chor, M.; Bouffi, C.; Qimron, U.; Bachelet, I.; Fulkerson, P.C.; Rothenberg, M.E.; et al. CD300f:IL-5 Cross-Talk Inhibits Adipose Tissue Eosinophil Homing and Subsequent IL-4 Production. Sci. Rep. 2017, 7, 5922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S.; Fingleton, B.; et al. Interleukin-5 Facilitates Lung Metastasis by Modulating the Immune Microenvironment. Cancer Res. 2015, 75, 1624. [Google Scholar] [CrossRef] [Green Version]
- Deetman, P.E.; Bakker, S.J.L.; Dullaart, R.P.F. High Sensitive C-Reactive Protein and Serum Amyloid A Are Inversely Related to Serum Bilirubin: Effect-Modification by Metabolic Syndrome. Cardiovasc. Diabetol. 2013, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Malle, E.; De Beer, F.C. Human Serum Amyloid A (SAA) Protein: A Prominent Acute-Phase Reactant for Clinical Practice. Eur. J. Clin. Investig. 1996, 26, 427–435. [Google Scholar] [CrossRef]
- Lucas, S.; Taront, S.; Magnan, C.; Fauconnier, L.; Delacre, M.; Macia, L.; Delanoye, A.; Verwaerde, C.; Spriet, C.; Saule, P.; et al. Interleukin-7 Regulates Adipose Tissue Mass and Insulin Sensitivity in High-Fat Diet-Fed Mice through Lymphocyte-Dependent and Independent Mechanisms. PLoS ONE 2012, 7, 40351. [Google Scholar] [CrossRef]
- Morre, M.; Beq, S. Interleukin-7 and Immune Reconstitution in Cancer Patients: A New Paradigm for Dramatically Increasing Overall Survival. Target. Oncol. 2012, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, B.; Wei, T.; Huang, Y.; Gong, Y.; Wu, G.; Liu, J.; Chen, X.; Shi, L. Interleukin-7 Resensitizes Non-Small-Cell Lung Cancer to Cisplatin via Inhibition of ABCG2. Mediat. Inflamm. 2019, 2019, 7241418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaman, S.; Hollmén, M.; Yoon, S.Y.; Alkan, H.F.; Alitalo, K.; Wolfrum, C.; Detmar, M. Transgenic Overexpression of VEGF-C Induces Weight Gain and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 31566. [Google Scholar] [CrossRef] [Green Version]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The Role of the VEGF-C/VEGFR-3 Axis in Cancer Progression. Br. J. Cancer 2006, 96, 541–545. [Google Scholar] [CrossRef]
- Wang, C.A.; Harrell, J.C.; Iwanaga, R.; Jedlicka, P.; Ford, H.L. Vascular Endothelial Growth Factor C Promotes Breast Cancer Progression via a Novel Antioxidant Mechanism That Involves Regulation of Superoxide Dismutase 3. Breast Cancer Res. 2014, 16, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobold, S.; Völk, S.; Clauditz, T.; Küpper, N.J.; Minner, S.; Tufman, A.; Düwell, P.; Lindner, M.; Koch, I.; Heidegger, S.; et al. Interleukin-22 Is Frequently Expressed in Small- and Large-Cell Lung Cancer and Promotes Growth in Chemotherapy-Resistant Cancer Cells. J. Thorac. Oncol. 2013, 8, 1032–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.Y.; Noh, H.M.; Choi, B.; Park, J.E.; Kim, J.E.; Jang, Y.; Lee, H.K.; Chang, E.J. Interleukin-22 Induces the Infiltration of Visceral Fat Tissue by a Discrete Subset of Duffy Antigen Receptor for Chemokine-Positive M2-Like Macrophages in Response to a High Fat Diet. Cells 2019, 8, 1578. [Google Scholar] [CrossRef] [Green Version]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β Induces Proliferation in Colorectal Cancer Cells and Resveratrol can down-modulate it. Exp. Biol. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef] [Green Version]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New Insights into IL-12-Mediated Tumor Suppression. Cell Death Differ. 2015, 22, 237. [Google Scholar] [CrossRef] [Green Version]
- Clish, C.B. Metabolomics: An Emerging but Powerful Tool for Precision Medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltais-Payette, I.; Boulet, M.M.; Prehn, C.; Adamski, J.; Tchernof, A. Circulating Glutamate Concentration as a Biomarker of Visceral Obesity and Associated Metabolic Alterations. Nutr. Metab. 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, G.; Campi, B.; Saba, A.; Codini, S.; Ciccarone, A.; Giusti, L.; Del Prato, S.; Esterline, R.L.; Ferrannini, E. Plasma N-Acetylaspartate Is Related to Age, Obesity, and Glucose Metabolism: Effects of Antidiabetic Treatment and Bariatric Surgery. Front. Endocrinol. 2020, 11, 216. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamamoto, K.; Sato, Y.; Inoue, S.; Morinaga, T.; Hirano, E. Combination of Aspartic Acid and Glutamic Acid Inhibits Tumor Cell Proliferation. Biomed. Res. 2016, 37, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding Adipocyte Differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [Green Version]
- Hama, K.; Fujiwara, Y.; Hayama, T.; Ozawa, T.; Nozawa, K.; Matsuda, K.; Hashiguchi, Y.; Yokoyama, K. Very Long-Chain Fatty Acids Are Accumulated in Triacylglycerol and Nonesterified Forms in Colorectal Cancer Tissues. Sci. Rep. 2021, 11, 6163. [Google Scholar] [CrossRef]
- Tomin, T.; Fritz, K.; Gindlhuber, J.; Waldherr, L.; Pucher, B.; Thallinger, G.G.; Nomura, D.K.; Schittmayer, M.; Birner-Gruenberger, R. Deletion of Adipose Triglyceride Lipase Links Triacylglycerol Accumulation to a More-Aggressive Phenotype in A549 Lung Carcinoma Cells. J. Proteome Res. 2018, 17, 1415–1425. [Google Scholar] [CrossRef]
- Palomo, I.; Contreras, A.; Alarcón, L.M.; Leiva, E.; Guzmán, L.; Mujica, V.; Icaza, G.; Díaz, N.; González, D.R.; Moore-Carrasco, R. Elevated Concentration of Asymmetric Dimethylarginine (ADMA) in Individuals with Metabolic Syndrome. Nitric Oxide 2011, 24, 224–228. [Google Scholar] [CrossRef]
- Hajer, G.R.; Van Der Graaf, Y.; Olijhoek, J.K.; Verhaar, M.C.; Visseren, F.L.J. Levels of Homocysteine Are Increased in Metabolic Syndrome Patients but Are Not Associated with an Increased Cardiovascular Risk, in Contrast to Patients without the Metabolic Syndrome. Heart 2007, 93, 216. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. New Insights into Purine Metabolism in Metabolic Diseases: Role of Xanthine Oxidoreductase Activity. Am. J. Physiol.—Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Wu, J.T. Hyperhomocysteinemia Is a Risk Factor for Cancer and a New Potential Tumor Marker. Clin. Chim. Acta 2002, 322, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, Y.; Kim, T.H.; Kim, D.H.; Lee, A. Plasma Metabolites as Possible Biomarkers for Diagnosis of Breast Cancer. PLoS ONE 2019, 14, e0225129. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Zhao, A.; Qiu, Y.; Xie, G.; Jiang, Q.; Zheng, X.; Zhong, W.; Sun, X.; Zhou, Z.; et al. Asymmetric Dimethylarginine Attenuates Serum Starvation-Induced Apoptosis via Suppression of the Fas (APO-1/CD95)/JNK (SAPK) Pathway. Cell Death Dis. 2013, 4, e830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Baliou, S.; Kyriakopoulos, A.M.; Spandidos, D.A.; Zoumpourlis, V. Role of Taurine, its Haloamines and its LncRNA TUG1 in Both Inflammation and Cancer Progression. On the Road to Therapeutics? (Review). Int. J. Oncol. 2020, 57, 631–664. [Google Scholar] [CrossRef]
- Wu, T.K.; Wei, C.W.; Pan, Y.R.; Hsu, R.J.; Wu, C.Y.; Yu, Y.L. The Uremic Toxin P-Cresyl Sulfate Induces Proliferation and Migration of Clear Cell Renal Cell Carcinoma via MicroRNA-21/ HIF-1α Axis Signals. Sci. Rep. 2019, 9, 3207. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.S.; Syu, J.P.; Wang, S.D.; Pan, P.C.; Kung, H.N. BSA-Bounded p-Cresyl Sulfate Potentiates the Malignancy of Bladder Carcinoma by Triggering Cell Migration and EMT through the ROS/Src/FAK Signaling Pathway. Cell Biol. Toxicol. 2020, 36, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Tian, W.; Yao, Y.; Fan, G.; Zhou, Y.; Wu, M.; Xu, D.; Deng, Y. Changes in Lipid Profiles during and after (Neo)Adjuvant Chemotherapy in Women with Early-Stage Breast Cancer: A Retrospective Study. PLoS ONE 2019, 14, e0221866. [Google Scholar] [CrossRef]
- Morad, S.A.F.; Cabot, M.C. Ceramide-Orchestrated Signalling in Cancer Cells. Nat. Rev. Cancer 2012, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Nagahashi, M.; Gabriel, E.; Takabe, K.; Wakai, T. Clinical Application of Ceramide in Cancer Treatment. Breast Cancer 2019, 26, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wu, P.W.; Liu, D.H.; Yan, S.J.; Shen, X.M.; Yang, L.Y. Prognostic Significance of High Triglyceride and Apolipoprotein B Levels in Patients with Stage III and High-Risk Stage II Colorectal Cancer Undergoing Curative Surgery. Oncol. Lett. 2020, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.D.F.; Andrade, L.N. de S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-Derived Lipid Mediators: The Crosstalk Between Cancer Cells and Immune Cells. Front. Immunol. 2022, 13, 215. [Google Scholar] [CrossRef]
- Rettig, T.C.D.; Verwijmeren, L.; Dijkstra, I.M.; Boerma, D.; Van De Garde, E.M.W.; Noordzij, P.G. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann. Surg. 2016, 263, 1207–1212. [Google Scholar] [CrossRef]
- Belizon, A.; Balik, E.; Feingold, D.L.; Bessler, M.; Arnell, T.D.; Forde, K.A.; Horst, P.K.; Jain, S.; Cekic, V.; Kirman, I.; et al. Major Abdominal Surgery Increases Plasma Levels of Vascular Endothelial Growth Factor: Open More So Than Minimally Invasive Methods. Ann. Surg. 2006, 244, 792. [Google Scholar] [CrossRef]
- Yang, C.; Bork, U.; Schölch, S.; Kulu, Y.; Kaderali, L.; Bolstorff, U.L.; Kahlert, C.; Weitz, J.; Rahbari, N.N.; Reissfelder, C. Postoperative Course and Prognostic Value of Circulating Angiogenic Cytokines after Pancreatic Cancer Resection. Oncotarget 2017, 8, 72315. [Google Scholar] [CrossRef] [Green Version]
- El-Ghonaimy, E.A.; El-Shinawi, M.; Ibrahim, S.A.; El-Ghazaly, H.; Abd-El-Tawab, R.; Nouh, M.A.; El-Mamlouk, T.; Mohamed, M.M. Positive Lymph-Node Breast Cancer Patients—Activation of NF-ΚB in Tumor-Associated Leukocytes Stimulates Cytokine Secretion That Promotes Metastasis via C-C Chemokine Receptor CCR7. FEBS J. 2015, 282, 271–282. [Google Scholar] [CrossRef]
- Davern, M.; Donlon, N.E.; O’Connell, F.; Gaughan, C.; O’Donovan, C.; Habash, M.; Sheppard, A.D.; MacLean, M.; Dunne, M.R.; Moore, J.; et al. Acidosis Significantly Alters Immune Checkpoint Expression Profiles of T Cells from Oesophageal Adenocarcinoma Patients. Cancer Immunol. Immunother. 2023, 72, 55–71. [Google Scholar] [CrossRef]
- Wang, Y. Cancer-Generated Lactic Acid: A Regulatory, Immunosuppressive Metabolite? J. Pathol. 2013, 230, 350. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Inoue, K.; Klein, S.; Halvorsen, S.; Chen, J.; Matsui, A.; Nikmaneshi, M.R.; Kitahara, S.; Hato, T.; Chen, X.; et al. Placental Growth Factor Promotes Tumour Desmoplasia and Treatment Resistance in Intrahepatic Cholangiocarcinoma. Gut 2022, 71, 185–193. [Google Scholar] [CrossRef] [PubMed]
| Patient Clinical Parameters | |
|---|---|
| Diagnosis | OAC = 13 OGJ = 19 |
| Sex | Male = 21 Female = 11 |
| Obesity Status via Visceral Fat Area | Obese = 16 Non-obese = 16 |
| Age at diagnosis | 51–83 (Mean = 67.83) |
| Post-treatment BMI | 22.34–43 (Mean = 32.391) Non-obese Mean = 28.4, Obese Mean = 30.025 Male Mean = 40.282, Female Mean = 29.822 |
| Weight | 57.2–176 kg (Mean = 86.697) Non-obese Mean = 82.865, Obese Mean = 94.093 Male Mean = 94.72, Female Mean = 76.79 |
| Mean VFA | 22.9–485.2 (Mean = 139.404) Non-obese Mean = 115.192, Obese Mean = 167.1 Male Mean = 148.436, Female Mean = 122.872 |
| Metabolic Dysfunction | n = 15 |
| High cholesterol or intervention for high cholesterol | n = 21 |
| High blood pressure or intervention for high blood pressure | n = 20 |
| High Triglycerides or intervention for high Triglycerides | n = 4 |
| Diabetes | n = 10 |
| Barrett’s oesophagus | n = 17 |
| ASA grade (risk-stratifying system to help predict preoperative risks) | Grade 1 → n = 3 |
| Grade 2 → n = 15 | |
| Grade 3 → n = 10 | |
| Clavien–Dindo classification (grading for adverse events which occur as a result of surgical procedures) | Classification 0 → n = 5 Classification 1 → n = 4 Classification 2 → n = 7 Classification 3 → n = 7 Classification 4 → n = 5 |
| Treatment | Naïve → n = 10 FLOT → n = 14 CROSS → n = 8 |
| Tumour Regression Grading (TRG) | TRG 1 → n = 3 (CROSS n = 2, FLOT n = 1) TRG 2 → n = 3 (CROSS n = 1, FLOT n = 2) TRG 3 → n = 8 (CROSS n = 3, FLOT n = 5) TRG 4 → n = 3 (CROSS n = 2, FLOT n = 1) TRG 5 → n = 3 (CROSS n = 0, FLOT n = 3) |
| Clinical Stage (T) | T1 → n = 10 T2 → n = 3 T3 → n = 19 |
| Clinical Stage (N) | N0 → n = 17 N1 → n = 8 N2 → n = 7 |
| Path stage (T) | T0 → n = 3 T1 → n = 9 T2 → n = 4 T3 → n = 13 T4 → n = 3 |
| Path Stage (N) | N0 → n = 17 N1 → n = 6 N2 → n = 6 N3 → n = 3 |
| Perineural Invasion | n = 9 |
| Lymph Involvement | n = 17 |
| Vascular Involvement | n = 11 |
| Evidence of Disease | n = 10 |
| Viscerally Obese | ||
|---|---|---|
| Factors | R | P |
| OCR | 0.575376009 | 0.000570727 |
| ECAR | 0.399378641 | 0.023543127 |
| IL-17D | 0.410806922 | 0.041356767 |
| MDC | 0.381263049 | 0.037637047 |
| Asp | −0.402873982 | 0.022243626 |
| Gln | 0.37914075 | 0.032351539 |
| Glu | −0.392645496 | 0.026222596 |
| Cer(d18:1/23:0) | 0.390706524 | 0.027038963 |
| PC aa C42:6 | −0.481757449 | 0.00524064 |
| H1 | −0.358763864 | 0.043756148 |
| TG(14:0_36:2) | 0.370140718 | 0.037047715 |
| TG(16:0_35:3) | 0.350214483 | 0.049411241 |
| TG(16:0_38:7) | 0.466737152 | 0.007083324 |
| TG(18:2_35:2) | 0.388990152 | 0.027778863 |
| TG(18:2_38:4) | 0.357422007 | 0.044607706 |
| TG(22:5_34:3) | 0.351685392 | 0.048398926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, F.; Mylod, E.; Donlon, N.E.; Heeran, A.B.; Butler, C.; Bhardwaj, A.; Ramjit, S.; Durand, M.; Lambe, G.; Tansey, P.; et al. Energy Metabolism, Metabolite, and Inflammatory Profiles in Human Ex Vivo Adipose Tissue Are Influenced by Obesity Status, Metabolic Dysfunction, and Treatment Regimes in Patients with Oesophageal Adenocarcinoma. Cancers 2023, 15, 1681. https://doi.org/10.3390/cancers15061681
O’Connell F, Mylod E, Donlon NE, Heeran AB, Butler C, Bhardwaj A, Ramjit S, Durand M, Lambe G, Tansey P, et al. Energy Metabolism, Metabolite, and Inflammatory Profiles in Human Ex Vivo Adipose Tissue Are Influenced by Obesity Status, Metabolic Dysfunction, and Treatment Regimes in Patients with Oesophageal Adenocarcinoma. Cancers. 2023; 15(6):1681. https://doi.org/10.3390/cancers15061681
Chicago/Turabian StyleO’Connell, Fiona, Eimear Mylod, Noel E. Donlon, Aisling B. Heeran, Christine Butler, Anshul Bhardwaj, Sinead Ramjit, Michael Durand, Gerard Lambe, Paul Tansey, and et al. 2023. "Energy Metabolism, Metabolite, and Inflammatory Profiles in Human Ex Vivo Adipose Tissue Are Influenced by Obesity Status, Metabolic Dysfunction, and Treatment Regimes in Patients with Oesophageal Adenocarcinoma" Cancers 15, no. 6: 1681. https://doi.org/10.3390/cancers15061681






