Sun-Protective Clothing Worn Regularly during Early Childhood Reduces the Number of New Melanocytic Nevi: The North Queensland Sun-Safe Clothing Cluster Randomized Controlled Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Rationale
2.2. Recruitment of Childcare Centers
2.3. Recruitment of Children
2.4. Questionnaires
2.5. Clinical Examination of Children
2.6. Intervention Design
2.7. Compliance
2.8. Control Childcare Centers and Children
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandeya, N.; Olsen, C.M.; Whiteman, D.C. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med. J. Aust. 2017, 207, 339–343. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer (IARC): Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 30 December 2022).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens; IARC Monographs Radiation; World Health Organization: Lyon, France, 2012; Volume 100D. [Google Scholar]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer. 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. In Sunlight, Vitamin D and Skin Cancer, 3rd ed.; Reichrath, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1268, pp. 123–139. [Google Scholar]
- Arnold, M.; de Vries, E.; Whiteman, D.C.; Jemal, A.; Bray, F.; Parkin, D.M.; Soerjomataram, I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int. J. Cancer 2018, 143, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.; Maitland, C.; Tabbakh, T.; Preston, P.; Wakefield, M.; Sinclair, C. Forty years of Slip! Slop! Slap! A call to action on skin cancer prevention for Australia. Public Health Res. Pract. 2022, 32, e3145211. [Google Scholar] [CrossRef]
- Queensland Health. The Health of Queenslanders 2020; Report of the Chief Health Officer Queensland; Queensland Government: Brisbane, Australia, 2020; pp. 94–96.
- Australian Radiation Protection and Nuclear Safety Agency (ARPANSA). Australian Government. Ultraviolet Radiation Dose. Available online: https://www.arpansa.gov.au/our-services/monitoring/ultraviolet-radiation-monitoring/ultraviolet-radiation-dose (accessed on 30 December 2022).
- Moise, A.F.; Harrison, S.L.; Gies, P. Solar ultraviolet radiation exposure of infants and small children. Photodermatol. Photoimmunol. Photomed. 1999, 15, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.F.; Büttner, P.G.; Harrison, S.L. Sun exposure at school. Photochem. Photobiol. 1999, 70, 269–274. [Google Scholar] [CrossRef]
- Downs, N.J.; Harrison, S.L.; Garzon-Chavez, D.R.; Parisi, A.V. Solar ultraviolet and the occupational radiant exposure of Queensland school teachers: A comparative study between teaching classifications and behavior patterns. J. Photochem. Photobiol. B Biology. 2016, 158, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Haenssle, H.A.; Mograby, N.; Ngassa, A.; Buhl, T.; Emmert, S.; Schon, M.P.; Rosenberger, A.; Bertsch, H.P. Association of Patient Risk Factors and Frequency of Nevus-Associated Cutaneous Melanomas. JAMA Dermatol. 2016, 152, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. J Am. Acad. Dermatol. 2017, 77, 938–945. [Google Scholar] [CrossRef]
- Dessiniotti, C.; Geller, A.C.; Stratigos, A.J. A review of nevus-associated melanoma: What is the evidence? J. European Acad. Dermatol. Venereol. 2022, 36, 1927–1936. [Google Scholar] [CrossRef]
- MacKie, R.M.; Watt, D.; Doherty, V.; Aitchison, T. Malignant melanoma occurring in those under 30 in the west of Scotland 1979-86: A study of incidence, clinical features, pathological features & survival. Br. J. Dermatol. 1991, 124, 560–564. [Google Scholar]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J Cancer. 2005, 41, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Scope, A.; Marchetti, M.A.; Marghoob, A.A.; Dusza, S.W.; Geller, A.C.; Satagopan, J.M.; Weinstock, M.A.; Berwick, M.; Halpern, A.C. The study of nevi in children: Principles learned and implications for melanoma diagnosis. J. Am. Acad. Dermatol. 2016, 75, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Berwick, M.; Buller, D.B.; Cust, A.; Gallagher, R.; Lee, T.K.; Meyskens, F.; Pandey, S.; Thomas, N.E.; Veierod, M.B.; Ward, S. Melanoma epidemiology and prevention. Cancer Treat. Res. 2016, 167, 17–49. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.M.; Newton-Bishop, J.A.; Bishop, D.T.; Armstrong, B.K.; Bataille, V.; Bergman, W.; Berwick, M.; Bracci, P.M.; Elwood, J.M.; Ernstoff, M.S.; et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int. J. Cancer. 2009, 124, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Garbe, C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 2003, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.L.; Zhu, G.; Li, X.; Sanna, M.; Iles, M.M.; Jacobs, L.C.; Evans, D.M.; Yazar, S.; Beesley, J.; Law, M.H.; et al. Novel pleiotropic risk loci for melanoma and nevus density implicate multiple biological pathways. Nat. Commun. 2018, 9, 4774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wachsmuth, R.C.; Turner, F.; Barrett, J.H.; Gaut, R.; Randerson-Moor, J.A.; Bishop, D.T.; Newton Bishop, J.A. The Effect of Sun Exposure in Determining Nevus Density in UK Adolescent Twins. J. Investig. Dermatol. 2005, 124, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Green, A.C.; Wallingford, S.C.; McBride, P. Childhood exposure to ultraviolet radiation and harmful skin effects: Epidemiological evidence. Prog. Biophys. Mol. Biol. 2011, 107, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Gefeller, O.; Diehl, K. Children and Ultraviolet Radiation. Children 2022, 9, 537. [Google Scholar] [CrossRef]
- Gefeller, O.; Tarantino, J.; Lederer, P.; Uter, W.; Pfahlberg, A.B. The relation between patterns of vacation sun exposure and the development of acquired melanocytic nevi in German children 6–7 years of age. Am. J. Epidemiol. 2007, 165, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.W.; Rivers, J.K.; MacLennan, R.; Harrison, S.L.; Lewis, A.E.; Tate, B.J. Sunlight: A major factor associated with the development of melanocytic naevi in Australian schoolchildren. J. Am. Acad. Dermatol. 1994, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; MacLennan, R.; Speare, R.; Wronski, I. Sun Exposure and melanocytic naevi in young Australian children. Lancet 1994, 344, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; MacLennan, R.; Buettner, P.G. Sun exposure and the incidence of melanocytic nevi in young Australian children. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2318–2324. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.L.; MacKie, R.M.; MacLennan, R. Development of melanocytic nevi in the first 3 years of life. J. Natl. Cancer Inst. 2000, 92, 1436–1438. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Hawk, J.L.; Honig, P.; Giam, Y.C.; Hoath, S.; Mack, M.C.; Stamatas, G.N. New insights about infant and toddler skin: Implications for sun protection. Pediatrics 2011, 128, 92–102. [Google Scholar] [CrossRef]
- Armstrong, B.K.; English, D.R. Epidemiologic studies. In Cutaneous Melanoma: Clinical management and Treatment Results Worldwide, 2nd ed.; Balch, C.M., Houghton, A.N., Milton, G.W., Sober, A.J., Soong, S.-J., Eds.; J.B. Lippincott: Philadelphia, PA, USA, 1992; pp. 12–26. [Google Scholar]
- Rivers, J.K.; MacLennan, R.; Kelly, J.W.; Lewis, A.E.; Tate, B.J.; Harrison, S.; McCarthy, W.H. The eastern Australian childhood nevus study: Prevalence of atypical nevi, congenital-nevus-like-nevi, and other pigmented lesions. J. Am. Acad. Dermatol. 1995, 32, 957–963. [Google Scholar] [CrossRef]
- Satagopan, J.M.; Oliveria, S.A.; Arora, A.; Marchetti, M.A.; Orlow, I.; Dusza, S.W.; Weinstock, M.A.; Scope, A.; Geller, A.C.; Marghoob, A.A.; et al. Sunburn, sun exposure, and sun sensitivity in the Study of Nevi in Children. Ann. Epidemiol. 2015, 25, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Barsoum, R.; Harrison, S.L. Clinical Characteristics in Early Childhood Associated with a Nevus-Prone Phenotype in Adults from Tropical Australia: Two Decades of Follow-Up of the Townsville Preschool Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 8680. [Google Scholar] [CrossRef]
- Dennis, L.K.; Vanbeek, M.J.; Beane Freeman, L.E.; Smith, B.J.; Dawson, D.V.; Coughlin, J.A. Sunburns and risk of cutaneous melanoma: Does age matter? A comprehensive meta-analysis. Ann. Epidemiol. 2008, 18, 614–627. [Google Scholar] [CrossRef] [Green Version]
- Stark, M.S.; Tan, J.-M.; Tom, L.; Jagirdar, K.; Lambie, D.; Schaider, H.; Soyer, H.P.; Sturm, R.A. Whole-Exome Sequencing of Acquired Nevi Identifies Mechanisms for Development and Maintenance of Benign Neoplasms. J. Investig. Dermatol. 2018, 138, 1636–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, N.G.; Scolyer, R.A.; Colebatch, A.J. Biology and genetics of acquired and congenital melanocytic naevi. Pathology 2023, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Cust, A.E. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma A perspective on Fears et al. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. American Journal of Epidemiology 1977; 105: 420–427. Cancer Epidemiol. 2017, 48, 147–156. [Google Scholar] [PubMed]
- Jayasinghe, D.; Nufer, K.L.; Betz-Stablein, B.; Soyer, H.P.; Janda, M. Body Site Distribution of Acquired Melanocytic Naevi and Associated Characteristics in the General Population of Caucasian Adults: A Scoping Review. Dermatol. Ther. 2022, 12, 2453–2488. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Watt, P.; Purdie, D.M.; Hughes, M.-C.; Kaywards, N.K.; Green, A.C. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J. Natl. Cancer Inst. 2003, 95, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Burnett, M.E.; Marghoob, A.A.; Scope, A. Nevogenesis: Changing Theories. In Nevogenesis; Marghoob, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–23. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Plasmeijer, E.; Nguyen, T.M.U.; Olsen, C.M.; Janda, M.; Soyer, H.P.; Green, A. The natural history of common melanocytic naevi: A systematic review of longitudinal studies in the general population. J. Investig. Dermatol. 2017, 137, 2017–2018. [Google Scholar] [CrossRef] [Green Version]
- Bataille, V.; Grulich, A.; Sasieni, P.; Swerdlow, A.; Newton-Bishop, J.; McCarthy, W.H.; Hersey, P.; Cuzick, J. The association between naevi and melanoma in populations with different levels of sun exposure: A joint case–control study of melanoma in the UK and Australia. Br. J. Cancer 1998, 77, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.L.; Buettner, P.G.; MacLennan, R. The North Queensland sun-safe clothing study: Design and baseline results of a randomized trial to determine the effectiveness of sun-protective clothing in preventing melanocytic nevi. Am. J. Epidemiol. 2005, 161, 536–545. [Google Scholar] [CrossRef]
- Milne, E.; Johnston, R.; Cross, D.; Giles-Corti, B.; English, D.R. Effect of a school-based sun-protection intervention on the development of melanocytic nevi in children. Am. J. Epidemiol 2002, 155, 739–745. [Google Scholar] [CrossRef] [Green Version]
- English, D.R.; Milne, E.; Jacoby, P.; Giles-Corti, B.; Cross, D.; Johnston, R. The effect of a school-based sun protection intervention on the development of melanocytic nevi in children: 6-year follow-up. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.; Saunders, V.; Nowak, M. Baseline Survey of Sun-Protection Knowledge, Practices and Policy in Early Childhood Settings in Queensland, Australia. Health Educ. Res. 2007, 22, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enta, T.; Kwan, T.Y. Melanocytic nevi in sun-protected Canadian Hutterite children. Arch. Dermatol. 1998, 134, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Doré, J.F.; Cattaruzza, M.S.; Renard, F.; Luther, H.; Gentiloni-Silverj, F.; Zantedeschi, E.; Mezzetti, M.; Monjaud, I.; Andry, M.; et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J. Natl. Cancer Inst. 1998, 90, 1873–1880. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Hu, J.Y.; Wang, S.Q. Sunscreens: A review of health benefits, regulations, and controversies. Dermatol. Clin. 2014, 32, 427–438. [Google Scholar] [CrossRef]
- Diaz, A.; Neale, R.E.; Kimlin, M.G.; Jones, L.; Janda, M. The children and sunscreen study: A crossover trial investigating children’s sunscreen application thickness and the influence of age and dispenser type. Arch. Dermatol. 2012, 148, 606–612. [Google Scholar] [CrossRef] [Green Version]
- AS/NZS 4399: 1996; Sun Protective Clothing—Evaluation and Classification. Standards Australia: Sydney, Australia, 1996; pp. 1–13.
- 1996 Census of Population and Housing—Socio-Economic Indexes For Areas (SEIFA); Catalog no. 2033.0; Australian Bureau of Statistics: Canberra, Australia, 1996.
- Australian New Zealand Clinical Trials Registry (ANZCTR). The North Queensland Sun-Safe Clothing Study: Effectiveness of Sun-Protective Clothing in Preventing or Delaying the Development of Pigmented Moles in Early Childhood. Available online: www.ANZCTR.org.au/ACTRN12617000621314.aspx (accessed on 30 December 2022).
- Campbell, M.K.; Piaggio, G.; Elbourne, D.R.; Altman, D.G. for the CONSORT GROUP. Consort 2010 Statement: Extension to cluster randomised trials. BMJ 2012, 345, e5661. [Google Scholar] [CrossRef] [Green Version]
- Boyd, E. The Growth of the Surface Area of the Human Body; Institute of Child Welfare Monograph Series 10; University of Minnesota Press: Minneapolis, MN, USA, 1935; pp. 53–60. [Google Scholar]
- Harrison, S.L.; Buettner, P.G.; MacLennan, R. Body-site distribution of melanocytic nevi in young Australian children. Arch. Dermatol. 1999, 135, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- English, D.R.; MacLennan, R.; Rivers, J.; Kelly, J.; Armstrong, B.K. Epidemiological Studies of Melanocytic Naevi: Protocol for Identifying and Recording Naevi; IARC Internal Report No. 90/002; International Agency for Research on Cancer: Lyon, France, 1990. [Google Scholar]
- Cancer Council Australia. SunSmart in Schools and Early Childhood. Available online: https://www.cancer.org.au/cancer-information/causes-and-prevention/sun-safety/be-sunsmart/sunsmart-in-schools (accessed on 30 December 2022).
- Gallagher, R.P.; Rivers, J.K.; Lee, T.K.; Bajdik, C.D.; McLean, D.I.; Coldman, A.J. Broad-spectrum sunscreen use and the development of new nevi in white children: A randomized controlled trial. JAMA 2000, 283, 2955–2960. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Büttner, P.; Wiecker, T.S.; Luther, H.; Garbe, C. Interventional study in 1232 young German children to prevent the development of melanocytic nevi failed to change sun exposure and sun protective behavior. Int. J. Cancer 2005, 116, 755–761. [Google Scholar] [CrossRef]
- Crane, L.A.; Deas, A.; Mokrohisky, S.T.; Ehrsam, G.; Jones, R.H.; Dellavalle, R.; Byers, T.E.; Morelli, J. A randomized intervention study of sun protection promotion in well-child care. Prev. Med. 2006, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Chatenoud, L.; Bertuccio, P.; Zinetti, C.; Di Landro, A.; Scotti, L.; La Vecchia, C. Improving Sun-Protection Behavior among Children: Results of a Cluster-Randomized Trial in Italian Elementary Schools. The ‘‘SoleSi SoleNo-GISED’’ Project. J. Investig. Dermatol. 2007, 127, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, L.A.; Asdigian, N.L.; Barón, A.E.; Aalborg, J.; Marcus, A.C.; Mokrohisky, S.T.; Byers, T.E.; Dellavalle, R.P.; Morelli, J.G. Mailed intervention to promote sun protection of children: A randomized controlled trial. Am. J. Prev. Med. 2012, 43, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Roetzheim, R.G.; Love-Jackson, K.M.; Hunter, S.G.; Lee, J.-H.; Chen, R.; Abdulla, R.; Wells, K.J. A cluster randomized trial of sun protection at elementary schools. Results from year 2. Am. J. Prev. Med. 2011, 41, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahé, E.; Beauchet, A.; de Paula Corrêa, M.; Godin-Beekmann, S.; Haeffelin, M.; Bruant, S.; Fay-Chatelard, F.; Jégou, F.; Saiag, P.; Aegerter, P. Outdoor sports and risk of ultraviolet radiation-related skin lesions in children: Evaluation of risks and prevention. Br. J. Dermatol. 2011, 165, 360–367. [Google Scholar] [CrossRef] [PubMed]
- English, D.R.; Milne, E.; Simpson, J.A. Sun Protection and the Development of Melanocytic Nevi in Children. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2873–2876. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Harrison, S.L.; Nowak, M.; Buettner, P.; MacLennan, R. Changes in the pattern of sun-exposure and sun-protection in young children from tropical Australia. J. Am. Acad. Dermatol. 2013, 68, 774–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, D.C.; Brown, R.M.; Purdie, D.M.; Hughes, M.-C. Melanocytic nevi in very young children: The role of phenotype, sun exposure, and sun protection. J. Am. Acad. Dermatol. 2005, 52, 40–47. [Google Scholar] [CrossRef]
- Bauer, J.; Buttner, P.; Wiecker, T.S.; Luther, H.; Garbe, C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1812 German children attending day care. Am. J. Epidemiol. 2005, 161, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Gies, H.P.; Roy, C.R.; Elliott, G.; Zongli, W. Ultraviolet radiation protection factors for clothing. Health Phys. 1994, 67, 131–139. [Google Scholar] [CrossRef]
- BS 7949:1999; Including Amendment 1. Children’s clothing—Requirements for protection against erythemally-weighted solar ultraviolet radiation; Replaced by BS EN 13758-2:2003. The British Standards Institution: London, UK, 1999; pp. 1–3.
- EN 13758-2:2003+A1:2007; Textiles—Solar UV Protective Properties—Part 2: Classification and Marking of Apparel. European Committee for Standardization: Brussels, Belgium, 2007.
- American Association of Textile Chemists and Colorists (AATCC). TM183(2020)e. Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation Through Fabrics. Available online: https://members.aatcc.org/store/tm183/579/ (accessed on 31 December 2022).
- ASTM D6603-12; Standard Specification for Labeling of UV-Protective Textiles. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Standards Australia. AS 4399:2020 Sun Protective Clothing—Evaluation and Classification, 3rd ed; Standards Australia: Homebush, NSW, Australia, 2020; pp. 1–21. ISBN 978 1 76072 815 1. [Google Scholar]
- Standards Australia. AS/NZS 4399:2017 Sun Protective Clothing—Evaluation and Classification, 2nd ed.; Standards Australia: Homebush, NSW, Australia, 2017; pp. 1–30. ISBN 978 1 76035 884 6. [Google Scholar]
- Downs, N.J.; Harrison, S.L. A comprehensive approach to evaluating and classifying sun-protective clothing. Br. J. Dermatol. 2018, 178, 958–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.L.; Downs, N. Development of a Reproducible Rating System for Sun Protective Clothing that Incorporates Body Surface Coverage. World J. Eng. Technol. 2015, 3, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.L.; Konovalov, D. A computer-vision approach for measuring garment-coverage, and implications for sun-protective clothing standards (Abstract 266, Session 3H, Non-communicable disease). In Proceedings of the Population Health Congress 2022 towards a Just, Safe and Sustainable Future for Australasia, Adelaide, Australia, 21–23 September 2022. [Google Scholar]
- Turner, D.; Harrison, S.L. Sun-protection provided by regulation school uniforms in Australian Schools: An opportunity to improve personal sun-protection during childhood. Photochem. Photobiol. 2014, 90, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L. Sun-Safe School Uniforms through Collaboration (Oral Presentation), Queensland Department of Education Research Showcase 2020 Evidence for Impact. Sun-Safe School Uniforms: A Collaborative Partnership. Brisbane, 18 February 2020. Available online: https://mediasite.eq.edu.au/mediasite/Play/4b9aa099aead44759fcf61ca200018931d (accessed on 30 December 2022).
- Skin Cancer Prevention Queensland. Towards a Future of Reduced Skin Cancer Burden for Queenslanders: Skin Cancer Prevention Targets (2022–2050); Queensland Health: Brisbane, Australia, 2022; pp. 15–16.
- Ettridge, K.A.; Bowden, J.A.; Rayner, J.M.; Wilson, C.J. The relationship between sun protection policy and associated practices in a national sample of early childhood services in Australia. Health Educ. Res. 2011, 26, 53–62. [Google Scholar] [CrossRef]
- Hunkin, H.; Morris, J.N. A decade of sun protection in Australian early-childhood services: Analysis of cross-sectional and repeated-measures data. Health Educ. Res. 2020, 35, 99–109. [Google Scholar] [CrossRef]
- Duignan, M.; Signal, L.; Thomson, G. “Good intentions, but inadequate practices”-sun protection in early childhood centres, a qualitative study from New Zealand. N. Z. Med. J. 2014, 127, 40–50. [Google Scholar]
- Fiessler, C.; Pfahlberg, A.B.; Uter, W.; Gefeller, O. Shedding light on the shade: How nurseries protect their children from ultraviolet radiation. Int. J. Environ. Res. Public Health 2018, 15, 1793. [Google Scholar] [CrossRef] [Green Version]
- Barrett, F.; Usher, K.; Woods, C.; Harrison, S.L.; Nikles, J.; Conway, J. Sun protective behaviours at an outdoor entertainment event in Australia. Nurs. HealthSci. 2017, 20, 132–138. [Google Scholar] [CrossRef]
- Nikles, J.; Harrison, S.L. An Observational Study of Sun-Protective Behaviour at an Outdoor Spectator Sporting Event in a Region of High Sun Exposure. Carcinog. Mutagen. 2014, S4, 003. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.L.; James Cook University, Townsville, Queensland, Australia. Personal communication, 2023.
- Choi, H.Y.; Harrison, S.L. Changes in Observed Sun Protective Behaviors in the United States Between 2000 and 2017. Photochem. Photobiol. 2023. submitted. [Google Scholar]
- Insight Economics; Melanoma Institute of Australia; Melanoma Patients Australia. State of the Nation: A Report into Melanoma—A National Health Priority; Insight Economics: Melbourne, Victoria, Australia, 2022; Available online: https://melanoma.org.au/wp-content/uploads/2022/03/MIA-and-MPA_SoN-Report_Final-Report_28-March-2022.pdf (accessed on 31 December 2022).
- Brough, D.; Baldwin, L. Re-think, Re-generate, Re-envisage fashionable sun-safe apparel. In Proceedings of the 2nd Global Advances & Controversies in Skin Cancer, Brisbane, Australia, 29–31 October 2015. [Google Scholar]

| Characteristics | Intervention Childcare Center (n = 13) | Control Childcare Center (n = 12) | p-Value |
|---|---|---|---|
| % childcare centers under private management | 61.5% | 58.3% | p > 0.999 |
| Median of max number of licensed places at the center (IQR) | 75 (74, 75) | 66 (46.5, 75) | p = 0.17 |
| % centers with a sun protection policy | 100% | 100% | p > 0.999 |
| % “SunSmart” centers | 7.7% | 8.3% | p > 0.999 |
| % centers in suburbs with moderate socioeconomic indicators * | 61.5% | 75.0% | p = 0.34 |
| Mean measured protection provided by shade structures ** (SD) | 54.2 (19.2) | 57.3 (14.3) | p = 0.67 |
| % centers offering moderate to good shade for outside areas | 61.5% | 50.0% | p = 0.60 |
| Characteristics | Intervention Baby Units (n = 44) # | Control Baby Units (n = 32) # | p-Value |
| % units that applied sunscreen routinely to all children | 68.2% | 68.8% | p = 0.97 |
| % units that provided sunscreen for children (of the 64 units who applied sunscreen) | 47.2% | 60.7% | p = 0.53 |
| % units that applied sunscreen to: | |||
| Face | 81.8% | 84.4% | p = 0.84 |
| Anterior Neck | 36.4% | 65.6% | p = 0.14 |
| Forearms | 81.8% | 87.5% | p = 0.66 |
| Lower Legs | 68.2% | 68.8% | p = 0.97 |
| All four sun-exposed body sites | 31.8% | 46.9% | p = 0.36 |
| Median hrs spent outside per day (IQR) | 3.1 (1.9, 4.4) | 2.7 (2.0, 5.0) | p = 0.57 p = 0.96§ |
| % units where children spent time outside with their back exposed to the sun (duration per day outside) | 4.5% (max 1 h/day) | 9.4% (max 40 min/day) | p = 0.54 |
| Phenotypic Characteristics of Children | Total (n = 544) | Intervention (n = 334) | Control (n = 210) | p-Value |
|---|---|---|---|---|
| Mean age [months] (±SD) | 15.4 (6.5) | 14.8 (6.3) | 16.3 (6.7) | p = 0.222 |
| % girls | 46.0% | 44.9% | 47.6% | p = 0.556 |
| % fair-skinned children | 97.2% | 97.0% | 97.6% | p = 0.659 |
| % children with blue or green eyes | 66.7% | 65.9% | 68.1% | p = 0.787 |
| % children with fair or blond hair | 66.7% | 63.5% | 71.9% | p = 0.167 |
| % skin ”always burns” after 30 min sun exposure ¶ | 33.7% | 33.9% | 33.3% | p = 0.702 |
| % skin ”never tans” after 30 min sun exposure ¶ | 14.9% | 14.1% | 16.3% | p = 0.694 |
| Mean % skin reflectance of the inner upper arm (±SD) | 72.1 (2.5) | 72.2 (2.5) | 72.1 (2.5) | p = 0.597 |
| % children with 4 Caucasian grandparents † | 83.0% | 83.9% | 81.6% | p = 0.209 |
| Demographic characteristics of children | ||||
| % children with 1+ parents with a degree † | 36.8% | 39.2% | 32.8% | p = 0.504 |
| % children living in low-SEIFA suburb ‡ | 28.9% | 24.9% | 35.2% | p = 0.200 |
| % children born in the tropics † | 91.7% | 92.6% | 90.3% | p = 0.579 |
| Sun exposure of children | ||||
| Median hours spent outside on a typical day [IQR] † | 2.3 [1.1,3.9] | 2.3 [1.1, 3.9] | 2.5 [1.3, 4.0] | p = 0.980 p = 0.817 § |
| Median total hours spent outside in warmer half of the previous year [IQR] † | 178 [31, 484] | 178 [27, 465] | 192 [38, 521] | p = 0.431 p = 0.630 § |
| Median number of hours spent playing in water on a typical day in warmer half of the previous year [IQR] † | 2 [1, 3.5] | 2 [1, 3.5] | 2 [1, 3.5] | p = 0.785 p = 0.810 § |
| % children who swim in an outdoor pool 1+/week during the warmer months † | 41.2% | 39.0% | 44.9% | p = 0.378 |
| % children who visit the beach 1+/week during the warmer months † | 8.5% | 9.9% | 6.1% | p = 0.596 |
| % children who played outside “almost every day” during the warmer months † | 44.5% | 41.2% | 50.0% | p = 0.299 |
| Sunburn characteristics | Total (n = 544) | Intervention (n = 334) | Control (n = 210) | p-value |
| % children with at least one sunburn with “redness without peeling” † | 43.8% | 42.4% | 46.2% | p = 0.404 |
| % children with at least one “peeling sunburn” † | 3.9% | 3.4% | 4.6% | p = 0.528 |
| % children with at least one sunburn that was “very painful with blistering” † | 0.6% | 0% | 1.5% | p = 0.022 |
| % children with a sunburn severity score of 2+ † | 4.2% | 3.4% | 5.6% | p = 0.403 |
| Median extent of sunburn score [IQR] † | 0 [0, 0.13] | 0 [0, 0.13] | 0.05 [0, 0.14] | p = 0.222 p = 0.184 § |
| Median sunburn history score weighted according to extent and severity [IQR] † | 0 [0, 0.13] | 0 [0, 0.13] | 0.05 [0,0.14] | p = 0.220 p = 0.128 § |
| Use of sun protection | ||||
| % parents who used sunscreen on child at home † | 91.3% | 90.1% | 93.4% | p = 0.288 |
| % children who use SPF 30+ sunscreen ∫ | 76.8% | 77.8% | 75.3% | p = 0.600 |
| % parents who “almost always” apply sunscreen to their child at home in summer † | 49.4% | 49.7% | 49.0% | p = 0.527 |
| % parents who “almost always” apply sunscreen to their child at home in winter † | 30.8% | 30.3% | 31.6% | p = 0.838 |
| Mean number of body sites to which sunscreen was usually applied (±SD) † | 7.5 (3.4) | 7.3 (3.5) | 7.8 (3.2) | p = 0.062 |
| Median BSA to which sunscreen was usually applied [IQR]† | 0.44 [0.3, 0.5] | 0.44 [0.3, 0.5] | 0.45 [0.3, 0.5] | p = 0.109 * p = 0.068 § |
| Median sunscreen score weighted by BSA and frequency of use [IQR] † | 2.1 [0.9, 3.4] | 2.0 [0.8, 3.4] | 2.1 [1.0, 3.4] | p = 0.397 * p = 0.162 § |
| Median hat use score weighted by hat style and frequency of use [IQR] † | 16 [8, 24] | 16 [9, 24] | 16 [8, 24] | p = 0.049 * p = 0.143§ |
| % children who usually wear Lycra suit in water † | 64.8% | 66.0% | 62.8% | p = 0.302 |
| Median swimwear protection score weighted by style and frequency of use [IQR] † | 25 [20, 25] | 25 [20, 25] | 25 [15, 25] | p = 0.259 * p = 0.091§ |
| Outcome | ||||
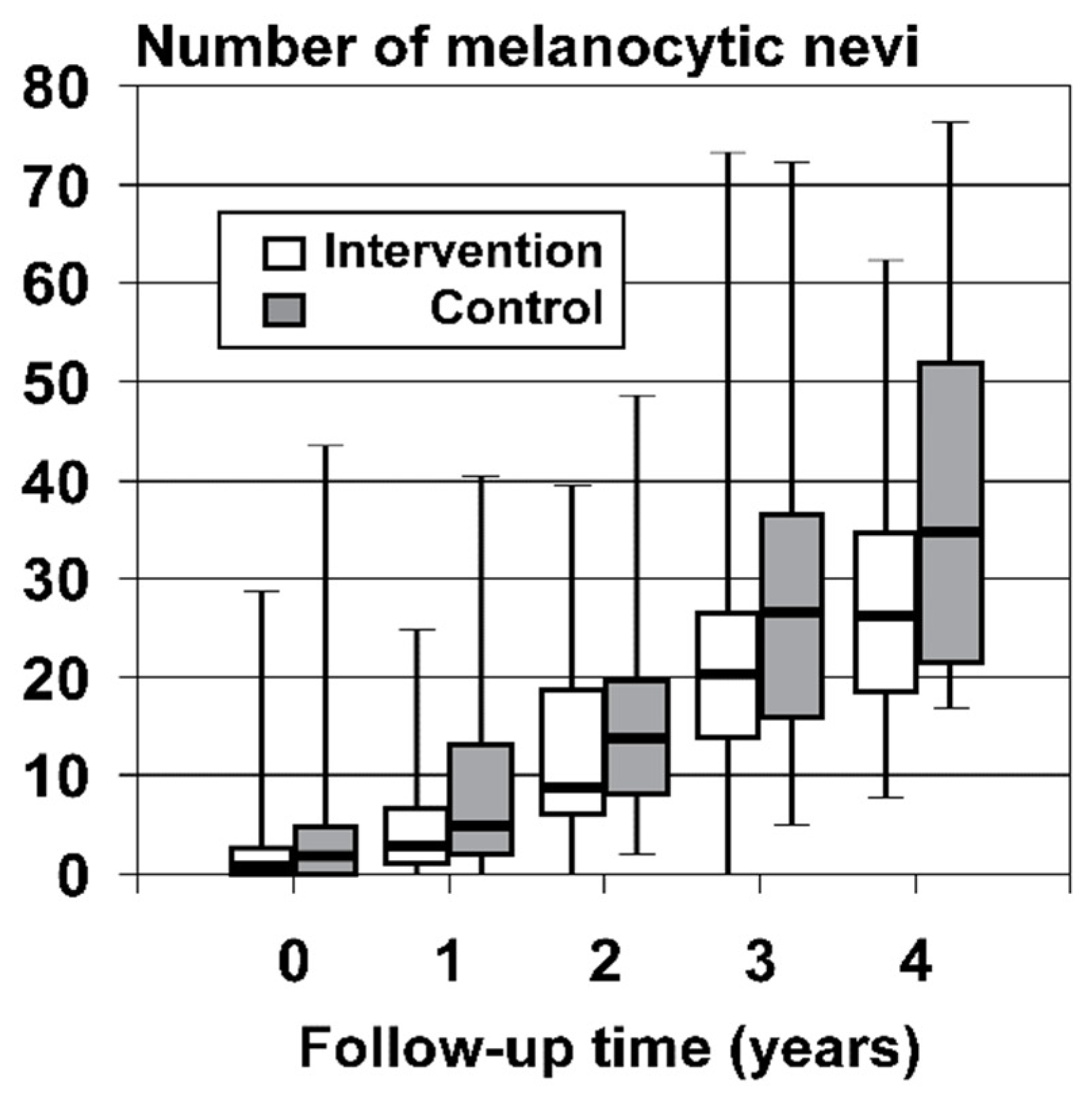
| Median total melanocytic nevus count [IQR] | 1 [0, 4]; range 0–44 | 1 [0, 3]; range 0–29 | 2 [0, 5]; range 0–44 | p = 0.028 * p = 0.183§ |
| Duration of Follow-Up | Intervention Arm (n = 334 Children) | Control Arm (n = 210 Children) | n p-Values |
|---|---|---|---|
| Median counts of incident MN present at final assessment [IQR] by duration of follow-up | |||
| <18 months | 3 [1, 7]; range 0–25 | 5 [2, 13.5]; range 0–41 | n = 151 |
| 18–29 months | 9 [6, 19]; range 0–40 | 14 [8.25, 20]; range 2–49 | n = 140 |
| 30–41 months | 20.5 [14, 27]; range 0–74 | 27 [16, 37]; range 5–73 | n = 207 |
| ≥42 months | 26.5 [18.75, 35]; range 8–63 | 35 [21.5, 52.25]; range 17–77 | n = 46 |
| Overall | 12.5 [5.75, 23]; range 0–74 | 16 [8, 30]; range 0–77 | p < 0.001; |
| p = 0.011; | |||
| p = 0.020§ | |||
| Median incidence rate [IQR] (i.e., new MN acquired per month) by duration of follow-up | |||
| <18 months | 0.25 [0.13, 0.55] | 0.46 [0.15, 1.13] | n = 151 |
| 18–29 months | 0.38 [0.25, 0.70] | 0.54 [0.35, 0.83] | n = 140 |
| 30–41 months | 0.58 [0.37, 0.79] | 0.75 [0.50, 1.13] | n = 207 |
| ≥42 months | 0.57 [0.43, 0.77] | 0.81 [0.50, 1.16] | n = 46 |
| Overall | 0.46 [0.25, 0.71] | 0.68 [0.37, 1.04] | p < 0.001; |
| p < 0.001; | |||
| p = 0.001§ | |||
| Median counts of new MN at sun-protected body sites [IQR] by duration of follow-up | |||
| <18 months | 1 [0.75, 3]; range 0–14 | 3 [0.5, 6.5]; range 0–16 | n = 151 |
| 18–29 months | 5 [2, 9]; range 0–22 | 6 [3.25, 11]; range 1–27 | n = 140 |
| 30–41 months | 8.5 [6, 14]; range 0–44 | 12 [8, 20]; range 0–41 | n = 207 |
| ≥42 months | 13 [10.25, 18]; range 2–32 | 19 [12, 26.25]; range 6–31 | n = 46 |
| Overall | 6 [2, 12]; range 0–44 | 8 [4, 15.25]; range 0–41 | p < 0.001; |
| p = 0.027; | |||
| p = 0.021§ | |||
| Study Name, Authors, and Study Date | Study Population at Baseline | n | Design, Sun Protection Strategies Used in Intervention, and Level of Randomization | MN Exam Methods | Outcome |
|---|---|---|---|---|---|
| Vancouver Mole Cohort Study 1993–1996 Gallagher et al. 2000 [62] | Grade 1 (6–7 yrs) and 4 (9–10 yrs) Caucasian children in British Columbia | Baseline (n = 483): controls (n = 236), S/S group (n = 222) 3 yr follow-up (n = 309) | RCT of S/S (children individually randomized) Treatment group supplied with SPF30 S/S and directions for use when the child was to be in the sun for at least 30 min. Main Strategy ¶ | Whole-body MN counts (all sizes) by dermatologist excl. buttocks, genitals, and scalp. Breast not examined in girls. | S/S group developed significantly fewer MN than control children (median 24 vs. 28; p = 0.048). Freckled S/S group developed fewer MN than freckled controls. |
| Kidskin Study 1995–1999 Milne et al. 2002 [47] English et al. 2005 [48] | Grade 1 (5–6 yrs) Caucasian children in Perth, Australia | Baseline (n = 1615) 4-year follow-up (n = 1453) 6-year follow-up in 2001 (n = 1116) comprised of 14 control schools (n = 484), 11 moderate intervention schools (n = 354), and 8 high intervention schools (n = 278) | Non-random cluster trial with 3 arms. School curriculum and policy-based sun protection intervention. Specially designed 4-year sun- protection curriculum and given guidelines on school sun protection policies. “High” group also received program materials in summer vacations and were offered discounted sun-protective swimwear. Main Strategies † § | Partial-body MN counts (all sizes) from projected image by lay examiners. MN counted on face, arms, and back (excl. shoulders) for both genders. MN on chest also counted in boys. MN on lower limbs not counted. | Mean new MN on back at follow-up: controls = 6.6 MN; moderate group = 5.2 MN; high group = 5.3 MN (p = 0.09; NS). Hat use improved in the high group but NS difference in MN on face and arms. Weak evidence of reduced counts of new MN. Mean new MN on boys’ backs at follow-up: controls = 7.9 MN; moderate group = 5.9 MN; high group = 6.4 MN; p = 0.0009. MN by gender: NS. |
| 1998–2001 Bauer et al. 2005 [63] | 2–7 year-olds at 78 public nursery schools (childcare centers) in Stuttgart and Bochum, Germany | Baseline (1998) n = 1812; 3-year follow-up n = 1232: control group (n = 398), education group (n = 369), S/S and education group (n = 465) | Cluster-RCT (3 arms). Moderate intervention group parents sent sun protection education letter 3x/year; high intervention group sent sun protection education and a free bottle of SPF25 S/S per year. Main Strategies ∫ ¶ | Whole-body MN (all sizes) counted by dermatologist. | NS difference in MN counts between groups. Median new MN [IQR]: controls = 8 [4,14]; education group = 8 [4,14]; education + S/S group =9 [6, 14]; p = 0.101 (NS). |
| 1998–2001 Crane et al. 2006 [64] | 0–6 month-olds recruited from 14 primary care practices servicing ~29% insured population in Denver- –Boulder area, USA | 728 infants and their parents 3-year follow-up of MN | Cluster-RCT. Sun protection advice provided to parents by healthcare providers at each well-child visit from 2–36 months old. Child sun hat provided at first visit; 2 small S/S samples provided each visit from 6 months old; sunglasses provided at 12 months old; parent–child activities to teach about sun protection provided at child’s 3-year visit. Main Strategies ∫ ¶ ‡ * | Whole-body MN counts (≥2 mm only) by dermatologist or pediatrician. | NS difference in MN counts: control = 5.64 MN, intervention = 6.3 MN, p = 0.56 (NS). |
| SoleSi SoleNo-GISED 2001–2004 Naldi et al. 2007 [65] | Grade 3–4 ~8-year-old children at 122 primary schools in Italy | Baseline (n = 4921 with MN counts) Follow-up 2005 (n = 3933) comprised of control group (n = 1661) and intervention group (n = 2272) | Cluster RCT. School-based intervention designed to reduce sunburn rates through use of a curriculum (median 6 hrs including video) and distribution of educational booklets on sun protection to children and parents. Main Strategies † ∫ | MN counted on upper limbs in subsample of 4921 children (44% of baseline sample). | NS impact of educational program on sunburn episodes or MN 1 year after intervention. Geometric MN at follow-up: controls, 6.4 MN; intervention group, 6.8 (NS difference). |
| Colorado Kids Sun Care Program 2004–2007 Crane et al. 2012 [66] | 6 year-olds (born 1998) recruited from pediatric offices and community settings in Denver, USA | Baseline 2003–2004 Follow-up of MN n = 677 white non-Hispanic children annually in 2005, 2006, and 2007 | RCT postal intervention. Educational newsletters posted to parents and children. Parent-reported use of S/S, protective clothing, hats, shade-seeking, and midday sun avoidance. Main Strategy ∫ | Whole-body MN counts (all sizes) by a team of 4–7 healthcare providers excl. buttocks, genitals, and scalp. | NS difference in MN < 2 mm. NS effect for presence of MN ≥ 2 mm (p = 0.09), with the intervention group having fewer large moles in 2006 only but not at the other 2 follow-ups. |
| Sun Protection for Florida’s Children (SPF) project 2006–2008 Roetzheim et al. 2011 [67] | Grade 4 primary children at 24 schools in Florida, USA | Baseline 2006–7 24 Florida schools Control group (n = 239 children) Intervention group (n = 200) | Cluster RCT of a school-based educational intervention focused on increasing hat use. Education session delivered in schools by researchers (2x hour sessions/yr) and parents given 2 wide-brimmed hats per child (for home and school). Main Strategies † ‡ | MN counted on head and neck by research assistants in a convenience subsample of 439 children. | Hat wearing at school improved from 2% (baseline) to 41% at 1-year follow-up and 19% at 2-year follow-up. NS difference between intervention and control groups wrt MN counts, tanning, and self-reported hat use outside of school. |
| RISC-UV project 2007–2009 (Tête Brûlée study) Mahé et al. 2011 [68] | Children at 52 Primary schools in greater Paris area | Baseline n = 828 with MN counts conducted 2007 Follow-up MN in 2009 n = 660 (mean age 10.8 yrs; 1:1 males:females)) | Cluster RCT of a school-based educational intervention evaluated by administering questionnaires to the player in 6 soccer teams about the sun protection they used during and between matches at a spring 2009 tournament. Main Strategies † | MN counted on the arms and back by 2 trained nurses (≤2 mm, >2 mm) in 2007 and again in 2009. | Sun protection use by soccer players and public inadequate. Total MN and new MN acquired after 2 years of study were higher in the 344 children who practiced outdoor sports. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, S.L.; Buettner, P.G.; Nowak, M.J. Sun-Protective Clothing Worn Regularly during Early Childhood Reduces the Number of New Melanocytic Nevi: The North Queensland Sun-Safe Clothing Cluster Randomized Controlled Trial. Cancers 2023, 15, 1762. https://doi.org/10.3390/cancers15061762
Harrison SL, Buettner PG, Nowak MJ. Sun-Protective Clothing Worn Regularly during Early Childhood Reduces the Number of New Melanocytic Nevi: The North Queensland Sun-Safe Clothing Cluster Randomized Controlled Trial. Cancers. 2023; 15(6):1762. https://doi.org/10.3390/cancers15061762
Chicago/Turabian StyleHarrison, Simone L., Petra G. Buettner, and Madeleine J. Nowak. 2023. "Sun-Protective Clothing Worn Regularly during Early Childhood Reduces the Number of New Melanocytic Nevi: The North Queensland Sun-Safe Clothing Cluster Randomized Controlled Trial" Cancers 15, no. 6: 1762. https://doi.org/10.3390/cancers15061762
APA StyleHarrison, S. L., Buettner, P. G., & Nowak, M. J. (2023). Sun-Protective Clothing Worn Regularly during Early Childhood Reduces the Number of New Melanocytic Nevi: The North Queensland Sun-Safe Clothing Cluster Randomized Controlled Trial. Cancers, 15(6), 1762. https://doi.org/10.3390/cancers15061762








