Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Radiotherapy-Based Treatment
2.1. Conventional Radiotherapy Alone
2.2. Concurrent Chemoradiotherapy
2.3. Radiotherapy with Hydrogen Peroxide Sensitization
2.4. Whole-Breast Radiotherapy Followed by Stereotactic or Intensity-Modulated Boost with or without Radiosensitization Strategy
2.5. Particle Therapy
3. Image-Guided Percutaneous Minimally Invasive Treatment
3.1. Radiofrequency Ablation
3.2. High-Intensity Focused Ultrasound
3.3. Cryoablation
3.4. Microwave Ablation
3.5. Laser Ablation
3.6. Other Modalities
3.7. Summary of Ablation Therapies
4. Current Recommendations and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Boyce-Fappiano, D.; Bedrosian, I.; Shen, Y.; Lin, H.; Gjyshi, O.; Yoder, A.; Shaitelman, S.F.; Woodward, W.A. Evaluation of overall survival and barriers to surgery for patients with breast cancer treated without surgery: A National Cancer Database analysis. NPJ Breast Cancer 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Dalal, M.; Sodhi, G.S. Estimation of Clinical Size of Breast Tumour Lesions Using Contrast Enhanced Magnetic Resonance Imaging: Delineation of Tumour Boundaries; University of Maryland, Baltimore County: Baltimore, MD, USA, 2021; 355p. [Google Scholar] [CrossRef]
- Singh, M.; Singh, T.; Soni, S. Pre-operative assessment of ablation margins for variable blood perfusion metrics in a magnetic resonance imaging based complex breast tumour anatomy: Simulation paradigms in thermal therapies. Comput. Methods Programs Biomed. 2020, 198, 105781. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Murai, T.; Suzuki, K.; Hashizume, C.; Ohta, K.; Yamada, Y.; Niwa, M.; Torii, A.; Shimohira, M. Definitive radiotherapy with SBRT or IMRT boost for breast cancer: Excellent local control and cosmetic outcome. Technol. Cancer Res. Treat. 2018, 17, 1533033818799355. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Takano, S.; Iida, M.; Urano, M.; Ohta, K.; Oguri, M.; Murai, T. Definitive radiotherapy with stereotactic or IMRT boost with or without radiosensitization strategy for operable breast cancer patients who refuse surgery. J. Radiat. Res. 2022, 63, 849–855. [Google Scholar] [CrossRef]
- Nimalasena, S.; Gothard, L.; Anbalagan, S.; Allen, S.; Sinnett, V.; Mohammed, K.; Kothari, G.; Musallam, A.; Lucy, C.; Yu, S.; et al. Intratumoral hydrogen peroxide with radiation therapy in locally advanced breast cancer: Results from a Phase 1 clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1019–1029. [Google Scholar] [CrossRef]
- Arriagada, R.; Mouriesse, H.; Sarrazin, D.; Clark, R.M.; Deboer, G. Radiotherapy alone in breast cancer. I. Analysis of tumor parameters, tumor dose and local control: The experience of the Gustave-Roussy Institute and the Princess Margaret Hospital. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 1751–1757. [Google Scholar] [CrossRef]
- Bedwinek, J.; Rao, D.V.; Perez, C.; Lee, J.; Fineberg, B. Stage III and localized stage IV breast cancer: Irradiation alone vs irradiation plus surgery. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 31–36. [Google Scholar] [CrossRef]
- Sheldon, T.; Hayes, D.F.; Cady, B.; Parker, L.; Osteen, R.; Silver, B.; Recht, A.; Come, S.; Henderson, I.C.; Harris, J.R. Primary radiation therapy for locally advanced breast cancer. Cancer 1987, 60, 1219–1225. [Google Scholar] [CrossRef]
- Harris, J.R.; Connolly, J.L.; Schnitt, S.J.; Cohen, R.B.; Hellman, S. Clinical-pathologic study of early breast cancer treated by primary radiation therapy. J. Clin. Oncol. 1983, 1, 184–189. [Google Scholar] [CrossRef]
- Courdi, A.; Ortholan, C.; Hannoun-Levi, J.M.; Ferrero, J.M.; Largillier, R.; Balu-Maestro, C.; Chapellier, C.; Ettore, F.; Birtwisle-Peyrottes, I. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiother. Oncol. 2006, 79, 156–161. [Google Scholar] [CrossRef]
- Kao, P.; Chi, M.-S.; Chi, K.-H.; Ko, H.-L. Primary chemo-radiotherapy for breast cancer patients who refused surgical treatment: A case series. Ther. Radiol. Oncol. 2019, 3, 24. [Google Scholar] [CrossRef]
- Mukai, H.; Watanabe, T.; Mitsumori, M.; Tsuda, H.; Nakamura, S.; Masuda, N.; Yamamoto, N.; Shibata, T.; Sato, A.; Iwata, H.; et al. Final results of a safety and efficacy trial of preoperative sequential chemoradiation therapy for the nonsurgical treatment of early breast cancer: Japan Clinical Oncology Group Study JCOG0306. Oncology 2013, 85, 336–341. [Google Scholar] [CrossRef]
- Ciérvide, R.; Montero, Á.; García-Rico, E.; García-Aranda, M.; Herrero, M.; Skaarup, J.; Benassi, L.; Barrera, M.J.; Vega, E.; Rojas, B.; et al. Primary chemoradiotherapy treatment (PCRT) for HER2+ and triple negative breast cancer patients: A feasible combination. Cancers 2022, 14, 4531. [Google Scholar] [CrossRef]
- Shanta, V.; Swaminathan, R.; Rama, R.; Radhika, R. Retrospective analysis of locally advanced noninflammatory breast cancer from Chennai, South India, 1990–1999. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 51–58. [Google Scholar] [CrossRef]
- Matuschek, C.; Boelke, E.; Roth, S.L.; Orth, K.; Lang, I.; Bojar, H.; Janni, J.W.; Audretsch, W.; Nestle-Kraemling, C.; Lammering, G.; et al. Long-term outcome after neoadjuvant radiochemotherapy in locally advanced noninflammatory breast cancer and predictive factors for a pathologic complete remission. Results of a multivariate analysis. Strahlenther. Onkol. 2012, 188, 777–781. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kubota, K.; Ue, H.; Kataoka, Y.; Tadokoro, M.; Miyatake, K.; Tsuzuki, K.; Yamanishi, T.; Itoh, S.; Hitomi, J.; et al. Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: A new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II). Int. J. Oncol. 2009, 34, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Kubota, K.; Aoyama, N.; Yamanashi, T.; Kariya, S.; Hamada, N.; Nogami, M.; Nishioka, A.; Onogawa, M.; Miyamura, M. Non-surgical breast-conserving treatment (KORTUC-BCT) using a new radiosensitization method (KORTUC II) for patients with Stage I or II breast cancer. Cancers 2015, 7, 2277–2289. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, N.; Ogawa, Y.; Yasuoka, M.; Ohgi, K.; Iwasa, H.; Miyatake, K.; Yoshimatsu, R.; Yamanashi, T.; Hamada, N.; Tamura, T.; et al. Therapeutic results of a novel enzyme-targeting radiosensitization treatment, Kochi oxydol-radiation therapy for unresectable carcinomas II, in patients with stage I primary breast cancer. Oncol. Lett. 2017, 13, 4741–4747. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, T.; Shibamoto, Y.; Matsuo, M.; Sugie, C.; Murai, T.; Ogawa, Y.; Miyakawa, A.; Manabe, Y.; Kondo, T.; Nakajima, K.; et al. Biological effects of hydrogen peroxide administered intratumorally with or without irradiation in murine tumors. Cancer Sci. 2017, 108, 1787–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimbo, T.; Nakata, M.; Yoshioka, H.; Sato, C.; Hori, A.; Kimura, K.; Iwamoto, M.; Yoshida, K.; Uesugi, Y.; Akiyama, H.; et al. New enzyme-targeting radiosensitizer (KORTUC II) treatment for locally advanced or recurrent breast cancer. Mol. Clin. Oncol. 2021, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Obata, S.; Ishimaru, Y.; Miyagi, S.; Nakatake, M.; Kuroiwa, A.; Ohta, Y.; Kan, T.; Kanegae, S.; Inoue, Y.; Nishizato, R.; et al. Actual practice of Kochi Oxydol Radiation Therapy for Unresectable Carcinomas by intra-tumoral administration of hydrogen peroxide as a radiosensitizer. Mol. Clin. Oncol. 2022, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Roman, O.; Kowalchuk, R.O.; Corbin, K.S.; Jimenez, R.B. Particle therapy for breast cancer. Cancers 2022, 14, 1066. [Google Scholar]
- Malouff, T.D.; Mahajan, A.; Mutter, R.W.; Krishnan, S.; Hoppe, B.S.; Beltran, C.; Trifiletti, D.M.; Vallow, L.A. Carbon ion radiation therapy in breast cancer: A new frontier. Breast Cancer Res. Treat. 2020, 181, 291–296. [Google Scholar] [CrossRef]
- Karasawa, K.; Omatsu, T.; Arakawa, A.; Yamamoto, N.; Ishikawa, T.; Saito, M.; Fukuda, S.; Kamada, T.; Working Group for Breast Cancer. A Phase I clinical trial of carbon ion radiotherapy for Stage I breast cancer: Clinical and pathological evaluation. J. Radiat. Res. 2019, 60, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, K.; Murata, K.; Mori, Y.; Okonogi, N.; Omatsu, N.; Wakatsuki, M.; Yamada, S. Clinical trials of carbon-ion therapy for early breast cancer. In Proceedings of the 35th Annual Meeting JASTRO, Hiroshima, Japan, 10–12 November 2022; Volume 1, p. 210. [Google Scholar]
- Karasawa, K.; Omatsu, T.; Shiba, S.; Irie, D.; Wakatsuki, M.; Fukuda, S. A clinical study of curative partial breast irradiation for stage I breast cancer using carbon ion radiotherapy. Radiat. Oncol. 2020, 15, 265. [Google Scholar] [CrossRef]
- Brock, R.M.; Beitel-White, N.; Davalos, R.V.; Allen, I.C. Starting a fire without flame: The induction of cell death and inflammation in electroporation-based tumor ablation strategies. Front. Oncol. 2020, 10, 1235. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, M.; Liu, J.; Ye, X.; Wei, Z.; Xu, J.; Xie, Q.; Liang, J. Crosstalk between microwave ablation and ferroptosis: The next hot topic? Front. Oncol. 2023, 13, 1099731. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Du, L.; Zhao, L.; Lei, F.; Ouyang, W.; Zhang, Y.; Liao, Y.; Tang, J. Effect of hyperthermia on the apoptosis and proliferation of CaSki cells. Mol. Med. Rep. 2011, 4, 187–191. [Google Scholar]
- Roknsharifi, S.; Wattamwar, K.; Fishman, M.D.C.; Ward, R.C.; Ford, K.; Faintuch, S.; Joshi, S.; Dialani, V. Image-guided microinvasive percutaneous treatment of breast lesions: Where do we stand? Radiographics 2021, 41, 945–966. [Google Scholar] [CrossRef]
- Grotenhuis, B.A.; Vrijland, W.W.; Klem, T.M.A.L. Radiofrequency ablation for early-stage breast cancer: Treatment outcomes and practical considerations. Eur. J. Surg. Oncol. 2013, 39, 1317–1324. [Google Scholar] [CrossRef]
- Dai, Y.; Ping Liang, P.; Yu, J. Percutaneous management of breast cancer: A systematic review. Curr. Oncol. Rep. 2022, 24, 1443–1459. [Google Scholar] [CrossRef]
- van der Voort, E.M.F.; Struik, G.M.; Birnie, E.; Moelker, A.; Verhoef, C.; Klem, T.M.A.L. Thermal ablation as an alternative for surgical resection of small (≤ 2 cm) breast cancers: A meta-analysis. Clin. Breast Cancer 2021, 21, e715–e730. [Google Scholar] [CrossRef]
- Pediconi, F.; Marzocca, F.; Marincola, B.C.; Napoli, A. MRI-guided treatment in the breast. J. Magn. Reson. Imaging 2018, 48, 1479–1488. [Google Scholar] [CrossRef]
- Xia, L.Y.; Hu, Q.L.; Xu, W.Y. Efficacy and safety of radiofrequency ablation for breast cancer smaller than 2 cm: A systematic review and meta-analysis. Front. Oncol. 2021, 11, 651646. [Google Scholar] [CrossRef]
- Schmitz, A.C.; Gianfelice, D.; Daniel, B.L.; Mali, W.P.T.M.; van der Bosch, M.A.A.J. Image-guided focused ultrasound ablation of breast cancer: Current status, challenges, and future directions. Eur. Radiol. 2008, 18, 1431–1441. [Google Scholar] [CrossRef]
- Peek, M.C.L.; Ahmed, M.; Napoli, A.; ten Haken, B.; McWilliams, S.; Usiskin, S.I.; Pinder, S.E.; van Hemelrijck, M.; Douek, M. Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. Br. J. Surg. 2015, 102, 873–882. [Google Scholar] [CrossRef]
- Feril, L.B.; Fernan, R.L.; Tachibana, K. High-intensity focused ultrasound in the treatment of breast cancer. Curr. Med. Chem. 2021, 28, 5179–5188. [Google Scholar] [CrossRef]
- Takada, M.; Toi, M. Cryosurgery for primary breast cancers, its biological impact, and clinical outcomes. Int. J. Clin. Oncol. 2019, 24, 608–613. [Google Scholar] [CrossRef]
- Pusceddu, C.; Paliogiannis, P.; Nigri, G.; Fancellu, A. Cryoablation in the management of breast cancer: Evidence to date. Breast Cancer 2019, 11, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Oura, S.; Nagamine, S.; Takahashi, M.; Yamamoto, N.; Yamamichi, N.; Earashi, M.; Doihara, H.; Imoto, S.; Mitsuyama, S.; et al. Radiofrequency ablation of breast cancer: A retrospective study. Clin. Breast Cancer 2018, 18, e495–e500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oura, S.; Tamaki, T.; Hirai, I.; Yoshimasu, T.; Ohta, F.; Nakamura, R.; Okamura, Y. Radiofrequency ablation therapy in patients with breast cancers two centimeters or less in size. Breast Cancer 2007, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Iwamoto, E.; Tsuda, H.; Seki, K. Radiofrequency ablation as local therapy for early breast carcinomas. Breast Cancer 2011, 18, 10–17. [Google Scholar] [CrossRef]
- Yamamoto, N.; Fujimoto, H.; Nakamura, R.; Arai, M.; Yoshii, A.; Kaji, S.; Itami, M. Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: Evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer 2011, 18, 3–9. [Google Scholar] [CrossRef]
- Palussière, J.; Henriques, C.; Mauriac, L.; Asad-Syed, M.; Valentin, F.; Brouste, V.; Mathoulin-Pélissier, S.; de Lara, C.T.; Debled, M. Radiofrequency ablation as a substitute for surgery in elderly patients with nonresected breast cancer: Pilot study with long-term outcomes. Radiology 2012, 264, 597–605. [Google Scholar] [CrossRef]
- Gianfelice, D.; Khiat, A.; Boulanger, Y.; Amara, M.; Belblidia, A. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J. Vasc. Intervent. Radiol. 2003, 14, 1275–1282. [Google Scholar] [CrossRef]
- Furusawa, H.; Namba, K.; Nakahara, H.; Tanaka, C.; Yasuda, Y.; Hirabara, E.; Imahariyama, M.; Komaki, K. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer 2007, 14, 55–58. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Zhu, H.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Li, K.Q.; Jin, C.B.; Xie, F.L.; Su, H.B. Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res. Treat. 2005, 92, 51–60. [Google Scholar] [CrossRef]
- Simmons, R.M.; Ballman, K.V.; Cox, C.; Carp, N.; Sabol, J.; Hwang, R.F.; Attai, D.; Sabel, M.; Nathanson, D.; Kenler, A.; et al. A Phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: Results from ACOSOG (Alliance) Z1072. Ann. Surg. Oncol. 2016, 23, 2438–2445. [Google Scholar] [CrossRef] [Green Version]
- Poplack, S.P.; Levine, G.M.; Henry, L.; Wells, W.A.; Heinemann, F.S.; Hanna, C.M.; Deneen, D.R.; Tosteson, T.D.; Barth, R.J., Jr. A pilot study of ultrasound-guided cryoablation of invasive ductal carcinomas up to 15 mm with MRI follow-up and subsequent surgical resection. Am. J. Roentgenol. 2015, 204, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Zha, X.; Liu, X.; Ding, Q.; Chen, L.; Ni, Y.; Zhang, Y.; Xu, Y.; Chen, L.; Zhao, Y.; et al. US-guided percutaneous microwave coagulation of small breast cancers: A clinical study. Radiology 2012, 263, 364–373. [Google Scholar] [CrossRef]
- Schwartzberg, B.; Lewin, J.; Abdelatif, O.; Bernard, J.; Bu-Ali, H.; Cawthorn, S.; Chen-Seetoo, M.; Feldman, S.; Govindarajulu, S.; Jones, L.; et al. Phase 2 open-label trial investigating percutaneous laser ablation for treatment of early-stage breast cancer: MRI, pathology, and outcome correlations. Ann. Surg. Oncol. 2018, 25, 2958–2964. [Google Scholar] [CrossRef] [Green Version]
- Dowlatshahi, K.; Francescatti, D.S.; Bloom, K.J. Laser therapy for small breast cancers. Am. J. Surg. 2002, 184, 359–363. [Google Scholar] [CrossRef]
- Copelan, A.; Hartman, J.; Chehab, M.; Venkatesan, A.M. High-intensity focused ultrasound: Current status for image-guided therapy. Semin. Intervent. Radiol. 2015, 32, 398–415. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, H.; Namba, K.; Thomsen, S.; Akiyama, F.; Bendet, A.; Tanaka, C.; Yasuda, Y.; Nakahara, H. Magnetic resonance-guided focused ultrasound surgery of breast cancer: Reliability and effectiveness. J. Am. Coll. Surg. 2006, 203, 54–63. [Google Scholar] [CrossRef]
- Baust, J.G.; Gage, A.A. The molecular basis of cryosurgery. BJU Int. 2005, 95, 1187–1191. [Google Scholar] [CrossRef]
- Roubidoux, M.A.; Yang, W.; Stafford, R.J. Image-guided ablation in breast cancer treatment. Tech. Vasc. Interv. Radiol. 2014, 17, 49–54. [Google Scholar] [CrossRef]
- Yang, Q.; Li, H.; Chen, B.H.; He, G.Z.; Wu, X.P.; Wang, L.X.; Wu, H.; Dou, J.P.; Han, Z.Y.; Zhang, J.; et al. Ultrasound-guided percutaneous microwave ablation for 755 benign breast lesions: A prospective multicenter study. Eur. Radiol. 2020, 30, 5029–5038. [Google Scholar] [CrossRef]
- Ayala-Orozco, C.; Urban, C.; Bishnoi, S.; Urban, A.; Charron, H.; Mitchell, T.; Shea, M.; Nanda, S.; Schiff, R.; Halas, N.; et al. Sub-100 nm gold nanomatryoshkas improve photo-thermal therapy efficacy in large and highly aggressive triple negative breast tumors. J. Control Release 2014, 191, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Moghaddam, F.; Sattarahmady, N.; Azarpira, N. Gold-curcumin nanostructure in photo-thermal therapy on breast cancer cell line: 650 and 808 nm diode lasers as light sources. J. Biomed. Phys. Eng. 2019, 9, 473–482. [Google Scholar]
- Cao, T.L.; Le, T.A.; Hadadian, Y.; Yoon, J. Theoretical analysis for using pulsed heating power in magnetic hypertheremia therapy of breast cancer. Int. J. Mol. Sci. 2021, 22, 8895. [Google Scholar] [CrossRef] [PubMed]
- Rahpeima, R.; Lin, C.A. Numerical study of magnetic hyperthermia ablation of breast tumor on an anatomically realistic breast phantom. PLoS ONE 2022, 17, e0274801. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological benefits of ultra-high dose rate FLASH radiotherapy: Sleeping beauty awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-high dose rate (FLASH) radiotherapy: Silver bullet or fool’s gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, T.; Inaniwa, T.; Mizushima, K.; Sato, S.; Hojo, S.; Kitamura, H.; Konishi, T.; Kodaira, S. Radiation chemical yields of 7-hydroxy-coumarin-3-carboxylic acid for proton- and carbon-ion beams at ultra-high dose rates: Potential roles in FLASH effects. Radiat. Res. 2022, 198, 255–262. [Google Scholar] [CrossRef]
- Iwata, H.; Ogino, H.; Hashimoto, S.; Yamada, M.; Shibata, H.; Yasui, K.; Toshito, T.; Omachi, C.; Tatekawa, K.; Manabe, Y.; et al. Spot scanning and passive scattering proton therapy: Relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 95–102. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Sitarz, M.K.; Ankjærgaard, C.; Johansen, J.; Andersen, C.E.; Kanouta, E.; Overgaard, C.; Grau, C.; Poulsen, P. In vivo validation and tissue sparing factor for acute damage of pencil beam scanning proton FLASH. Radiother. Oncol. 2022, 167, 109–115. [Google Scholar] [CrossRef]
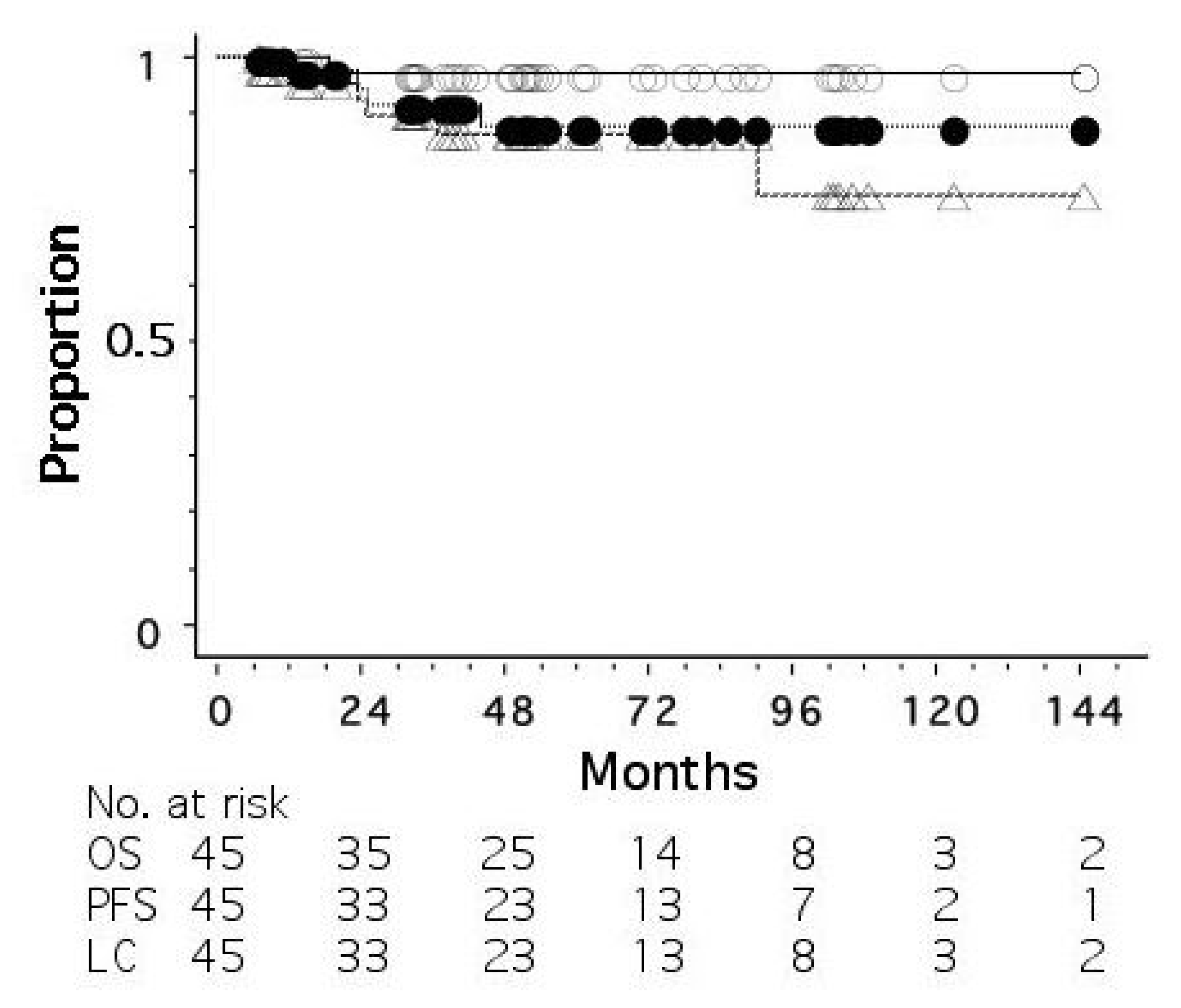


| 1st Author | n | Stage | Radiation (Gy/Fraction) | OS (%) | PFS (%) | LC (%) |
|---|---|---|---|---|---|---|
| Ogawa [20] | 72 | I/II | 44/16 + 9/3 | 100 (5 y) | 97.1 (5 y) | 97.1 (5 y) |
| Shimbo [23] | 30 | IIIA–IV & R | 44–67/16–30 | 60 (3 y) | 24 (2 y) | 75 (3 y) |
| Obata [24] * | 5 | I | 44/16 + 9/3 | 100 (5 y) | 100 (5 y) | 100 (5 y) |
| Nimalasena [8] | 13 | I–IV | 36/6 or 49.5/18 | 92 (1 y) | NA | 100 (1 y) |
| Shibamoto [7] ** | 15 | I–III | 50/25 + 21/3 or 20/8 | 93 (5 y) | 87 (5 y) | 93 (5 y) |
| 1st Author | Modality | n | Size * (cm) | Other Therapy | Complete Necrosis Rate (%) | Local Control (%) | Survival (%) |
|---|---|---|---|---|---|---|---|
| Ito [44] | RFA | 244 | ≤1.0 | R (91%) | NA | 97 | NA |
| 111 | 1.1–2.0 | NA | 94 | NA | |||
| 30 | >2.0 | NA | 87 | NA | |||
| Oura [45] | RFA | 52 | 0.5–2.0 (1.3) | S, R | 42 | 100 | 100 (6–30 M) |
| Kinoshita [46] | RFA | 49 | <3.0 (1.7) | S | 61 | NA | NA |
| Yamamoto [47] | RFA | 29 | 0.5–1.9 (1.3) | S, C, H | 92 | NA | NA |
| Palussière [48] | RFA | 21 | <3.0 | H, S (-) | 95 | NA | |
| Gianfelice [49] | HIFU | 24 | <2.0 | H, S (-) | 79 | NA | NA |
| Furusawa [50] | HIFU | 21 | 0.5–5.0 (1.5) | R, C | 95 (~26 M) | NA | |
| Wu [51] | HIFU | 22 | 2.0–4.8 (3.4) | R, C, H | 89 (5 year) | NA | |
| Simmons [52] | Cryo | 87 | 0–1.9 (1.1) | S | 76 | NA | NA |
| Poplack [53] | Cryo | 20 | ≤1.5 | S | 85 | NA | NA |
| Zhou [54] | Microwave | 41 | 1.3–6.4 (2.2) | S | 90 | NA | NA |
| Schwartzberg [55] | Laser | 61 | 0.4–1.9 (1.1) | S, R, C | 84 | 96 (34–65 M) | NA |
| Dowlatshahi [56] | Laser | 54 | 0.5–2.3 (1.2) | S | 70 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibamoto, Y.; Takano, S. Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects. Cancers 2023, 15, 1864. https://doi.org/10.3390/cancers15061864
Shibamoto Y, Takano S. Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects. Cancers. 2023; 15(6):1864. https://doi.org/10.3390/cancers15061864
Chicago/Turabian StyleShibamoto, Yuta, and Seiya Takano. 2023. "Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects" Cancers 15, no. 6: 1864. https://doi.org/10.3390/cancers15061864
APA StyleShibamoto, Y., & Takano, S. (2023). Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects. Cancers, 15(6), 1864. https://doi.org/10.3390/cancers15061864





