Normal Pressure Hydrocephalus Following Cranial Radiation: Identification of Shunting Responders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Neuropsychological Assessment
2.3. Lumbar Infusion Test and Data Acquisition
2.4. MRI Data and Image Processing for Volumetric CSF Analysis
3. Results
3.1. Demographic, Clinical, LIT and MRI Characteristics
3.2. Neuropsychological Assessment
3.3. Ventriculoperitoneal Shunting
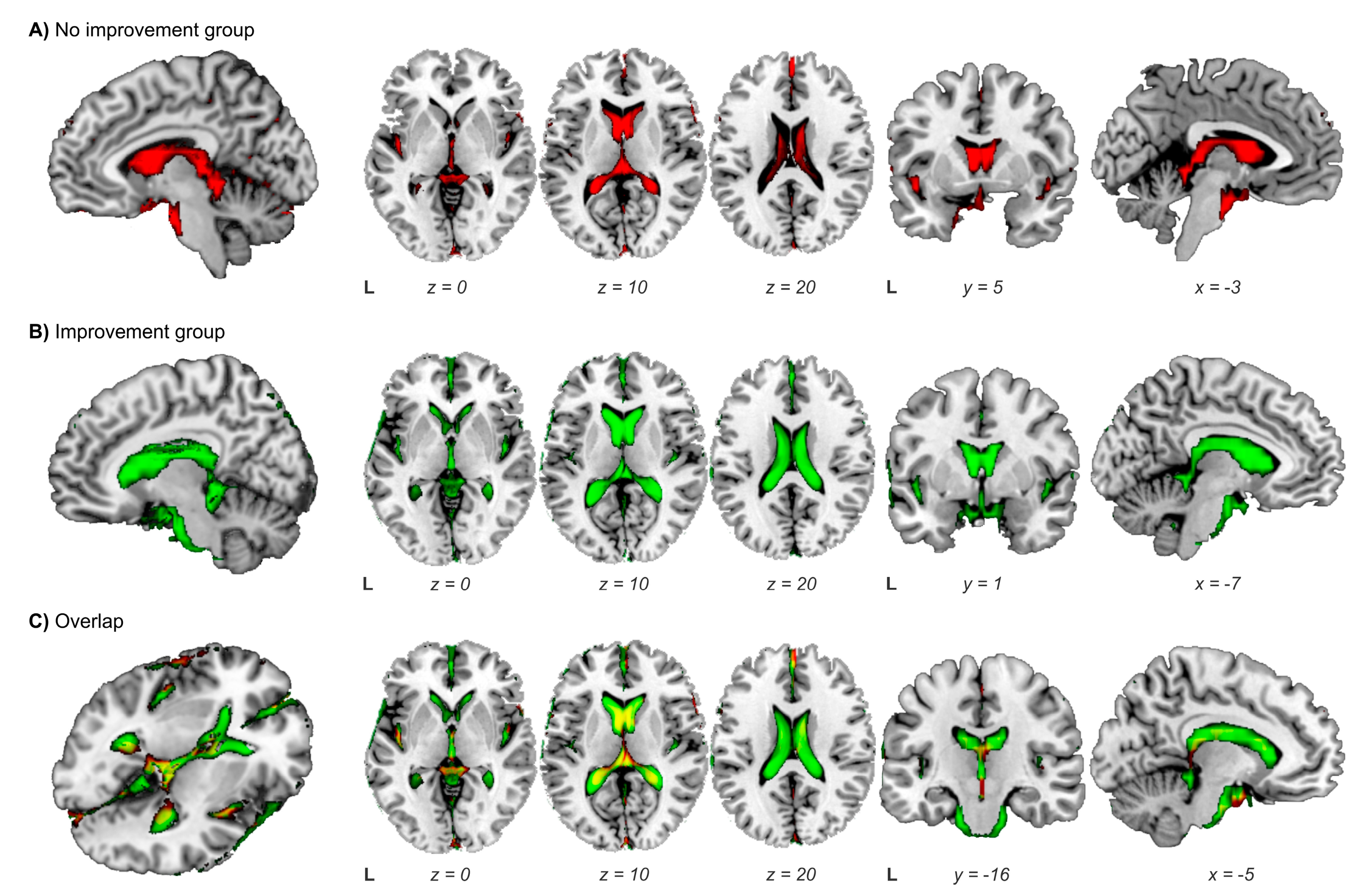
3.4. Ventriculoperitoneal Shunting: Identification of Responders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S.; EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Hwan Bae, G.; Kyung Kim, W.; Yoo, C.J.; Wan Park, C.; Kim, S.K.; Cha, J.; Wook Kim, J.; Jung, J. Radiotherapy for brain metastasis and long-term survival. Sci. Rep. 2021, 11, 8046. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, G.; Wang, M.; Shaw, E.; Jenkins, R.; Brachman, D.; Buckner, J.; Fink, K.; Shouhami, L.; Laperriere, N.; Curran, W.; et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J. Clin. Oncol. 2013, 31, 337–343. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Beanger, K.; et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a Randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douw, L.; Klein, M.; Fagel, S.S.; vanden Heuvel, J.; Taphoorn, M.J.B.; Aaronon, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A Randomized controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Bentzen, S.M.; Renschler, M.; Mehta, M.P. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Simó, M.; Vaquero, L.; Ripollés, P.; Jové, J.; Fuentes, R.; Cardenal, F.; Rodriguez-Fornells, A.; Bruna, J. Brain damage following prophylactic cranial irradiation in lung cancer survivors. Brain Imaging Behav. 2016, 10, 283–295. [Google Scholar] [CrossRef]
- Cayuela, N.; Jaramillo-Jiménez, E.; Càmara, E.; Majós, C.; Vidal, N.; Lucas, A.; Gil-Gil, M.; Graus, F.; Bruna, J.; Simó, M. Cognitive and brain structural changes in long-term oligodendroglial tumor survivors. Neuro. Oncol. 2019, 21, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Blair, R.M.; Moody, D.M.; Thore, C.R.; Ahmed, S.; Robbing, M.E.; Wheeler, K.T. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: A potential rat model of vascular dementia. J. Neurol. Sci. 2007, 257, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Giglio, P.; Gilbert, M.R. Cerebral radiation necrosis. Neurologist 2003, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Kumar, S.; Raj, M.K.; Das, K.J.M.; Sapru, S.; Behari, S.; Rathore, R.K.S.; Narayana, P.A.; Gupta, R.K. Serial diffusion tensor imaging to characterize radiation-induced changes in normal-appearing white matter following radiotherapy in patients with adult low-grade gliomas. Radiat. Med. 2008, 26, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, B.; DeAngelis, L.M. Hydrocephalus in radiation leukoencephalopathy: Results of ventriculoperitoneal shunting. Arch Neurol. 1998, 55, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Montano, N.; D’Alessandris, Q.G.; Bianchi, F.; Lauretti, L.; Doglietto, F.; Fernandez, E.; Maira, G.; Pallini, R. Communicating hydrocephalus following surgery and adjuvant radiochemotherapy for glioblastoma. J. Neurosurg. 2011, 115, 1126–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, O.; Szeifert, G.T.; Fazekas, I.; Vitanovics, D.; Csonka, E.; Kocsis, B.; Bori, Z.; Kemeny, A.A.; Nagy, Z. Effect of a single high-dose gamma irradiation on cultured cells in human cerebral arteriovenous malformation. J. Neurosurg. 2002, 97 (Suppl. 5), 459–463. [Google Scholar] [CrossRef]
- Shao, C.; Prise, K.M.; Folkard, M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat. Res. 2008, 638, 139–145. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Delattre, J.-Y.; Posner, J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Matsutani, M.; Kohno, T.; Nakamura, O.; Tanaka, H.; Fujimaki, T.; Funada, N.; Matsuda, T.; Nagata, K.; Takakura, K. Subacute brain atropthy after radiation therapy for malignant brain tumor. Cancer 1989, 63, 1962–1974. [Google Scholar] [CrossRef]
- Nakajima, M.; Yamada, S.; Miyajima, M.; Ishii, K.; Kuriyama, N.; Kazui, H.; Kanemoto, H.; Suehiro, T.; Yoshiyama, K.; Kaeda, M.; et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2021, 61, 63–97. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; McL Black, P. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005, 57 (Suppl. 3), S4–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Ishikawa, M.; Mori, E.; Kuwana, N. Study of INPH on neurological improvement (SINPHONI): Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: A prospective cohort study. Cereb. Fluid Res. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, D.C.; Adib, S.D.; Shuhmann, M.U.; Brendle, C. Paradigm-shift: Radiological changes in the asymptomatic Inph-patient to be: An observational study. Fluids Barriers CNS 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craven, C.L.; Toma, A.K.; Mostafa, T.; Patel, N.; Watkins, L.D. The predictive value of DESH for shunt responsiveness in idiopathic normal pressure hydrocephalus. J. Clin. Neurosci. 2016, 34, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Nitrini, R.; Román, G.C. Normal-pressure hydrocephalus: A critical review. Dement. Neuropsychol. 2019, 13, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kazui, H.; Miyajima, M.; Mori, E.; Ishikawa, M.; SINPHONI-2 Invesigators. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): An open-label randomised trial. Lancet Neurol. 2015, 14, 585–594. [Google Scholar] [CrossRef]
- Tisell, M.; Tullberg, M.; Hellström, P.; Edsbagge, M.; Högfeldt, M.; Wikkelsö, C. Shunt surgery in patients with hydrocephalus and white matter changes. J. Neurosurg. 2011, 114, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Palau, F.; Franco, M.; Jimenez, F.; Parra, E.; Bernate, M.; Solis, A. Clinical utility of the hopkins Verbal Test-Revised for detecting Alzheimer's disease and mild cognitive impairment in Spanish population. Arch. Clin. Neuropsychol. 2013, 28, 245. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Atxukarro, O.; Del Pino, R.; Peña, J.; Schretlen, D.J.; Ibarretxe-Bilbao, N.; Ojeda, N. Hopkins Verbal Learning Test-revised: Normalization and standardization for Spanish population. Rev. Neurol. 2021, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Palomo, R.; Casals-Coll, M.; Sanchez-Benavides, G.; Quintana, M.; Manero, R.M.; Rognoni, T.; Calvo, L.; Aranciva, F.; Tamayo, F.; Peña-Casanova, J. Spanish normative studies in young adults (NEURONORMA young adults project): Norms for the Rey-Osterrieth Complex Figure (copy and memory) and Free and Cued Selective Reminding Test. Neurologia 2013, 28, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Jak, A.J.; Clark, R.L.; Delano-Wood, L.; McDonald, C.R.; Nation, D.A.; Libon, D.J.; Au, R.; Galasko, D.; et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimers Dis. 2014, 42, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Blesa, R.; Pujol, M.; Aguilar, M.; Santacruz, P.; Bertran-Serra, I.; Hernández, G.; Sol, J.M.; Peña-Casanova, J.; NORMACODEM Group. NORMAlisation of Cognitive and Functional Instruments for DEMentia. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia 2001, 39, 1150–1157. [Google Scholar] [CrossRef]
- Katzman, R.; Hussey, F. A simple constant-infusion manometric test for measurement of CSF absorption. I. Rationale and method. Neurology 1970, 20, 534–544. [Google Scholar] [CrossRef]
- Wikkelso, C.; Hellstrom, P.; Klinge, P.M.; Tans, J.T. European iNPH Multicentre Study Group. The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF tap test in patients with idiopathic normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2013, 84, 562–568. [Google Scholar] [CrossRef]
- Sorteberg, A.; Eide, P.K.; Fremming, A.D. A prospective study on the clinical effect of surgical treatment of normal pressure hydrocephalus: The value of hydrodynamic evaluation. Br. J. Neurosurg. 2004, 18, 149–157. [Google Scholar] [CrossRef]
- Halperin, J.J.; Kurlan, R.; Schwalb, J.M.; Cusimano, M.D.; Gronseth, G.; Gloss, D. Practice guideline: Idiopathic normal pressure hydrocephalus: Response to shunting and predictors of response: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2015, 85, 2063–2071. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurting, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, H.; Kim, Y.T.; Yoon, B.C.; Czosnyka, Z.; Park, K.W.; Czosnyka, M. Thresholds of resistance to CSF outflow in predicting shunt responsiveness. Neurol. Res. 2015, 37, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Soma, T.; Shimada, K.; Oda, H.; Terashima, A.; Kawasaki, R. Automatic volumetry of the cerebrospinal fluid space in idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Dis. Extra. 2013, 3, 489–496. [Google Scholar] [CrossRef]
- Marmarou, A.; Bergsneider, M.; Klinge, P.; Relkin, N.; Black, P.M. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57, S17–S28. [Google Scholar] [CrossRef]
- Peterson, K.A.; Savulich, G.; Jackson, D.; Killikelly, C.; Pickard, J.D.; Sahakian, B.J. The effect of shunt surgery on neuropsychological performance in normal pressure hydrocephalus: A systematic review and meta-analysis. J. Neurol. 2016, 263, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Nishio, Y.; Kanno, S.; Uchiyama, M.; Hayashi, A.; Takagi, M.; Kikuchi, H.; Yamasaki, H.; Shimomura, Y.; Iizuka, O.; et al. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Dis Extra. 2011, 1, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kamagata, K.; Ueda, R.; Nakazawa, M.; Andica, C.; Irie, R.; Nakajima, M.; Miyajima, M.; Hori, M.; Taaka, F.; et al. Ventricular volumetry and free-water corrected diffusion tensor imaging of the anterior thalamic radiation in idiopathic normal pressure hydrocephalus. J. Neuroradiol. 2020, 47, 312–317. [Google Scholar] [CrossRef]
- Kanno, S.; Abe, N.; Saito, M.; Takagi, M.; Nishio, Y.; Hayashi, A.; Uchiyama, M.; Hanaki, R.; Kikuchi, H.; Hiraoka, K.; et al. White matter involvement in idiopathic normal pressure hydrocephalus: A voxel-based diffusion tensor imaging study. J. Neurol. 2011, 258, 1949–1957. [Google Scholar] [CrossRef]
- Serulle, Y.; Rusinek, H.; Kirov, I.I.; Milch, H.; Fieremans, E.; Baxter, A.B.; McMenamy, J.; Jain, R.; Wisoff, J.; Golomb, J.; et al. Differentiating shun-responsive normal pressure hydrocephalus from Alzheimer disease and normal aging: Pilot study using automated MRI brain tissue segmentation. J. Neurol. 2014, 261, 1994–2002. [Google Scholar] [CrossRef]
- Moore, D.W.; Kovanlikaya, I.; Heier, A.; Raj, A.; Huang, C.; Chu, K.W.; Relkin, N. A pilot study of quantitative MRI measurements of ventricular volume and cortical atrophy for the differential diagnosis of normal pressure hydrocephalus. Neurol. Res. Int. 2012, 2012, 718150. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Moghekar, A.; Shi, W.; Blitz, A.M.; Mor, S. Systematic volumetric analysis predicts response to CSF drainage and outcome to shunt surgery in idiopathic normal pressure hydrocephalus. Eur. Radiol. 2021, 31, 4972–4980. [Google Scholar] [CrossRef] [PubMed]

| Gait | Dementia | Urinary Incontinence | |||
|---|---|---|---|---|---|
| Wheelchair, bedridden | 5 | Institutionalized due to dementia. Patient may no longer survive without assistance | 5 | Permanent urinary catheter | 5 |
| Support of another person to walk | 4 | Severe dementia. Partial loss of independence | 4 | Constant incontinence | 4 |
| Support of a cane to walk | 3 | Moderate decreased memory and behaviour but independent patient | 3 | Occasional incontinence | 3 |
| Abnormal, but walking possible without support | 2 | Subjective feeling of decreased memory | 2 | Rare incontinence | 2 |
| Normal | 1 | None | 1 | None | 1 |
| Age, years at diagnosis (mean ± SD) | 56 ± 13.52 |
| Years of education (mean ± SD) | 13.33 ± 2.73 |
| Level of education, n (%) | |
| Primary School | 2 (5.6) |
| Lower Secondary School | 26 (72.2) |
| Upper Secondary School | 5 (13.9) |
| College | 3 (8.3) |
| Gender, n (%) | |
| Male | 18 (50) |
| Female | 18 (50) |
| NPH clinical score, median (range) | |
| Gait abnormalities | 4 (1–5) |
| Cognitive deficits | 2 (1–3) |
| Urinary incontinence | 2 (1–5) |
| Brain Tumour diagnoses, n (%) | |
| Primary | 20 (55.6) |
| Metastases | 8 (22.2) |
| Prophylactic cranial irradiation | 8 (22.2) |
| Surgery, n (%) | 23 (63.9) |
| Extent of surgical resection | |
| Gross total resection | 12 (52.2) |
| Partial resection | 11 (47.8) |
| RT type, n (%) | |
| Whole-brain RT | 16 (44.4) |
| Partial-brain RT | 18 (50) |
| Combination of cranial RT techniques | 2 (5.6) a |
| Total cranial RT dose Gy, median (range) | 50 (32–95) |
| Time between cranial RT and hydrocephalus suspicion (years), median (range) | 4 (2–19) |
| MRI features | |
| Fazekas scale, median (range) | 3 (1–3) |
| EI ratio (mean ± SD) | 0.33 ± 0.03 |
| Presence of DESH, n (%) | 16 (44.4) |
| CSF volume/TIV, mL (mean ± SD) b | 0.37 ± 0.06 |
| LIT features: | |
| Time between cranial RT and LIT (years), median (range) | 4 (2–20) |
| CSF opening pressure, mmHg (mean ± SD) | 10.37 ± 3.63 |
| ROUT, mmHg/ml/min (mean ± SD) | 13.20 ± 4.18 |
| Positive Tap Test, n (%) | 15 (41.7) |
| Time between cranial RT and neuropsychological tests (years), median (range) | 4 (2–23) |
| Cognitive impairment, n (%) | 26 (81.3) |
| ≥1 cognitive domain impaired | 21 (80.8) |
| MMSE score < 27 | 5 (19.2) |
| Phonemic fluency, mean ± SD | |
| COWA | −1.02 ± 0.79 |
| Visuospatial abilities, mean ± SD | |
| ROCF First Copy | 1.10 ± 1.53 |
| Visual memory, mean ± SD | |
| ROCF Delayed Copy | −0.08 ± 0.77 |
| Verbal memory, mean ± SD | |
| HVLT total recall | −0.85 ± 1.01 |
| HVLT delayed recall | −0.56 ± 1.33 |
| HVLT delayed recognition | 0.28 ± 1.64 |
| Processing speed/executive functions, mean ± SD | |
| Trail Making Test A | −1.33 ± 0.83 |
| Trail Making Test B | −1.51 ± 0.81 |
| Clinical Improvement Post-VPS | No Clinical Improvement Post-VPS | p Value | |
|---|---|---|---|
| (n = 10) | (n = 4) | ||
| Age, years at diagnosis (mean ± SD) | 57.60 ± 11.57 | 54.75 ± 16.70 | 0.72 |
| Gender, n (%) | 1.00 | ||
| Male | 5 (50) | 2 (50) | |
| Female | 5 (50) | 2 (50) | |
| NPH clinical score, median (range) | |||
| Gait abnormalities | 4 (3–5) | 4 (4–5) | 0.17 |
| Cognitive deficits | 2 (1–2) | 2 (2–3) | 0.23 |
| Urinary incontinence | 2 (1–4) | 3 (3–4) | 0.16 |
| Brain Tumour diagnoses, n (%) | 0.11 | ||
| Primary | 3 (30) | 4 (100) | |
| Metastases | 4 (40) | 0 (0) | |
| Prophylactic cranial irradiation | 3 (30) | 0 (0) | |
| Surgery, n (%) | 5 (50) | 4 (100) | 0.22 |
| Extent of surgical resection | 0.42 | ||
| Gross total resection | 1 (25) | 2 (50) | |
| Partial resection | 4 (75) | 2 (50) | |
| Total cranial RT dose Gy, median (range) | 45 (25–60) | 60 (60–95) a | 0.02 |
| MRI features | |||
| EI (mean ± SD) | 0.34 ± 0.04 | 0.37 ± 0.07 | 0.35 |
| DESH, n (%) | 6 (60) | 1 (25) | 0.56 |
| CSF volume / TIV, mL (mean ± SD) | 0.39 ± 0.08 | 0.30 ± 0.04 | 0.04 |
| LIT features | |||
| ROUT, mmHg/mL/min (mean ± SD) | 17.32 ± 2.10 | 16.75 ± 1.72 | 0.62 |
| Positive Tap Test, n (%) | 5 (50) | 1 (25) | 0.58 |
| Time between cranial RT and VPS (years) median (range) | 4 (2–20) | 8 (4–11) | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayuela, N.; Domínguez-Lizarbe, M.; Plans, G.; Alemany, M.; Sánchez, J.J.; Andrés, B.; Lucas, A.; Bruna, J.; Simó, M. Normal Pressure Hydrocephalus Following Cranial Radiation: Identification of Shunting Responders. Cancers 2023, 15, 1949. https://doi.org/10.3390/cancers15071949
Cayuela N, Domínguez-Lizarbe M, Plans G, Alemany M, Sánchez JJ, Andrés B, Lucas A, Bruna J, Simó M. Normal Pressure Hydrocephalus Following Cranial Radiation: Identification of Shunting Responders. Cancers. 2023; 15(7):1949. https://doi.org/10.3390/cancers15071949
Chicago/Turabian StyleCayuela, Nuria, Manuel Domínguez-Lizarbe, Gerard Plans, Montserrat Alemany, Juan José Sánchez, Begoña Andrés, Anna Lucas, Jordi Bruna, and Marta Simó. 2023. "Normal Pressure Hydrocephalus Following Cranial Radiation: Identification of Shunting Responders" Cancers 15, no. 7: 1949. https://doi.org/10.3390/cancers15071949
APA StyleCayuela, N., Domínguez-Lizarbe, M., Plans, G., Alemany, M., Sánchez, J. J., Andrés, B., Lucas, A., Bruna, J., & Simó, M. (2023). Normal Pressure Hydrocephalus Following Cranial Radiation: Identification of Shunting Responders. Cancers, 15(7), 1949. https://doi.org/10.3390/cancers15071949








