Identification of Metastatic Lymph Nodes Using Indocyanine Green Fluorescence Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Preoperative Imaging
2.3. Animal Model and Near-Infrared (NIR) Fluorescence Imaging
2.4. Patients and Preoperative Imaging
2.5. Surgical Procedures
2.5.1. Lung Cancer
2.5.2. Esophageal Cancer
2.6. Statistical Analysis
3. Results
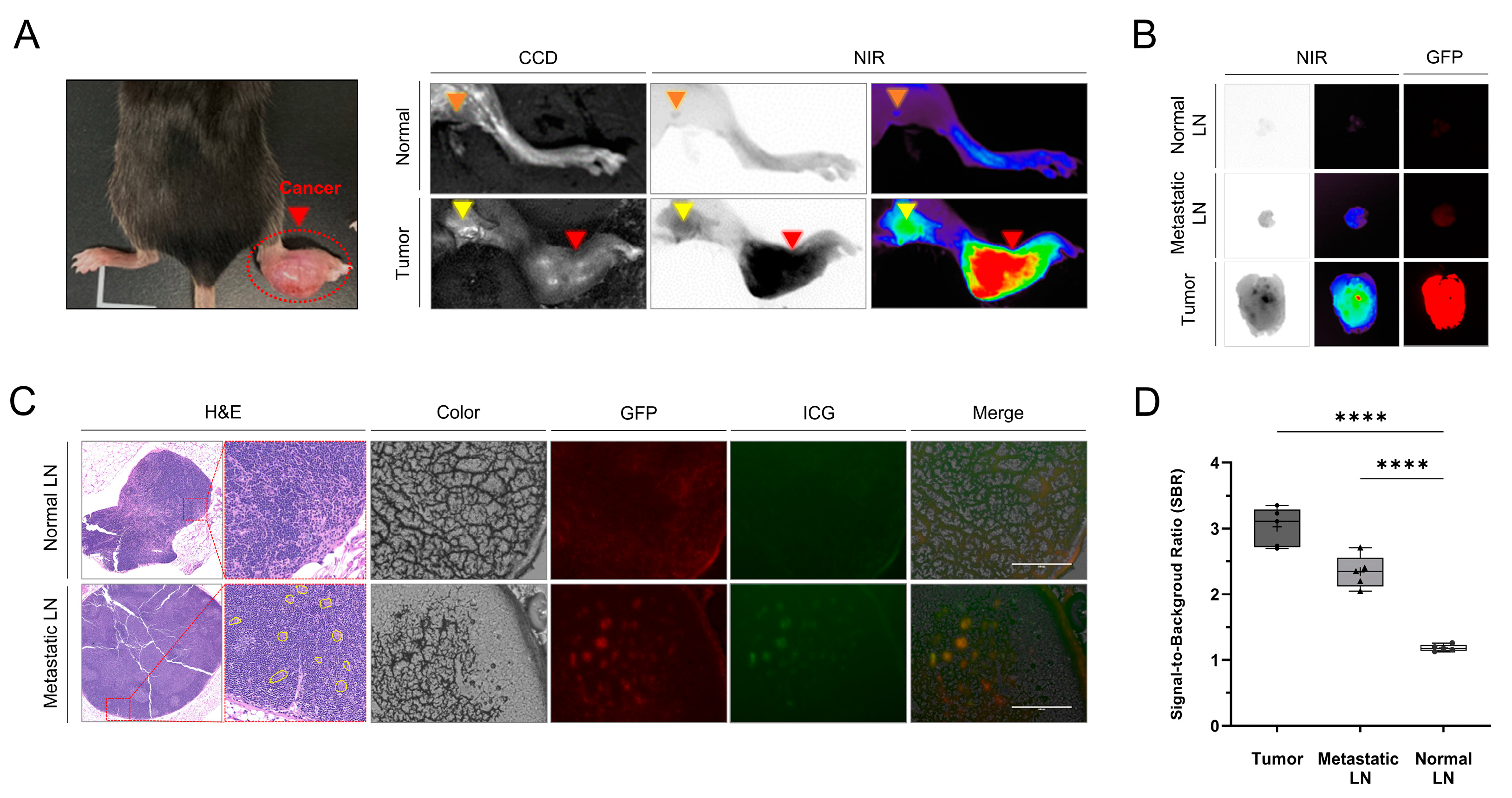
3.1. Identification of Tumors and Metastatic LNs Using ICG Fluorescence Imaging in the Mouse Model
3.2. Characteristics of Patients
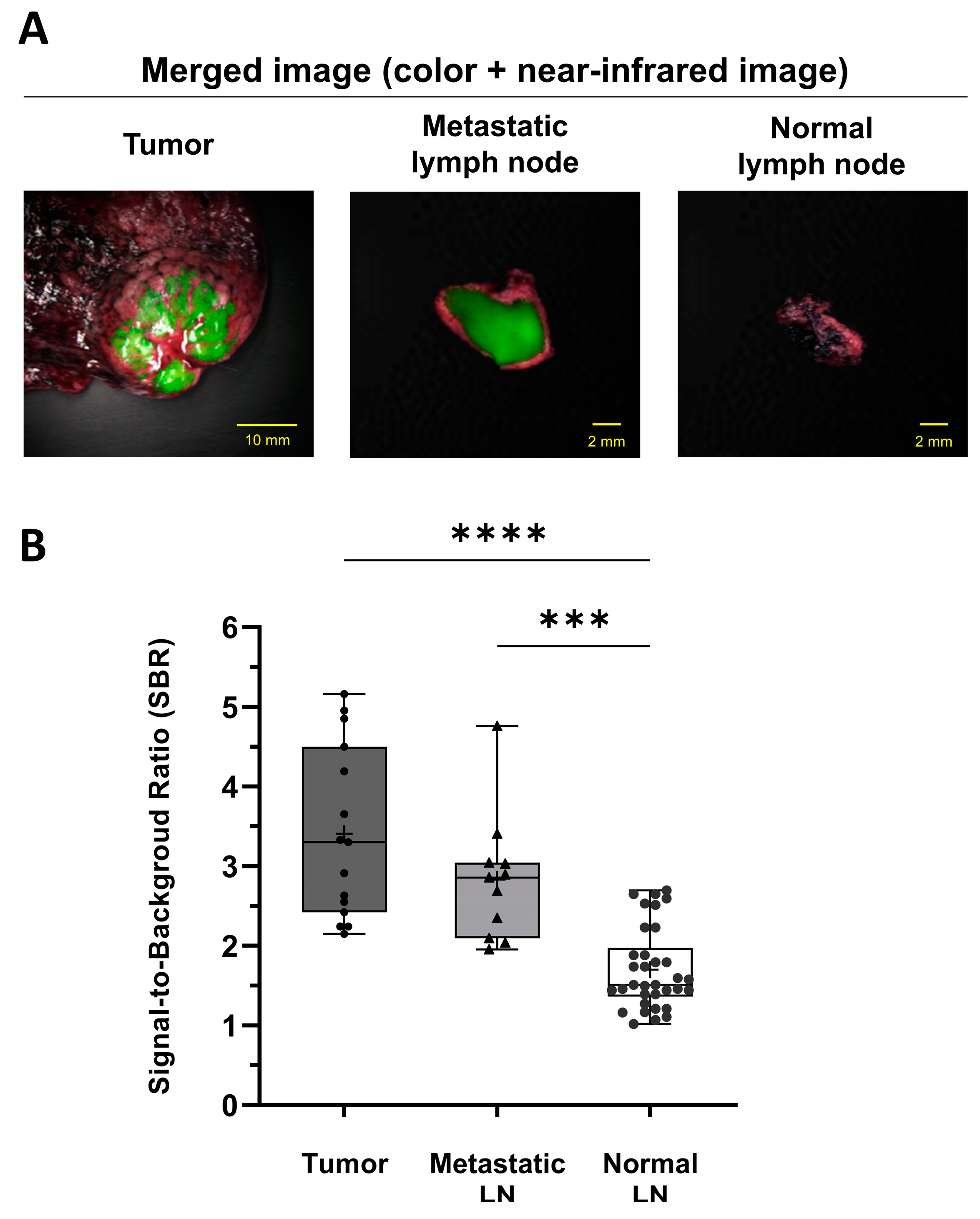
3.3. Identification of Tumors and Metastatic LNs Using ICG Fluorescence Imaging in the Patients
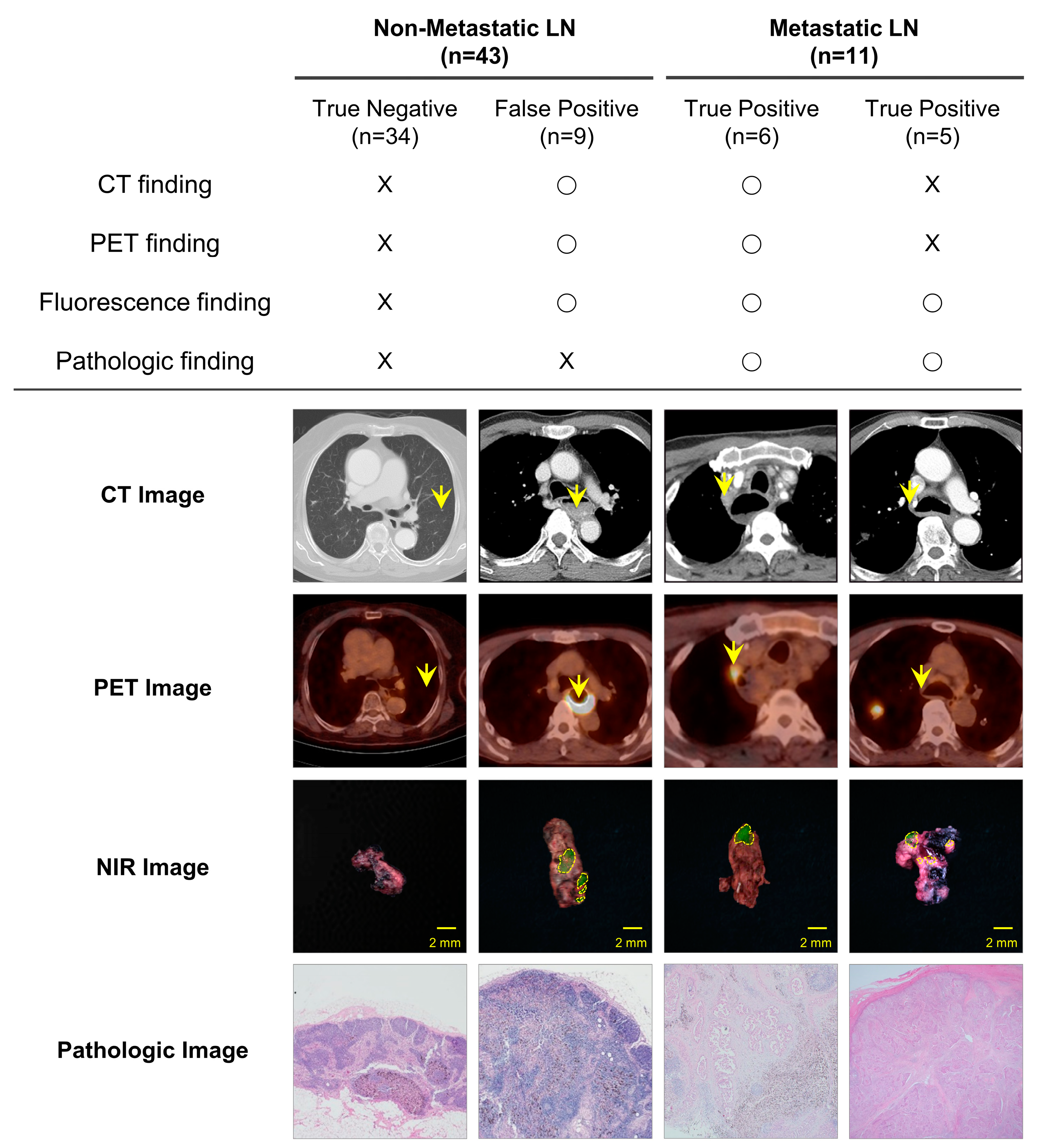
3.4. Comparison of Metastatic LN Detection Efficiency between PET/CT and NIR Fluorescence Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Borggreve, A.S.; Kingma, B.F.; Domrachev, S.A.; Koshkin, M.A.; Ruurda, J.P.; van Hillegersberg, R.; Takeda, F.R.; Goense, L. Surgical treatment of esophageal cancer in the era of multimodality management. Ann. N. Y. Acad. Sci. 2018, 1434, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lorente, D.; Navarro-Ripoll, R.; Guzman, R.; Moises, J.; Gimeno, E.; Boada, M.; Molins, L. Prehabilitation in thoracic surgery. J. Thorac. Dis. 2018, 10, S2593–S2600. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Quan, Y.H.; Choi, B.H.; Park, J.H.; Han, K.N.; Choi, Y.; Kim, B.M.; Choi, Y.H. Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur. J. Cardiothorac. Surg. 2016, 49, 1497–1502. [Google Scholar] [CrossRef]
- Xiao, Z.F.; Yang, Z.Y.; Miao, Y.J.; Wang, L.H.; Yin, W.B.; Gu, X.Z.; Zhang, D.C.; Sun, K.L.; Chen, G.Y.; He, J. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: Report of 549 cases. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 82–90. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva. Diagnostics 2021, 11, 912. [Google Scholar] [CrossRef]
- Varlotto, J.M.; Recht, A.; Nikolov, M.; Flickinger, J.C.; Decamp, M.M. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009, 115, 851–858. [Google Scholar] [CrossRef]
- Akthar, A.S.; Ferguson, M.K.; Koshy, M.; Vigneswaran, W.T.; Malik, R. Limitations of PET/CT in the Detection of Occult N1 Metastasis in Clinical Stage I(T1-2aN0) Non-Small Cell Lung Cancer for Staging Prior to Stereotactic Body Radiotherapy. Technol. Cancer Res. Treat. 2017, 16, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Hawes, D.; Decker, P.A.; Martin, S.E.; Abati, A.; Landreneau, R.J.; Patterson, G.A.; Inculet, R.I.; Jones, D.R.; Malthaner, R.A.; et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: Report of the ACOSOG Z0040 trial. J. Clin. Oncol. 2011, 29, 4313–4319. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, H.S.; Hyun, S.H.; Choi, Y.S.; Zo, J.I.; Shim, Y.M.; Lee, K.H.; Kim, B.T.; Choi, J.Y. Prediction of occult lymph node metastasis by metabolic parameters in patients with clinically N0 esophageal squamous cell carcinoma. J. Nucl. Med. 2014, 55, 743–748. [Google Scholar] [CrossRef] [PubMed]
- McGuill, M.J.; Byrne, P.; Ravi, N.; Reynolds, J. The prognostic impact of occult lymph node metastasis in cancer of the esophagus or esophago-gastric junction: Systematic review and meta-analysis. Dis. Esophagus 2008, 21, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, L.; Tessitore, A.; Casiraghi, M.; Guarize, J.; Solli, P.; Borri, A.; Gasparri, R.; Petrella, F.; Maisonneuve, P.; Galetta, D. Survival after extended resection for mediastinal advanced lung cancer: Lessons learned on 167 consecutive cases. Ann. Thorac. Surg. 2013, 95, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Shigematsu, H.; Okazaki, M.; Sakao, N.; Mori, Y.; Yukumi, S.; Izutani, H. Hoarseness after radical surgery with systematic lymph node dissection for primary lung cancer. Eur. J. Cardiothorac. Surg. 2019, 55, 280–285. [Google Scholar] [CrossRef]
- Adachi, H.; Maehara, T.; Nakayama, H.; Masuda, M. Mediastinal lymph node dissection in surgical treatment for early stage non-small-cell lung cancer: Lobe-specific or systematic? J. Thorac. Dis. 2017, 9, 2728–2731. [Google Scholar] [CrossRef]
- Veronesi, G.; Petrella, F.; Leo, F.; Solli, P.; Maissoneuve, P.; Galetta, D.; Gasparri, R.; Pelosi, G.; De Pas, T.; Spaggiari, L. Prognostic role of lymph node involvement in lung metastasectomy. J. Thorac. Cardiovasc. Surg. 2007, 133, 967–972. [Google Scholar] [CrossRef]
- Li, H.; Hu, H.; Li, B.; Sun, X.; Sun, Y.; Chen, H. What is the appropriate surgical strategy for pulmonary metastasis of colorectal cancer? Medicine 2020, 99, e21368. [Google Scholar] [CrossRef]
- Hernández, J.; Molins, L.; Fibla, J.J.; Heras, F.; Embún, R.; Rivas, J.J. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann. Oncol. 2016, 27, 850–855. [Google Scholar] [CrossRef]
- Religioni, J.; Orłowski, T. Surgical treatment of metastatic diseases to the lung. Kardiochirurgia Torakochirurgia Pol. 2020, 17, 52–60. [Google Scholar] [CrossRef]
- Forster, C.; Ojanguren, A.; Perentes, J.Y.; Zellweger, M.; Krueger, T.; Abdelnour-Berchtold, E.; Gonzalez, M. Survival prognostic and recurrence risk factors after single pulmonary metastasectomy. J. Cardiothorac. Surg. 2021, 16, 357. [Google Scholar] [CrossRef]
- Morton, D.L.; Cochran, A.J. The case for lymphatic mapping and sentinel lymphadenectomy in the management of primary melanoma. Br. J. Dermatol. 2004, 151, 308–319. [Google Scholar] [CrossRef]
- Ahmed, M.; Purushotham, A.D.; Douek, M. Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol. 2014, 15, e351–e362. [Google Scholar] [CrossRef]
- Soltesz, E.G.; Kim, S.; Laurence, R.G.; DeGrand, A.M.; Parungo, C.P.; Dor, D.M.; Cohn, L.H.; Bawendi, M.G.; Frangioni, J.V.; Mihaljevic, T. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann. Thorac. Surg. 2005, 79, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, L.; Wang, N.; Chen, P. Indocyanine green detects sentinel lymph nodes in early breast cancer. J. Int. Med. Res. 2017, 45, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Mizuguchi, S.; Izumi, N.; Chung, K.; Hanada, S.; Inoue, H.; Suehiro, S.; Nishiyama, N. Sialyl Lewis X as a predictor of skip N2 metastasis in clinical stage IA non-small cell lung cancer. World J. Surg. Oncol. 2013, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Prenzel, K.L.; Bollschweiler, E.; Schröder, W.; Mönig, S.P.; Drebber, U.; Vallboehmer, D.; Hölscher, A.H. Prognostic relevance of skip metastases in esophageal cancer. Ann. Thorac. Surg. 2010, 90, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Cavallin, F.; Alfieri, R.; Scarpa, M.; Cagol, M.; Ruol, A.; Fassan, M.; Rugge, M.; Ancona, E.; Castoro, C. Nodal skip metastasis in thoracic esophageal squamous cell carcinoma: A cohort study. BMC Surg. 2017, 17, 49. [Google Scholar] [CrossRef]
- Xu, Q.R.; Zhuge, X.P.; Zhang, H.L.; Ping, Y.M.; Chen, L.Q. The N-classification for esophageal cancer staging: Should it be based on number, distance, or extent of the lymph node metastasis? World J. Surg. 2011, 35, 1303–1310. [Google Scholar] [CrossRef]
- Chung, H.H.; Kim, J.W.; Han, K.H.; Eo, J.S.; Kang, K.W.; Park, N.H.; Song, Y.S.; Chung, J.K.; Kang, S.B. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol. Oncol. 2011, 120, 270–274. [Google Scholar] [CrossRef]
- Fischer, B.; Lassen, U.; Mortensen, J.; Larsen, S.; Loft, A.; Bertelsen, A.; Ravn, J.; Clementsen, P.; Høgholm, A.; Larsen, K.; et al. Preoperative staging of lung cancer with combined PET-CT. N. Engl. J. Med. 2009, 361, 32–39. [Google Scholar] [CrossRef]
- Yasufuku, K.; Nakajima, T.; Motoori, K.; Sekine, Y.; Shibuya, K.; Hiroshima, K.; Fujisawa, T. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006, 130, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Fukasawa, I.; Inaba, N.; Kaji, Y.; Sugimura, K. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am. J. Roentgenol. 2008, 190, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Yamada, T.; Ishikawa, O.; Takahashi, H.; Eguchi, H.; Yano, M.; Ohigashi, H.; Tomita, Y.; Miyamoto, Y.; Imaoka, S. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J. Surg. Oncol. 2009, 100, 75–79. [Google Scholar] [CrossRef]
- Keating, J.; Judy, R.; Newton, A.; Singhal, S. Near-infrared operating lamp for intraoperative molecular imaging of a mediastinal tumor. BMC Med. Imaging 2016, 16, 15. [Google Scholar] [CrossRef]
- Stubbs, V.C.; Jaffe, S.; Rajasekaran, K.; Cannady, S.B.; Shanti, R.M.; Lee, J.Y.K.; Newman, J.G. Intraoperative Imaging with Second Window Indocyanine Green for Head and Neck Lesions and Regional Metastasis. Otolaryngol. Head Neck Surg. 2019, 161, 539–542. [Google Scholar] [CrossRef]
- Batirel, H.F. Uniportal video-assisted thoracic surgery for esophageal cancer. J. Vis. Surg. 2017, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liang, W.; Liu, J.; He, J.; Ng, C.S.H.; Liu, C.C.; Petersen, R.H.; Rocco, G.; D’Amico, T.; Brunelli, A.; et al. Esophageal cancer in elderly patients: A population-based study. J. Thorac. Dis. 2018, 10, 448–457. [Google Scholar] [CrossRef]
- Venuta, F.; Diso, D.; Onorati, I.; Anile, M.; Mantovani, S.; Rendina, E.A. Lung cancer in elderly patients. J. Thorac. Dis. 2016, 8, S514–S908. [Google Scholar] [CrossRef]
- Jimenez-Lillo, J.; Villegas-Tovar, E.; Momblan-Garcia, D.; Turrado-Rodriguez, V.; Ibarzabal-Olano, A.; De Lacy, B.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Martinez-Portilla, R.J.; Lacy, A. Performance of Indocyanine-Green Imaging for Sentinel Lymph Node Mapping and Lymph Node Metastasis in Esophageal Cancer: Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2021, 28, 4869–4877. [Google Scholar] [CrossRef]
- Manny, T.B.; Krane, L.S.; Hemal, A.K. Indocyanine green cannot predict malignancy in partial nephrectomy: Histopathologic correlation with fluorescence pattern in 100 patients. J. Endourol. 2013, 27, 918–921. [Google Scholar] [CrossRef]
- Ludwig, M.S.; Goodman, M.; Miller, D.L.; Johnstone, P.A. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005, 128, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
| Variable | Data (n = 15) |
|---|---|
| Median Age (Years) | |
| Mean ± SD (Range) | 66 ± 10 (49–84) |
| Gender | |
| Female | 8 (54%) |
| Male | 7 (46%) |
| Cancer Type | |
| Lung Cancer (n = 10) | |
| Surgery | |
| Lobectomy | 9 (90%) |
| Wedge Resection | 1 (10%) |
| Histological Type | |
| Squamous Cell Carcinoma | 2 (20%) |
| Adenocarcinoma | 8 (80%) |
| TNM Stage | |
| T1N0M0 | 4 (40%) |
| T2N0M0 | 2 (20%) |
| T1N1M0 | 1 (10%) |
| T2N1M0 | 3 (30%) |
| Dissected Lymph Nodes | |
| Mean (Range) | 16 (1–31) |
| Metastatic Lung Cancer (n = 3) | |
| Surgery | |
| Wedge Resection | 3 (100%) |
| Histological Type | |
| Adenocarcinoma from Colon Cancer | 3 (100%) |
| Dissected Lymph Nodes | |
| Mean (Range) | 2 (1–3) |
| Esophageal Cancer (n = 2) | |
| Surgery | |
| Esophagectomy | 2 (100%) |
| Histological Type | |
| Squamous Cell Carcinoma | 2 (100%) |
| TNM Stage | |
| T2N0M0 | 1 (50%) |
| T3N2M0 | 1 (50%) |
| Dissected Lymph Nodes | |
| Mean (Range) | 49 (37–61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Han, K.N.; Choi, B.H.; Rho, J.; Lee, J.H.; Eo, J.S.; Kim, C.; Kim, B.-M.; Jeon, O.H.; Kim, H.K. Identification of Metastatic Lymph Nodes Using Indocyanine Green Fluorescence Imaging. Cancers 2023, 15, 1964. https://doi.org/10.3390/cancers15071964
Kim K, Han KN, Choi BH, Rho J, Lee JH, Eo JS, Kim C, Kim B-M, Jeon OH, Kim HK. Identification of Metastatic Lymph Nodes Using Indocyanine Green Fluorescence Imaging. Cancers. 2023; 15(7):1964. https://doi.org/10.3390/cancers15071964
Chicago/Turabian StyleKim, Kyungsu, Kook Nam Han, Byeong Hyeon Choi, Jiyun Rho, Jun Hee Lee, Jae Seon Eo, Chungyeul Kim, Beop-Min Kim, Ok Hwa Jeon, and Hyun Koo Kim. 2023. "Identification of Metastatic Lymph Nodes Using Indocyanine Green Fluorescence Imaging" Cancers 15, no. 7: 1964. https://doi.org/10.3390/cancers15071964
APA StyleKim, K., Han, K. N., Choi, B. H., Rho, J., Lee, J. H., Eo, J. S., Kim, C., Kim, B.-M., Jeon, O. H., & Kim, H. K. (2023). Identification of Metastatic Lymph Nodes Using Indocyanine Green Fluorescence Imaging. Cancers, 15(7), 1964. https://doi.org/10.3390/cancers15071964






