Critical Appraisal of Leibovich 2018 and GRANT Models for Prediction of Cancer-Specific Survival in Non-Metastatic Chromophobe Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Variables Definition
2.2. Statistical Analyses
3. Results
3.1. Descriptive Characteristics
3.2. Application of Leibovich 2018 and GRANT Risk Categories within the Development Cohort to Predict Cancer-Specific Survival
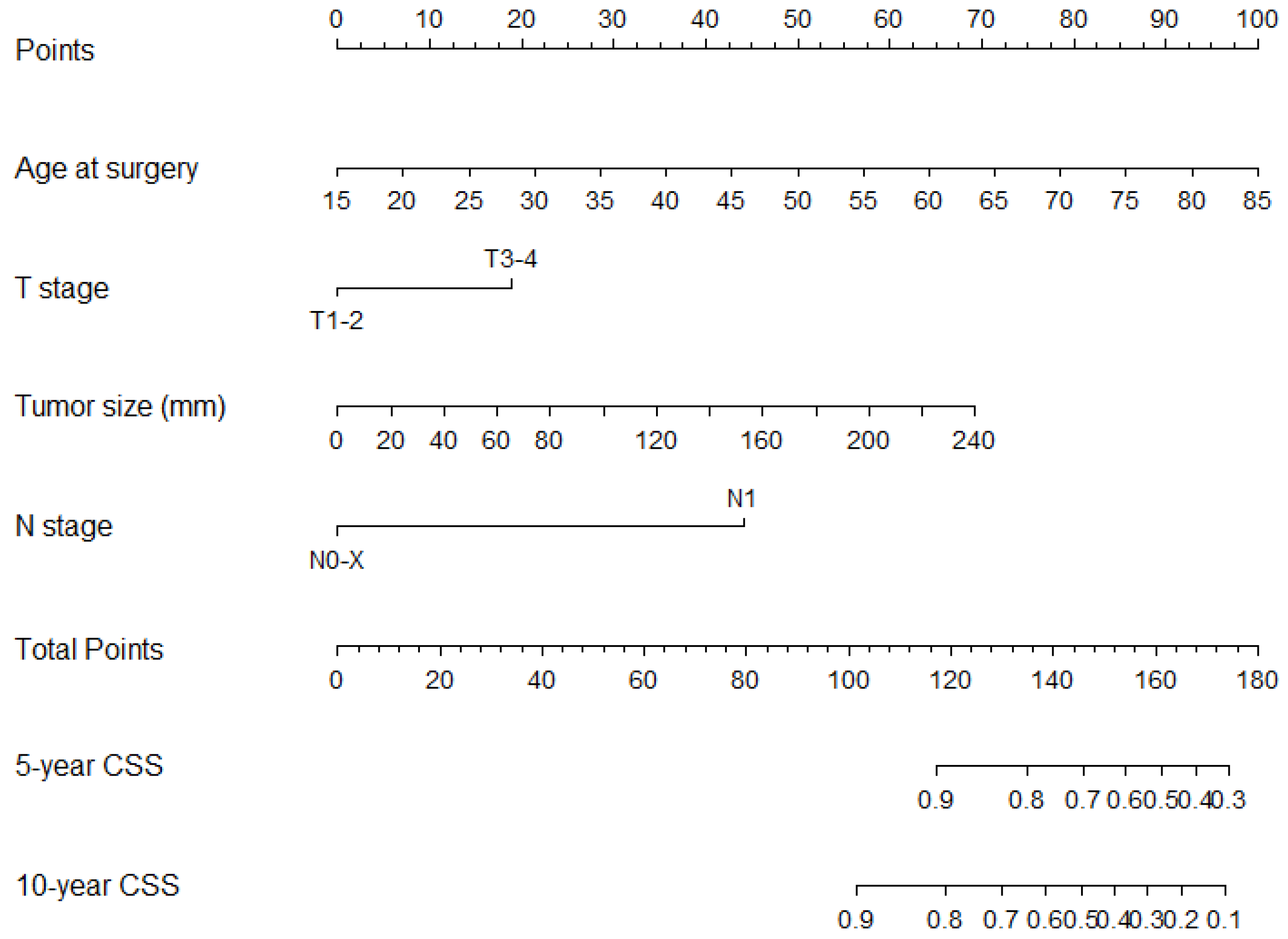
3.3. Development of a Novel Nomogram to Predict Cancer-Specific Survival in Chromophobe Kidney Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albiges, L.; Flippot, R.; Rioux-Leclercq, N.; Choueiri, T.K. Non–Clear Cell Renal Cell Carcinomas: From Shadow to Light. J. Clin. Oncol. 2018, 36, 3624–3631. [Google Scholar] [CrossRef] [PubMed]
- Leibovich, B.C.; Lohse, C.M.; Cheville, J.C.; Zaid, H.B.; Boorjian, S.A.; Frank, I.; Thompson, R.H.; Parker, W.P. Predicting Oncologic Outcomes in Renal Cell Carcinoma after Surgery. Eur. Urol. 2018, 73, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Buti, S.; Puligandla, M.; Bersanelli, M.; DiPaola, R.S.; Manola, J.; Taguchi, S.; Haas, N.B. Validation of a New Prognostic Model to Easily Predict Outcome in Renal Cell Carcinoma: The GRANT Score Applied to the ASSURE Trial Population. Ann. Oncol. 2017, 28, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W.; Reuter, V.; Motzer, R.J.; Katz, J.; Russo, P. A Postoperative Prognostic Nomogram for Renal Cell Carcinoma. J. Urol. 2001, 166, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Karakiewicz, P.I.; Briganti, A.; Chun, F.K.H.; Trinh, Q.D.; Perrotte, P.; Ficarra, V.; Cindolo, L.; de La Taille, A.; Tostain, J.; Mulders, P.F.A.; et al. Multi-Institutional Validation of a New Renal Cancer-Specific Survival Nomogram. J. Clin. Oncol. 2007, 25, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Egleston, B.L.; Wong, Y.N.; Uzzo, R.G. Evaluating Overall Survival and Competing Risks of Death in Patients with Localized Renal Cell Carcinoma Using a Comprehensive Nomogram. J. Clin. Oncol. 2010, 28, 311–317. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Dorey, F.; Said, J.W.; Shvarts, O.; Quintana, D.; Gitlitz, B.J.; deKernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Improved Prognostication of Renal Cell Carcinoma Using an Integrated Staging System. J. Clin. Oncol. 2001, 19, 1649–1657. [Google Scholar] [CrossRef]
- Wang, J.; Zhanghuang, C.; Tan, X.; Mi, T.; Liu, J.; Jin, L.; Li, M.; Zhang, Z.; He, D. Development and Validation of a Competitive Risk Model in Elderly Patients with Chromophobe Cell Renal Carcinoma: A Population-Based Study. Front. Public Health 2022, 10, 840525. [Google Scholar] [CrossRef]
- Li, S.; Zhu, J.; He, Z.; Ashok, R.; Xue, N.; Liu, Z.; Ding, L.; Zhu, H. Development and Validation of Nomograms Predicting Postoperative Survival in Patients with Chromophobe Renal Cell Carcinoma. Front. Oncol. 2022, 12, 982833. [Google Scholar] [CrossRef]
- Chen, C.; Geng, X.; Liang, R.; Zhang, D.; Sun, M.; Zhang, G.; Hou, J. Nomograms-Based Prediction of Overall and Cancer-Specific Survivals for Patients with Chromophobe Renal Cell Carcinoma. Exp. Biol. Med. 2021, 246, 729–739. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Li, L.; Roberts, L.; Harrison, H.; Rossi, S.H.; Sharp, S.J.; Coupland, C.; Hippisley-Cox, J.; Griffin, S.J.; Klatte, T.; et al. Risk Models for Recurrence and Survival after Kidney Cancer: A Systematic Review. BJU Int. 2022, 130, 562–579. [Google Scholar] [CrossRef]
- Correa, A.F.; Jegede, O.; Haas, N.B.; Flaherty, K.T.; Pins, M.R.; Messing, E.M.; Manola, J.; Wood, C.G.; Kane, C.J.; Jewett, M.A.S.; et al. Predicting Renal Cancer Recurrence: Defining Limitations of Existing Prognostic Models with Prospective Trial-Based Validation. J. Clin. Oncol. 2019, 37, 2062–2071. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Wieder, J.; Chao, D.H.; Dorey, F.; Said, J.W.; DeKernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Risk Group Assessment and Clinical Outcome Algorithm to Predict the Natural History of Patients with Surgically Resected Renal Cell Carcinoma. J. Clin. Oncol. 2002, 20, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.F.; Jegede, O.A.; Haas, N.B.; Flaherty, K.T.; Pins, M.R.; Adeniran, A.; Messing, E.M.; Manola, J.; Wood, C.G.; Kane, C.J.; et al. Predicting Disease Recurrence, Early Progression, and Overall Survival following Surgical Resection for High-Risk Localized and Locally Advanced Renal Cell Carcinoma. Eur. Urol. 2021, 80, 20–31. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y. Renal Cell Carcinoma EAU Guidelines On Renal Cell Carcinoma: 2020. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Rosiello, G.; Larcher, A.; Fallara, G.; Giancristofaro, C.; Martini, A.; Re, C.; Cei, F.; Musso, G.; Tian, Z.; Karakiewicz, P.I.; et al. Head-to-Head Comparison of All the Prognostic Models Recommended by the European Association of Urology Guidelines to Predict Oncologic Outcomes in Patients with Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 271.e19–271.e27. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, R.; Caminiti, C.; Buti, S.; Porta, C.; Camisa, R.; Braglia, L.; Tomasello, G.; Vaglio, A.; Labianca, R.; Rondini, E.; et al. Adjuvant Low-Dose Interleukin-2 (IL-2) plus Interferon-α (IFN-α) in Operable Renal Cell Carcinoma (RCC): A Phase III, Randomized, Multicentre Trial of the Italian Oncology Group for Clinical Research (GOIRC). J. Immunother. 2014, 37, 440–447. [Google Scholar] [CrossRef]
- Juul, S.; Donskov, F.; Clark, P.E.; Lund, L.; Azawi, N.H. GRade, Age, Nodes, and Tumor (GRANT) Compared with Leibovich Score to Predict Survival in Localized Renal Cell Carcinoma: A Nationwide Study. Int. J. Urol. 2022, 29, 641–645. [Google Scholar] [CrossRef]
- Cortellini, A.; Buti, S.; Bersanelli, M.; Cannita, K.; Pinterpe, G.; Venditti, O.; Verna, L.; Porzio, G.; Natoli, C.; Tinari, N.; et al. Predictive Ability for Disease-Free Survival of the GRade, Age, Nodes, and Tumor (GRANT) Score in Patients with Resected Renal Cell Carcinoma. Curr. Urol. 2020, 14, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Buti, S.; Karakiewicz, P.I.; Bersanelli, M.; Capitanio, U.; Tian, Z.; Cortellini, A.; Taguchi, S.; Briganti, A.; Montorsi, F.; Leonardi, F.; et al. Validation of the GRade, Age, Nodes and Tumor (GRANT) Score within the Surveillance Epidemiology and End Results (SEER) Database: A New Tool to Predict Survival in Surgically Treated Renal Cell Carcinoma Patients. Sci. Rep. 2019, 9, 13218. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 10 January 2023).
- Palumbo, C.; Perri, D.; Zacchero, M.; Bondonno, G.; Di Martino, J.; D’Agate, D.; Volpe, A. Risk of Recurrence after Nephrectomy: Comparison of Predictive Ability of Validated Risk Models. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 167.e1–167.e7. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2008, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. Net Benefit Approaches to the Evaluation of Prediction Models, Molecular Markers, and Diagnostic Tests. BMJ 2016, 352, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef]
- Shuch, B.; Singer, E.A.; Bratslavsky, G. The Surgical Approach to Multifocal Renal Cancers: Hereditary Syndromes, Ipsilateral Multifocality, and Bilateral Tumors. Urol. Clin. North Am. 2012, 39, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Prognostic/Clinical Prediction Models: Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Tutor. Biostat. Stat. Methods Clin. Stud. 2005, 1, 223–249. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Zheng, Y. Survival Model Predictive Accuracy and ROC Curves. Biometrics 2005, 61, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.; Norden, N. The R Project for Statistical Computing The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 January 2023).
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur. Urol. 2015, 67, 1142–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Surgically Treated Non-Metastatic chRCC n = 5522 | Development n = 2761 | External Validation n = 2761 |
|---|---|---|
| Age at surgery (years) | ||
| Median (IQR) | 59 (49–68) | 60 (50–70) |
| 18–35 | 140 (5%) | 152 (6%) |
| 36–50 | 613 (22%) | 545 (20%) |
| 51–70 | 1451 (53%) | 1426 (51%) |
| ≥71 | 557 (20%) | 638 (23%) |
| Sex | ||
| Male | 1580 (57%) | 1521 (55%) |
| Race/ethnicity | ||
| Caucasian | 1871 (68%) | 1838 (67%) |
| African American | 341 (12%) | 379 (14%) |
| Hispanic | 376 (14%) | 369 (13%) |
| Asian or Pacific Islander | 141 (5%) | 143 (5%) |
| Other | 32 (1%) | 32 (1%) |
| Treatment | ||
| Radical nephrectomy | 1772 (64%) | 1731 (63%) |
| Partial nephrectomy | 989 (36%) | 1030 (37%) |
| Grade | ||
| G1 | 231 (8%) | 237 (9%) |
| G2 | 1547 (56%) | 1486 (54%) |
| G3 | 828 (30%) | 864 (31%) |
| G4 | 155 (6%) | 174 (6%) |
| Sarcomatoid features | 31 (1%) | 23 (1%) |
| T stage | ||
| T1 | 1722 (62%) | 1769 (64%) |
| T2 | 491 (18%) | 476 (17%) |
| T3 | 503 (18%) | 471 (17%) |
| T4 | 45 (2%) | 45 (2%) |
| Size (mm) | 45 (30–75) | 45 (30–70) |
| Median (IQR) | ||
| N stage | ||
| N0-X | 2737 (99%) | 2728 (99%) |
| N1 | 24 (1%) | 33 (1%) |
| Leibovich 2018 risk categories | ||
| Group 1 | 2340 (85%) | 2343 (85%) |
| Group 2 | 368 (13%) | 367 (13%) |
| Group 3 | 53 (2%) | 51 (2%) |
| GRANT risk categories | ||
| Favourable | 2251 (82%) | 2199 (80%) |
| Unfavourable | 510 (18%) | 562 (20%) |
| Models Tested | Hazard Ratio | 95% CI | p-Value | External Validation | |
|---|---|---|---|---|---|
| 5-Year c-Index | 10-Year c-Index | ||||
| Leibovich 2018 risk categories | 0.68 | 0.65 | |||
| Group 1 | Ref | ||||
| Group 2 | 3.3 | (2.3–4.7) | <0.001 | ||
| Group 3 | 17.0 | (10.6–27.5) | <0.001 | ||
| GRANT risk categories | 0.64 | 0.64 | |||
| Favourable | Ref | ||||
| Unfavourable | 3.0 | (2.2–4.2) | <0.001 | ||
| Univariable | Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables Tested | HR | 95% CI | p-Value | Internal Validation 5-Year c-Index | Internal Validation 10-Year c-Index | HR | 95% CI | p-Value | External Validation 5-Year c-Index | External Validation 5-Year c-Index |
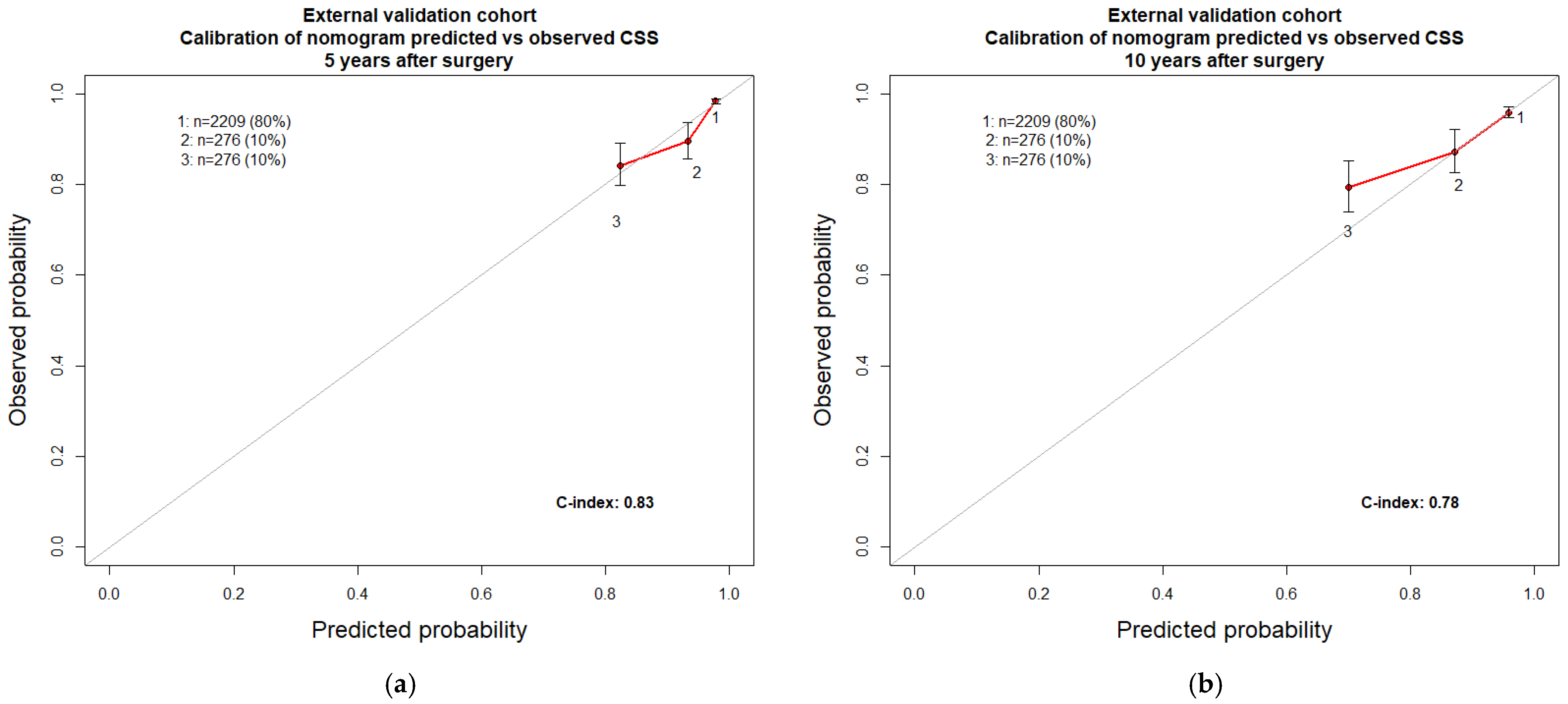
| Age at surgery (years) | 1.05 | (1.04–1.06) | <0.001 | 0.65 | 0.69 | 1.06 | (1.05–1.08) | <0.001 | 0.83 | 0.78 |
| T stage | 0.65 | 0.62 | ||||||||
| T1-2 | Ref | Ref | ||||||||
| T3-4 | 3.4 | (2.5–4.6) | <0.001 | 2.3 | (1.6–3.1) | <0.001 | ||||
| Tumour size (mm) | 1.01 | (1.01–1.02) | <0.001 | 0.70 | 0.64 | |||||
| N stage | 0.54 | 0.54 | ||||||||
| N0-X | Ref | Ref | ||||||||
| N1 | 10.8 | (5.9–20.0) | <0.001 | 6.6 | (3.4–12.6) | <0.001 | ||||
| Sex | 0.52 | 0.51 | ||||||||
| Male | Ref | |||||||||
| Female | 0.8 | (0.6–1.1) | 0.2 | |||||||
| Race/ethnicity | 0.51 | 0.55 | ||||||||
| Caucasian | Ref | |||||||||
| African American | 1.6 | (1.1–2.4) | 0.02 | |||||||
| Hispanic | 0.9 | (0.6–1.5) | 0.7 | |||||||
| Asian or Pacific Islander | 0.96 | (0.5–2.1) | 0.9 | |||||||
| Grade | 0.61 | 0.59 | ||||||||
| G1 | Ref | |||||||||
| G2 | 0.7 | (0.4–1.3) | 0.3 | |||||||
| G3 | 1.0 | (0.5–1.8) | 0.99 | |||||||
| G4 | 3.0 | (1.5–5.9) | 0.001 | |||||||
| Sarcomatoid features | 0.54 | 0.56 | ||||||||
| No | Ref | |||||||||
| Yes | 14.0 | (7.5–26.1) | <0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccinelli, M.L.; Morra, S.; Tappero, S.; Cano Garcia, C.; Barletta, F.; Incesu, R.-B.; Scheipner, L.; Baudo, A.; Tian, Z.; Luzzago, S.; et al. Critical Appraisal of Leibovich 2018 and GRANT Models for Prediction of Cancer-Specific Survival in Non-Metastatic Chromophobe Renal Cell Carcinoma. Cancers 2023, 15, 2155. https://doi.org/10.3390/cancers15072155
Piccinelli ML, Morra S, Tappero S, Cano Garcia C, Barletta F, Incesu R-B, Scheipner L, Baudo A, Tian Z, Luzzago S, et al. Critical Appraisal of Leibovich 2018 and GRANT Models for Prediction of Cancer-Specific Survival in Non-Metastatic Chromophobe Renal Cell Carcinoma. Cancers. 2023; 15(7):2155. https://doi.org/10.3390/cancers15072155
Chicago/Turabian StylePiccinelli, Mattia Luca, Simone Morra, Stefano Tappero, Cristina Cano Garcia, Francesco Barletta, Reha-Baris Incesu, Lukas Scheipner, Andrea Baudo, Zhe Tian, Stefano Luzzago, and et al. 2023. "Critical Appraisal of Leibovich 2018 and GRANT Models for Prediction of Cancer-Specific Survival in Non-Metastatic Chromophobe Renal Cell Carcinoma" Cancers 15, no. 7: 2155. https://doi.org/10.3390/cancers15072155
APA StylePiccinelli, M. L., Morra, S., Tappero, S., Cano Garcia, C., Barletta, F., Incesu, R.-B., Scheipner, L., Baudo, A., Tian, Z., Luzzago, S., Mistretta, F. A., Ferro, M., Saad, F., Shariat, S. F., Carmignani, L., Ahyai, S., Tilki, D., Briganti, A., Chun, F. K. H., ... Karakiewicz, P. I. (2023). Critical Appraisal of Leibovich 2018 and GRANT Models for Prediction of Cancer-Specific Survival in Non-Metastatic Chromophobe Renal Cell Carcinoma. Cancers, 15(7), 2155. https://doi.org/10.3390/cancers15072155








