The Role of Molecular Imaging in Patients with Brain Metastases: A Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. [18F]-FDG
4. Amino Acid Radiotracers
4.1. [11C]-MET
4.2. [18F]-FET
4.3. [18F]-DOPA
5. Other Radiotracers
6. Future Perspectives
6.1. Radiomics
6.2. Therapy with Immune Checkpoint Inhibitors
6.3. Theranostics
6.4. Novel Targeted Therapies and PET Imaging
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brindle, K.M.; Izquierdo-García, J.L.; Lewis, D.Y.; Mair, R.J.; Wright, A.J. Brain Tumor Imaging. J. Clin. Oncol. 2017, 35, 2432–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiesel, B.; Thomé, C.M.; Weiss, T.; Jakola, A.S.; Darlix, A.; Pellerino, A.; Furtner, J.; Kerschbaumer, J.; Freyschlag, C.F.; Wick, W.; et al. Perioperative Imaging in Patients Treated with Resection of Brain Metastases: A Survey by the European Association of Neuro-Oncology (EANO) Youngsters Committee. BMC Cancer 2020, 20, 410. [Google Scholar] [CrossRef] [PubMed]
- Putz, F.; Mengling, V.; Perrin, R.; Masitho, S.; Weissmann, T.; Rösch, J.; Bäuerle, T.; Janka, R.; Cavallaro, A.; Uder, M.; et al. Magnetic Resonance Imaging for Brain Stereotactic Radiotherapy: A Review of Requirements and Pitfalls. Strahlenther. Onkol. 2020, 196, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response Assessment Criteria for Brain Metastases: Proposal from the RANO Group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of Brain Tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Lausch, A.; Yeung, T.P.; Chen, J.; Law, E.; Wang, Y.; Urbini, B.; Donelli, F.; Manco, L.; Fainardi, E.; Lee, T.Y.; et al. A generalized parametric response mapping method for analysis of multi-parametric imaging: A feasibility study with application to glioblastoma. Med. Phys. 2017, 44, 6074–6084. [Google Scholar] [CrossRef]
- Derks, S.H.A.E.; van der Veldt, A.A.M.; Smits, M. Brain Metastases: The Role of Clinical Imaging. Br. J. Radiol. 2022, 95, 20210944. [Google Scholar] [CrossRef]
- Malani, R.; Bhatia, A.; Wolfe, J.; Grommes, C. Staging Identifies Non-CNS Malignancies in a Large Cohort with Newly Diagnosed Lymphomatous Brain Lesions. Leuk. Lymphoma 2019, 60, 2278–2282. [Google Scholar] [CrossRef]
- Krebs, S.; Mauguen, A.; Yildirim, O.; Hatzoglou, V.; Francis, J.H.; Schaff, L.R.; Mellinghoff, I.K.; Schöder, H.; Grommes, C. Prognostic Value of [(18)F]FDG PET/CT in Patients with CNS Lymphoma Receiving Ibrutinib-Based Therapies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3940–3950. [Google Scholar] [CrossRef]
- Werner, J.-M.; Lohmann, P.; Fink, G.R.; Langen, K.-J.; Galldiks, N. Current Landscape and Emerging Fields of PET Imaging in Patients with Brain Tumors. Molecules 2020, 25, 1471. [Google Scholar] [CrossRef] [Green Version]
- Langen, K.J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Castellani, M.; Florimonte, L.; Ciccariello, G.; Mansi, L.; Lopci, E. PET radiotracers in glioma: A review of clinical indications and evidence. Clin. Transl. Imaging 2022, 10, 535–551. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. NeuroOncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Habert, M.-O.; Rozenblum, L. Contribution of PET-MRI in Brain Diseases in Clinical Practice. Curr. Opin. Neurol. 2020, 33, 430–438. [Google Scholar] [CrossRef]
- Krüger, S.; Mottaghy, F.M.; Buck, A.K.; Maschke, S.; Kley, H.; Frechen, D.; Wibmer, T.; Reske, S.N.; Pauls, S. Brain Metastasis in Lung Cancer. Comparison of Cerebral MRI and 18F-FDG-PET/CT for Diagnosis in the Initial Staging. Nuklearmedizin-NuclearMedicine 2011, 50, 101–106. [Google Scholar] [CrossRef]
- Bochev, P.; Klisarova, A.; Kaprelyan, A.; Chaushev, B.; Dancheva, Z. Brain Metastases Detectability of Routine Whole Body (18)F-FDG PET and Low Dose CT Scanning in 2502 Asymptomatic Patients with Solid Extracranial Tumors. Hell. J. Nucl. Med. 2012, 15, 125–129. [Google Scholar] [CrossRef]
- Manohar, K.; Bhattacharya, A.; Mittal, B.R. Low Positive Yield from Routine Inclusion of the Brain in Whole-Body 18F-FDG PET/CT Imaging for Noncerebral Malignancies: Results from a Large Population Study. Nucl. Med. Commun. 2013, 34, 540–543. [Google Scholar] [CrossRef]
- Nia, E.S.; Garland, L.L.; Eshghi, N.; Nia, B.B.; Avery, R.J.; Kuo, P.H. Incidence of Brain Metastases on Follow-up (18)F-FDG PET/CT Scans of Non-Small Cell Lung Cancer Patients: Should We Include the Brain? J. Nucl. Med. Technol. 2017, 45, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Saito, G.; Kono, M.; Koyanagi, Y.; Miyashita, K.; Tsutsumi, A.; Kobayashi, T.; Miki, Y.; Hashimoto, D.; Nakamura, T.; Nozue, M.; et al. Significance of Brain Imaging for Staging in Patients with Clinical Stage T1-2 N0 Non-Small-Cell Lung Cancer on Positron Emission Tomography/Computed Tomography. Clin. Lung Cancer 2021, 22, 562–569. [Google Scholar] [CrossRef]
- Li, Y.; Jin, G.; Su, D. Comparison of Gadolinium-Enhanced MRI and 18FDG PET/PET-CT for the Diagnosis of Brain Metastases in Lung Cancer Patients: A Meta-Analysis of 5 Prospective Studies. Oncotarget 2017, 8, 35743–35749. [Google Scholar] [CrossRef] [Green Version]
- Oldan, J.D.; Glaubiger, S.A.; Khandani, A.H.; Jewells, V.L. Detectable Size of Melanoma Metastases to Brain on PET/CT. Ann. Nucl. Med. 2020, 34, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chung, J.-K.; Jeong, J.M.; Lee, D.S.; Kim, D.G.; Jung, H.W.; Lee, M.C. Comparison of FDG-PET Findings of Brain Metastasis from Non-Small-Cell Lung Cancer and Small-Cell Lung Cancer. Ann. Nucl. Med. 2008, 22, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Meric, K.; Killeen, R.P.; Abi-Ghanem, A.S.; Soliman, F.; Novruzov, F.; Cakan, E.; Cayci, Z. The Use of 18F-FDG PET Ratios in the Differential Diagnosis of Common Malignant Brain Tumors. Clin. Imaging 2015, 39, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Purandare, N.C.; Puranik, A.; Shah, S.; Agrawal, A.; Gupta, T.; Moiyadi, A.; Shetty, P.; Shridhar, E.; Jalali, R.; Rangarajan, V. Common Malignant Brain Tumors: Can 18F-FDG PET/CT Aid in Differentiation? Nucl. Med. Commun. 2017, 38, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Boethius, J.; Ericson, K. FDG-PET on Irradiated Brain Tumor: Ten Years’ Summary. Acta Radiol. 2006, 47, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Torrens, M.; Malamitsi, J.; Karaiskos, P.; Valotassiou, V.; Laspas, F.; Andreou, J.; Stergiou, C.; Prassopoulos, V. Although Non-Diagnostic Between Necrosis and Recurrence, FDG PET/CT Assists Management of Brain Tumours After Radiosurgery. In Vivo 2016, 30, 513–520. [Google Scholar] [PubMed]
- Horky, L.L.; Hsiao, E.M.; Weiss, S.E.; Drappatz, J.; Gerbaudo, V.H. Dual Phase FDG-PET Imaging of Brain Metastases Provides Superior Assessment of Recurrence versus Post-Treatment Necrosis. J. Neurooncol. 2011, 103, 137–146. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A Prospective Trial of Dynamic Contrast-Enhanced MRI Perfusion and Fluorine-18 FDG PET-CT in Differentiating Brain Tumor Progression from Radiation Injury after Cranial Irradiation. Neuro. Oncol. 2016, 18, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Salinas, C.; Muttikkal, T.J.E.; Flors, L.; Puig, J.; Wintermark, M.; Patrie, J.T.; Rehm, P.K.; Sheehan, J.P.; Schiff, D. FDG PET/MRI Coregistration Helps Predict Response to Gamma Knife Radiosurgery in Patients with Brain Metastases. AJR Am. J. Roentgenol. 2019, 212, 425–430. [Google Scholar] [CrossRef]
- Stockham, A.L.; Tievsky, A.L.; Koyfman, S.A.; Reddy, C.A.; Suh, J.H.; Vogelbaum, M.A.; Barnett, G.H.; Chao, S.T. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J. Neurooncol. 2012, 109, 149–158. [Google Scholar] [CrossRef]
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE 2016, 11, e0157139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamimoto, R.; Saginoya, T.; Kondo, C.; Tomura, N.; Ito, K.; Matsuo, Y.; Matsunaga, S.; Shuto, T.; Akabane, A.; Miyata, Y.; et al. Differentiation of Brain Tumor Recurrence from Post-Radiotherapy Necrosis with 11C-Methionine PET: Visual Assessment versus Quantitative Assessment. PLoS ONE 2015, 10, e0132515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaerts, C.W.; van Dijken, B.R.; Stormezand, G.N.; van der Weide, H.L.; Wagemakers, M.; Enting, R.H.; van der Hoorn, A. (11)C-Methyl-L-Methionine PET Measuring Parameters for the Diagnosis of Tumour Progression against Radiation-Induced Changes in Brain Metastases. Br. J. Radiol. 2021, 94, 20210275. [Google Scholar] [CrossRef] [PubMed]
- Yomo, S.; Oguchi, K. Prospective Study of (11)C-Methionine PET for Distinguishing between Recurrent Brain Metastases and Radiation Necrosis: Limitations of Diagnostic Accuracy and Long-Term Results of Salvage Treatment. BMC Cancer 2017, 17, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, M.; Miwa, K.; Shinoda, J.; Kako, N.; Nishibori, H.; Sakurai, K.; Yano, H.; Iwama, T.; Kanematsu, M. Target Definition by C11-Methionine-PET for the Radiotherapy of Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Nariai, T.; Kawabe, T.; Inaji, M.; Tanaka, Y.; Watanabe, S.; Maehara, T.; Oda, K.; Ishii, K.; Ishiwata, K.; et al. Clinical Benefit of 11C Methionine PET Imaging as a Planning Modality for Radiosurgery of Previously Irradiated Recurrent Brain Metastases. Clin. Nucl. Med. 2014, 39, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Rottenburger, C.; Hentschel, M.; Kelly, T.; Trippel, M.; Brink, I.; Reithmeier, T.; Meyer, P.T.; Nikkhah, G. Comparison of C-11 Methionine and C-11 Choline for PET Imaging of Brain Metastases: A Prospective Pilot Study. Clin. Nucl. Med. 2011, 36, 639–642. [Google Scholar] [CrossRef]
- Tran, T.T.; Gallezot, J.-D.; Jilaveanu, L.B.; Zito, C.; Turcu, G.; Lim, K.; Nabulsi, N.; Huang, H.; Huttner, A.; Kluger, H.M.; et al. [(11)C]Methionine and [(11)C]PBR28 as PET Imaging Tracers to Differentiate Metastatic Tumor Recurrence or Radiation Necrosis. Mol. Imaging 2020, 19, 1536012120968669. [Google Scholar] [CrossRef]
- Cicuendez, M.; Lorenzo-Bosquet, C.; Cuberas-Borrós, G.; Martinez-Ricarte, F.; Cordero, E.; Martinez-Saez, E.; Castell-Conesa, J.; Sahuquillo, J. Role of [(11)C] Methionine Positron Emission Tomography in the Diagnosis and Prediction of Survival in Brain Tumours. Clin. Neurol. Neurosurg. 2015, 139, 328–333. [Google Scholar] [CrossRef]
- Lahoutte, T.; Caveliers, V.; Camargo, S.M.R.; Franca, R.; Ramadan, T.; Veljkovic, E.; Mertens, J.; Bossuyt, A.; Verrey, F. SPECT and PET Amino Acid Tracer Influx via System L (H4F2hc-HLAT1) and Its Transstimulation. J. Nucl. Med. 2004, 45, 1591–1596. [Google Scholar]
- Wang, L.; Lieberman, B.P.; Ploessl, K.; Kung, H.F. Synthesis and Evaluation of 18F Labeled FET Prodrugs for Tumor Imaging. Nucl. Med. Biol. 2014, 41, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.-C.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.-J.; Albert, N.L. (18)F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2017, 58, 584–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-(18)F-Fluoroethyl)-L-Tyrosine PET for Differentiation of Local Recurrent Brain Metastasis from Radiation Necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.P.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.I.; Kocher, M.; et al. Dynamic O-(2-18F-Fluoroethyl)-L-Tyrosine Positron Emission Tomography Differentiates Brain Metastasis Recurrence from Radiation Injury after Radiotherapy. Neuro. Oncol. 2017, 19, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Kebir, S.; Rauschenbach, L.; Galldiks, N.; Schlaak, M.; Hattingen, E.; Landsberg, J.; Bundschuh, R.A.; Langen, K.-J.; Scheffler, B.; Herrlinger, U.; et al. Dynamic O-(2-[18F]Fluoroethyl)-L-Tyrosine PET Imaging for the Detection of Checkpoint Inhibitor-Related Pseudoprogression in Melanoma Brain Metastases. Neuro. Oncol. 2016, 18, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Romagna, A.; Unterrainer, M.; Schmid-Tannwald, C.; Brendel, M.; Tonn, J.-C.; Nachbichler, S.B.; Muacevic, A.; Bartenstein, P.; Kreth, F.-W.; Albert, N.L. Suspected Recurrence of Brain Metastases after Focused High Dose Radiotherapy: Can [(18)F]FET- PET Overcome Diagnostic Uncertainties? Radiat. Oncol. 2016, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Grosu, A.-L.; Astner, S.T.; Riedel, E.; Nieder, C.; Wiedenmann, N.; Heinemann, F.; Schwaiger, M.; Molls, M.; Wester, H.-J.; Weber, W.A. An Interindividual Comparison of O-(2-[18F]Fluoroethyl)-L-Tyrosine (FET)- and L-[Methyl-11C]Methionine (MET)-PET in Patients with Brain Gliomas and Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1049–1058. [Google Scholar] [CrossRef]
- Gempt, J.; Bette, S.; Buchmann, N.; Ryang, Y.-M.; Förschler, A.; Pyka, T.; Wester, H.-J.; Förster, S.; Meyer, B.; Ringel, F. Volumetric Analysis of F-18-FET-PET Imaging for Brain Metastases. World Neurosurg. 2015, 84, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The Role of LAT1 in (18)F-DOPA Uptake in Malignant Gliomas. J. Neurooncol. 2013, 111, 11–18. [Google Scholar] [CrossRef]
- Lizarraga, K.J.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.A.F.; Yong, W.H.; Phelps, M.E.; Chen, W. (18)F-FDOPA PET for Differentiating Recurrent or Progressive Brain Metastatic Tumors from Late or Delayed Radiation Injury after Radiation Treatment. J. Nucl. Med. 2014, 55, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and Perfusion-MRI for Differentiating Radionecrotic from Progressive Brain Metastases after Radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Carideo, L.; Scaringi, C.; Romano, A.; Mamede, M.; Papa, A.; Tofani, A.; Cascini, G.L.; Bozzao, A.; Scopinaro, F.; et al. Long-Term Metabolic Evolution of Brain Metastases with Suspected Radiation Necrosis Following Stereotactic Radiosurgery: Longitudinal Assessment by F-DOPA PET. Neuro. Oncol. 2021, 23, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Bourg, V.; Mondot, L.; Gal, J.; Bondiau, P.-Y.; Fontaine, D.; Barriere, J.; Saada-Bouzid, E.; Paquet, M.; Chardin, D.; et al. Correction to: (18)F-DOPA PET/CT in Brain Tumors: Impact on Multidisciplinary Brain Tumor Board Decisions. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamson, D.O.; Mittal, S.; Buth, A.; Muzik, O.; Kupsky, W.J.; Robinette, N.L.; Barger, G.R.; Juhász, C. Differentiation of Glioblastomas from Metastatic Brain Tumors by Tryptophan Uptake and Kinetic Analysis: A Positron Emission Tomographic Study with Magnetic Resonance Imaging Comparison. Mol. Imaging 2013, 12, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, H.; Liu, F.; Zhang, Y.; Yang, J.; Zhang, L.; Zhu, L.; Li, N.; Kung, H.F.; Yang, Z. Imaging Brain Metastasis Patients with 18F-(2S,4 R)-4-Fluoroglutamine. Clin. Nucl. Med. 2018, 43, e392–e399. [Google Scholar] [CrossRef]
- Yu, C.; Pan, D.; Mi, B.; Xu, Y.; Lang, L.; Niu, G.; Yang, M.; Wan, W.; Chen, X. (18)F-Alfatide II PET/CT in Healthy Human Volunteers and Patients with Brain Metastases. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2021–2028. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, E.; Lazzeri, P.; Milano, A.; Gaeta, M.C.; Ciarmiello, A. Clinical Applications of Choline PET/CT in Brain Tumors. Curr. Pharm. Des. 2015, 21, 121–127. [Google Scholar] [CrossRef]
- Grkovski, M.; Kohutek, Z.A.; Schöder, H.; Brennan, C.W.; Tabar, V.S.; Gutin, P.H.; Zhang, Z.; Young, R.J.; Beattie, B.J.; Zanzonico, P.B.; et al. (18)F-Fluorocholine PET Uptake Correlates with Pathologic Evidence of Recurrent Tumor after Stereotactic Radiosurgery for Brain Metastases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1446–1457. [Google Scholar] [CrossRef]
- Morikawa, A.; Grkovski, M.; Patil, S.; Jhaveri, K.L.; Tang, K.; Humm, J.L.; Holodny, A.; Beal, K.; Schöder, H.; Seidman, A.D. A Phase I Trial of Sorafenib with Whole Brain Radiotherapy (WBRT) in Breast Cancer Patients with Brain Metastases and a Correlative Study of FLT-PET Brain Imaging. Breast Cancer Res. Treat. 2021, 188, 415–425. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Lindenberg, M.; Bryla, C.; Patronas, N.; Peer, C.J.; Amiri-Kordestani, L.; Davarpanah, N.; Gonzalez, E.M.; Burotto, M.; Choyke, P.; et al. ANG1005 for breast cancer brain metastases: Correlation between 18F-FLT-PET after first cycle and MRI in response assessment. Breast Cancer Res. Treat. 2016, 160, 51–59. [Google Scholar]
- Allen, A.M.; Ben-Ami, M.; Reshef, A.; Steinmetz, A.; Kundel, Y.; Inbar, E.; Djaldetti, R.; Davidson, T.; Fenig, E.; Ziv, I. Assessment of Response of Brain Metastases to Radiotherapy by PET Imaging of Apoptosis with 18F-ML-10. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1400–1408. [Google Scholar] [CrossRef]
- Øen, S.K.; Johannessen, K.; Pedersen, L.K.; Berntsen, E.M.; Totland, J.A.; Johansen, H.; Bogsrud, T.V.; Solheim, T.S.; Karlberg, A.; Eikenes, L. Diagnostic Value of 18 F-FACBC PET/MRI in Brain Metastases. Clin. Nucl. Med. 2022, 47, 1030–1039. [Google Scholar]
- O’Brien, S.R.; Edmonds, C.E.; Katz, D.; Mankoff, D.A.; Pantel, A.R. 18F-Fluoroestradiol (FES) PET/CT: Review of current practice and future directions. Clin. Transl. Imaging 2022, 10, 331–341. [Google Scholar]
- Lee, Y.; Yoo, I.R.; Ha, S. 18F-FES PET/CT for Characterization of Brain and Leptomeningeal Metastasis in Double Primary Cancer Patient. Clin. Nucl. Med. 2022, 47, e554–e556. [Google Scholar] [PubMed]
- Ulaner, G.A.; Mankoff, D.A.; Clark, A.S.; Fowler, A.M.; Linden, H.M.; Peterson, L.M.; Dehdashti, F.; Kurland, B.F.; Mortimer, J.; Mouabbi, J.; et al. Summary: Appropriate Use Criteria for Estrogen Receptor-Targeted PET Imaging with 16α-18F-Fluoro-17β-Fluoroestradiol. J. Nucl. Med. 2023, 64, 351–354. [Google Scholar] [PubMed]
- Iqbal, R.; Yaqub, M.; Bektas, H.O.; Oprea-Lager, D.E.; de Vries, E.G.E.; Glaudemans, A.W.; Aftimos, P.; Gebhart, G.; Beelen, A.P.; Schuit, R.C.; et al. [18F]FDG and [18F]FES PET/CT Imaging as a Biomarker for Therapy Effect in Patients with Metastatic ER+ Breast Cancer Undergoing Treatment with Rintodestrant. Clin. Cancer Res. 2023, CCR-22-2720. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Castello, A.; Castellani, M.; Florimonte, L.; Urso, L.; Mansi, L.; Lopci, E. The Role of Radiomics in the Era of Immune Checkpoint Inhibitors: A New Protagonist in the Jungle of Response Criteria. J. Clin. Med. 2022, 11, 1740. [Google Scholar] [CrossRef]
- Peng, L.; Parekh, V.; Huang, P.; Lin, D.D.; Sheikh, K.; Baker, B.; Kirschbaum, T.; Silvestri, F.; Son, J.; Robinson, A.; et al. Distinguishing True Progression from Radionecrosis After Stereotactic Radiation Therapy for Brain Metastases With Machine Learning and Radiomics. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1236–1243. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ruge, M.I.; Visser-Vandewalle, V.; Shah, N.J.; Fink, G.R.; Langen, K.-J.; Galldiks, N. PET/MRI Radiomics in Patients with Brain Metastases. Front. Neurol. 2020, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Tan, D.; Liu, Z.; Liao, M.; Kan, Y.; Yao, R.; Zhang, L.; Nie, L.; Liao, R.; Chen, S.; et al. Differentiating Solitary Brain Metastases from Glioblastoma by Radiomics Features Derived from MRI and 18F-FDG-PET and the Combined Application of Multiple Models. Sci. Rep. 2022, 12, 5722. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ceccon, G.; Bauer, E.K.; Stoffels, G.; Viswanathan, S.; Ruge, M.I.; Neumaier, B.; Shah, N.J.; Fink, G.R.; et al. Combined FET PET/MRI Radiomics Differentiates Radiation Injury from Recurrent Brain Metastasis. NeuroImage Clin. 2018, 20, 537–542. [Google Scholar] [CrossRef]
- Lohmann, P.; Stoffels, G.; Ceccon, G.; Rapp, M.; Sabel, M.; Filss, C.P.; Kamp, M.A.; Stegmayr, C.; Neumaier, B.; Shah, N.J.; et al. Radiation Injury vs. Recurrent Brain Metastasis: Combining Textural Feature Radiomics Analysis and Standard Parameters May Increase (18)F-FET PET Accuracy without Dynamic Scans. Eur. Radiol. 2017, 27, 2916–2927. [Google Scholar] [CrossRef]
- Stefano, A.; Comelli, A.; Bravatà, V.; Barone, S.; Daskalovski, I.; Savoca, G.; Sabini, M.G.; Ippolito, M.; Russo, G. A Preliminary PET Radiomics Study of Brain Metastases Using a Fully Automatic Segmentation Method. BMC Bioinform. 2020, 21, 325. [Google Scholar] [CrossRef]
- Akhoundova, D.; Hiltbrunner, S.; Mader, C.; Förster, R.; Kraft, J.; Schwanhäusser, B.; Bankel, L.; Kollias, S.; Treyer, V.; Rushing, E.J.; et al. 18F-FET PET for Diagnosis of Pseudoprogression of Brain Metastases in Patients with Non-Small Cell Lung Cancer. Clin. Nucl. Med. 2020, 45, 113–117. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, D.; Ehlerding, E.B.; Luo, Q.; Cai, W. Noninvasive PET Imaging of T cells. Trends Cancer 2018, 4, 359–373. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, F.; Timens, W.; Brouwers, A.H.; et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: A phase 1/2 trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef]
- Schwenck, J.; Tabatabai, G.; Skardelly, M.; Reischl, G.; Beschorner, R.; Pichler, B.; La Fougère, C. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 170–171. [Google Scholar] [CrossRef]
- Kasoha, M.; Unger, C.; Solomayer, E.-F.; Bohle, R.M.; Zaharia, C.; Khreich, F.; Wagenpfeil, S.; Juhasz-Böss, I. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin. Exp. Metastasis 2017, 34, 479–490. [Google Scholar] [CrossRef]
- Kelly, W.J.; Shah, N.J.; Subramaniam, D.S. Management of Brain Metastases in Epidermal Growth Factor Receptor Mutant Non-Small-Cell Lung Cancer. Front. Oncol. 2018, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Weber, B.; Winterdahl, M.; Memon, A.; Sorensen, B.S.; Keiding, S.; Sorensen, L.; Nexo, E.; Meldgaard, P. Erlotinib accumulation in brain metastases fromnon-small cell lung cancer: Visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J. Thorac. Oncol. 2011, 6, 1287–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Hu, Y.; Liu, W.; Chen, L.; Zhao, Y.; Ma, H.; Yang, J.; Yang, Y.; Liao, J.; Cai, J.; et al. A radiopharmaceutical [(89)Zr]Zr-DFO-nimotuzumab for immunoPET with epidermal growth factor receptor expression in vivo. Nucl. Med. Biol. 2019, 70, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cai, L.; Zhang, K.; Zhang, A.; Pu, P.; Yang, W.; Gao, S. A pilot study on EGFR-targeted molecular imaging of PET/CT With 11C-PD153035 in human gliomas. Clin. Nucl. Med. 2014, 39, e20–e26. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [Green Version]
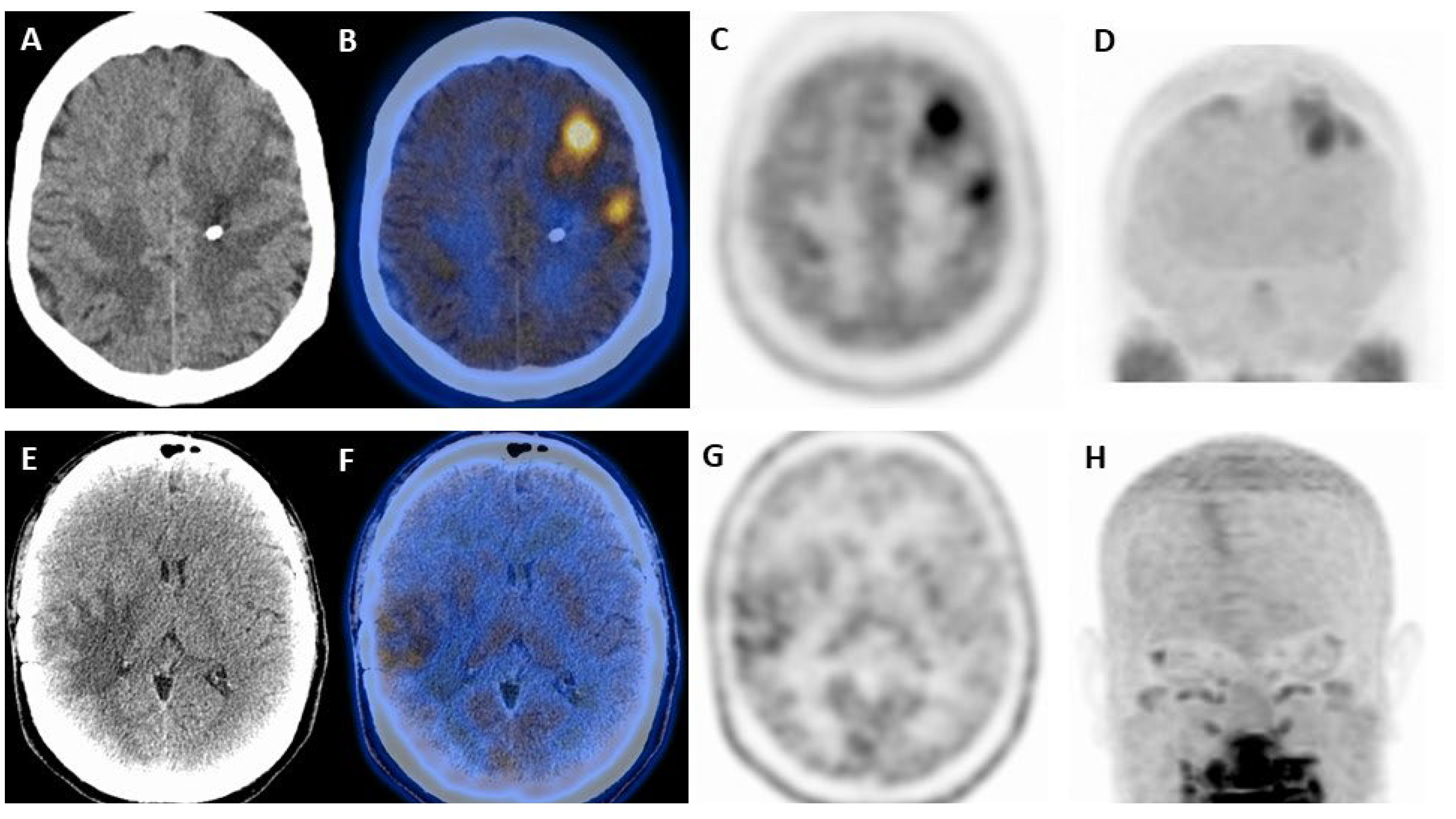
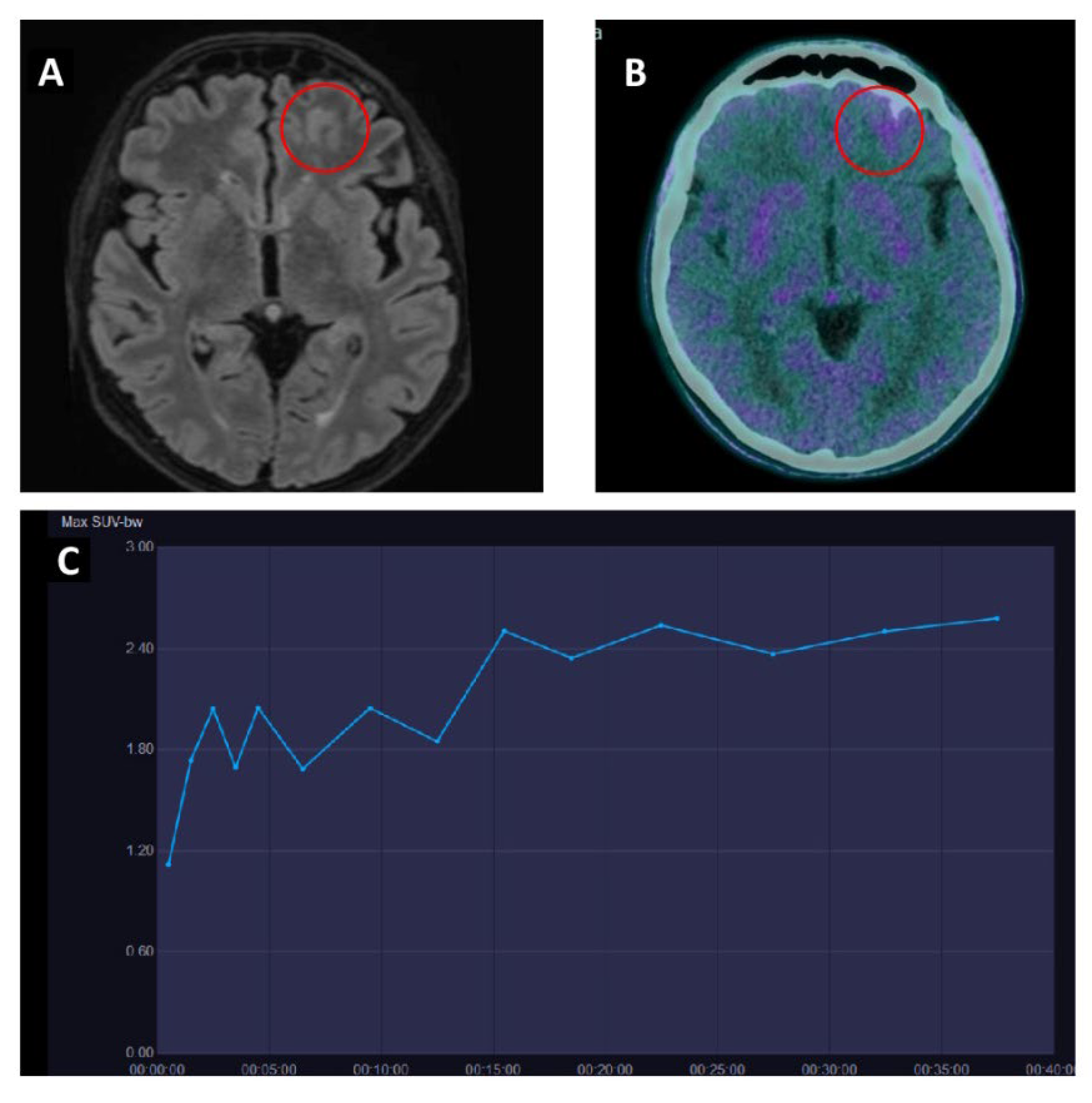
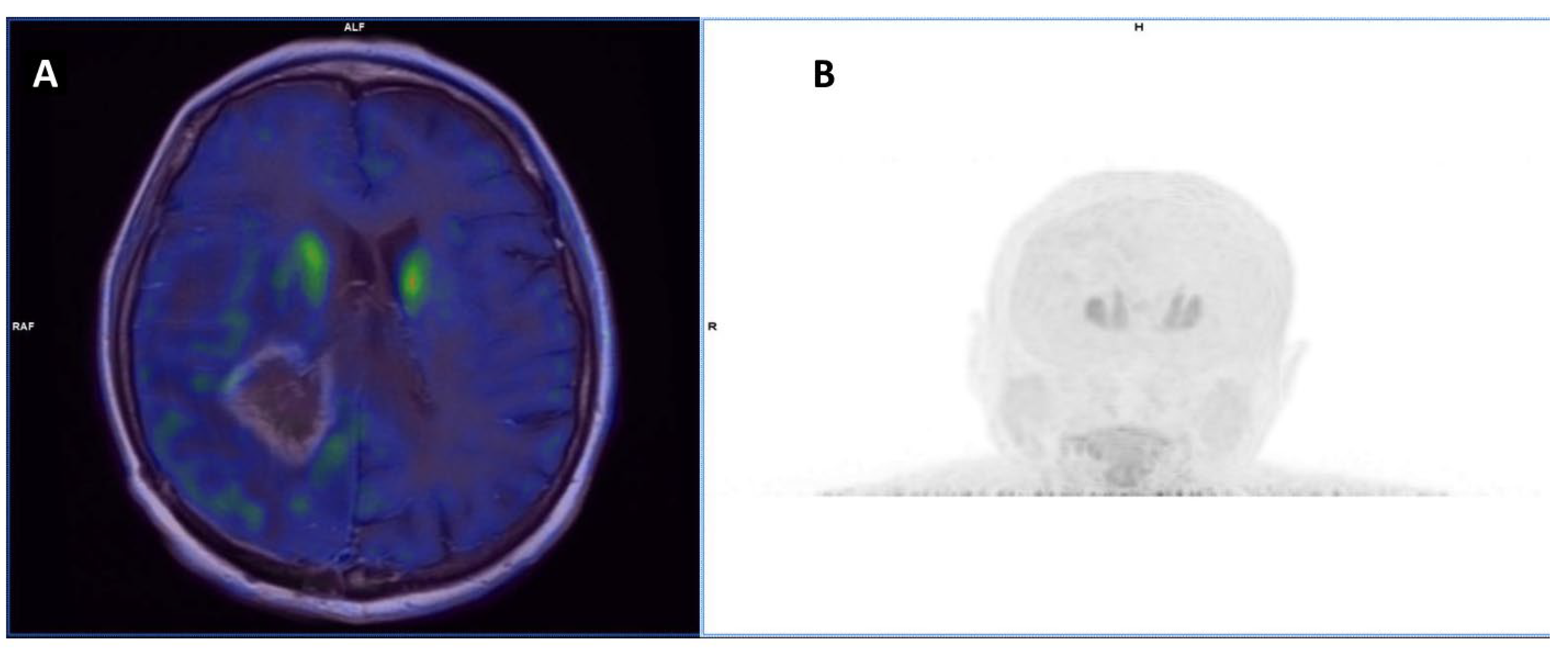
| Authors | Year | Study Design | Primary Malignancy | Patients M/F | Aim | Comments |
|---|---|---|---|---|---|---|
| Krüger et al. [15] | 2011 | P | Lung cancer | 104 (77/27) | To compare MRI and PET/CT for diagnosis BM | PET/CT showed a sensitivity of 27%, with a high number of false positive for BM |
| Bochev et al. [16] | 2012 | P | Solid neoplasms | 2502 (NR) | To assess the role of PET/CT for detecting BM | PET/CT detected BM in 1% of all patients |
| Manohar et al. [17] | 2013 | R | Solid neoplasms | 5110 (3322/1788) | To assess the role of PET/CT for detecting BM | PET/CT detected BM in only 0.7% of cases |
| Nia et al. [18] | 2017 | R | NSCLC | 227 (NR) | To assess the role of follow-up PET/CT for detecting BM | Only 5/227 patients were found to have BM |
| Saito et al. [19] | 2021 | R | T1-T2 N0 NSCLC | 466 (272/194) | To assess the frequency of BM | Screening of brain by PET/CT is unnecessary in patients with early stage NSCLC |
| Li et al. [20] | 2017 | Meta-analysis | Lung cancer | 941 (NR) | To compare MRI and PET/CT for diagnosis BM | Gadolinium-enhanced MRI had higher sensitivity than PET/CT |
| Oldan et al. [21] | 2020 | R | Melanoma | 212 (NR) | To evaluate at what size BM are detectable by PET/CT | Lesions over about 2 cm were detectable by PET/CT |
| Lee et al. [22] | 2008 | R | Lung cancer | 48 (31/17) | To compare FDG uptake between NSCLC and SCLC BM | NSCLC BM were more frequently hypermetabolic than those from SCLC |
| Meric et al. [23] | 2015 | R | BM, CNS lymphomas, gliomas | 76 (37/39) | To characterize the nature of brain masses | SUVmax and Tmax:Wmimax seem useful parameters to discriminate brain masses |
| Purandare et al. [24] | 2017 | R | GBM, CNS lymphoma, BM | 106 (70/36) | To characterize the nature of brain masses | CNS lymphomas showed higher metabolic activity than GBM and BM |
| Wang et al. [25] | 2006 | R | BM, gliomas | 117 (58/59) | To differentiate between recurrence and radionecrosis | PET/CT demonstrated: PPV 96% NPV 56% |
| Torrens et al. [26] | 2016 | R | BM, glioblastoma | 16 (11/5) | To differentiate between recurrence and radionecrosis co-registering PET/CT and MRI | PET/MRI co-registration determined: Sensitivity 65% Specificity 100% |
| Horky et al. [27] | 2011 | R | Solid neoplasms | 32 (10/22) | Dual phase PET/CT to differentiate recurrence from radionecrosis | Variation of L/GM > 0.19 between early and delayed: Sensitivity 955% Specificity 100% Accuracy 96% |
| Hatzoglou et al. [28] | 2016 | P | BM, gliomas | 53 (35/18) | To differentiate between recurrence and radionecrosis using PET/CT and DCE MRI | Vp ratio = 2.1 showed highest accuracy: Sensitivity 92% Specificity 77% |
| Leiva-Salinas et al. [29] | 2019 | R | BM | 85 (37/48) | To determinate if PET/MRI predicts recurrence after radiosurgery | Relative SUV = 1.75: Sensitivity 87% Specificity 32% |
| Authors | Year | Study Design | RP | Primary Malignancy | Patients M/F | Aim | Comments |
|---|---|---|---|---|---|---|---|
| Minamoto et al. [32] | 2015 | R | [11C]-MET | BM and gliomas | 70 (38/32) | To differentiate between recurrence and radionecrosis | Visual analysis was comparable to quantitative assessment by L/Nmax and L/Nmean |
| Govaerts et al. [33] | 2021 | R | [11C]-MET | Solid neoplasms | 26 (13/13) | To differentiate between recurrence and radionecrosis | SUVmax of 3.9 was the best parameter: AUC = 0.834 sensitivity 78.6% specificity 70.6% PPV 74.3% NPV 75.3% |
| Yomo et al. [34] | 2017 | P | [11C]-MET | Solid neoplasms | 32 (19/13) | To differentiate between recurrence and radionecrosis | LNR of 1.40 showed: AUC 0.84 sensitivity 82% specificity 75% |
| Matsuo et al. [35] | 2009 | P | [11C]-MET | Solid neoplasms | 19 (14/5) | To delineate and to compare target volumes with MRI | Tumor volume on PET imaging was significantly larger than that on MRI for lesions >0.5 mL |
| Momose et al. [36] | 2014 | R | [11C]-MET | NR | 88 (48/40) | To differentiate between recurrence and radionecrosis | [11C]-MET-PET was predictive for longer OS after stereotactic radiosurgery |
| Rottenburger et al. [37] | 2011 | P | [11C]-MET, [11C]-choline | Solid neoplasms | 8 (NR) | To compare [11C]-MET and [11C]-choline PET | [11C]-choline showed a higher LNR |
| Tran et al. [38] | 2020 | P | [11C]-MET, [11C]PBR28 | Melanoma, NSCLC | 5 (3/2) | To compare [11C]-MET and [11C]PBR28 PET | [11C]PBR28 was not a valid biomarker to detect radionecrosis |
| Cicuendez et al. [39] | 2015 | P | [11C]-MET | NR, gliomas | 43 (24/19) | To evaluate [11C]-MET uptake and relationship with histopathological grade | T/C was higher in BM and high grade gliomas; T/C < 1.9 was associated with longer OS |
| Unterrainer et al. [42] | 2017 | R | [18F]-FET | Solid neoplasms | 30 (NR) | To evaluate the uptake characteristics of untreated BM | All BM > 1cm were [18F]-FET positive |
| Galldiks et al. [43] | 2012 | P | [18F]-FET | Solid neoplasms | 31 (5/26) | To differentiate between recurrence and radionecrosis | TBRmax of 2.55: AUC 0.822 sensitivity 79% specificity 76% TBRmean of 1.95: AUC 0.851 sensitivity 74% specificity 90% |
| Ceccon et al. [44] | 2017 | R | [18F]-FET | Solid neoplasms | 62 (14/48) | To role of dynamic PET scan to differentiate recurrence from radiation injury | TBRmean > 1.95 + a slope < 0.37 SUV/ h: accuracy 88% sensitivity 83% specificity 93% |
| Kebir et al. [45] | 2016 | R | [18F]-FET | melanoma | 5 (NR) | To evaluate pseudoprogression in patients treated with ICI | TBRmax was higher in patients with true progression (5.4 vs. 2.5), as well as time to peak was significantly shorter (17 min vs. 45 min) |
| Romagna et al. [46] | 2016 | R | [18F]-FET | Solid neoplasms | 22 (11/11) | To differentiate between recurrence and radionecrosis | TBRmax of 2.15 and TBRmean of 1.95: AUC 0.84 sensitivity 86% specificity 79% TBRs + decreasing TAC: AUC 0.79 sensitivity 91% specificity 83% |
| Grosu et al. [47] | 2011 | P | [18F]-FET, [11C]-MET | Solid neoplasms, gliomas | 42 (NR) | To compare [18F]-FET and [11C]-MET uptake in gliomas and BM; To compare volumes between PET and MRI | [18F]-FET and [11C]-MET strongly correlated; Both radiotracers: sensitivity 91% specificity 100% |
| Gempt et al. [48] | 2015 | R | [18F]-FET | Solid neoplasms | 41 (NR) | To delineate and to compare target volumes with MRI | Tumor volumes by [18F]-FET and MRI were only partially overlapped |
| Papin-Michault [31] | 2016 | R | [18F]-DOPA | Solid neoplasms, non-tumoral tissue | 67 BM 53 control | LAT-1 and CD68 expression in BM | LAT-1 expression level and [18F]-DOPA uptake were significantly correlated |
| Lizarraga et al. [50] | 2014 | R | [18F]-DOPA | Solid neoplasms | 32 (26/6) | To differentiate between recurrence and radionecrosis | Visual scoring ≥ 2: sensitivity 81% specificity 84% |
| Cicone et al. [51] | 2015 | R | [18F]-DOPA | Solid neoplasms | 42 (NR) | To differentiate between recurrence and radionecrosis and to compare with MRI | SUVLmax/Bkgrmax of 1.59: sensitivity 90% specificity 92% |
| Cicone et al. [52] | 2021 | P | [18F]-DOPA | Solid neoplasms | 30 (13/17) | To characterize the long-term metabolic evolution of radionecrosis | rSUV of 1.92: sensitivity 90% specificity 96% |
| Humbert et al. [53] | 2019 | P | [18F]-DOPA | Solid neoplasms, glioblastoma | 106 | To evaluate the impact of [18F]-DOPA on the therapeutic decision | For suspicions of tumor recurrence, [18F]-DOPA improved diagnostic accuracy for both BM and glioblastomas |
| Authors | Year | Study Design | RF | Primary Malignancy | Patients (M/F) | Aim | Comments |
|---|---|---|---|---|---|---|---|
| Kamson et al. [54] | 2013 | P | [11C]-AMT | Solid neoplasms, glioblastoma | 36 (20/16) | To discriminate between BM and glioblastomas | BM had lower tumoral SUVs, lower mean tumor/cortex SUVratio, and tumor/cortex VD′-ratio |
| Xu et al. [55] | 2018 | P | [18F]-FGln | Solid neoplasms, gliomas | 14 (7/7) | To compare [18F]-Fgln and [18F]-FDG | Detection rates for BM [18F]-Fgln 82% [18F]-FDG 37% |
| Yu et al. [56] | 2015 | P | [18F]-Alfatide II | Solid neoplasms, gliomas | 9 (5/4) | To compare [18F]-Alfatide II and [18F]-FDG | All 20 brain lesions were visualized by [18F]-Alfatide II, while only 10 by [18F]-FDG, and 13 by CT. |
| Grkovski et al. [58] | 2020 | P | [18F]-choline | Solid neoplasms | 14 (NR) | To evaluate [18F]-choline uptake correlation from surgical samples with pathologic evidence of recurrent tumor | Surgical samples with viable tumor had higher uptake than those without tumor, although inflammation and gliosis also increase the uptake |
| Morikawa et al. [59] | 2021 | P | [18F]-FLT | Breast | 15 (NR) | To assess early response to sorafenib and whole-brain radiation therapy | [18F]-FLT seems a valid imaging tool for early response assessment |
| O’Sullivan et al. [59] | 2016 | P | [18F]-FLT | Breast | 10 (NR) | To evaluate therapy response | A total of 52% of target lesions showed a reduction in [18F]-FLT SUV ≥20% after treatment |
| Allen et al. [61] | 2012 | P | [18F]-ML-10 | Solid neoplasms | 10 (NR) | To evaluate therapy response after radiation therapy | High correlation between early changes on [18F]-ML-10 PET and later changes on MRI |
| Øen et al. [62] | 2022 | R | [18F]-fluciclovine | Solid neoplasms | 18 (11/7) | To compare diagnostic accuracy for tumor recurrence between PET/MRI and MRI alone | PET volumes correlated and were comparable in size with those from MRI, but were only partially congruent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, L.; Bonatto, E.; Nieri, A.; Castello, A.; Maffione, A.M.; Marzola, M.C.; Cittanti, C.; Bartolomei, M.; Panareo, S.; Mansi, L.; et al. The Role of Molecular Imaging in Patients with Brain Metastases: A Literature Review. Cancers 2023, 15, 2184. https://doi.org/10.3390/cancers15072184
Urso L, Bonatto E, Nieri A, Castello A, Maffione AM, Marzola MC, Cittanti C, Bartolomei M, Panareo S, Mansi L, et al. The Role of Molecular Imaging in Patients with Brain Metastases: A Literature Review. Cancers. 2023; 15(7):2184. https://doi.org/10.3390/cancers15072184
Chicago/Turabian StyleUrso, Luca, Elena Bonatto, Alberto Nieri, Angelo Castello, Anna Margherita Maffione, Maria Cristina Marzola, Corrado Cittanti, Mirco Bartolomei, Stefano Panareo, Luigi Mansi, and et al. 2023. "The Role of Molecular Imaging in Patients with Brain Metastases: A Literature Review" Cancers 15, no. 7: 2184. https://doi.org/10.3390/cancers15072184
APA StyleUrso, L., Bonatto, E., Nieri, A., Castello, A., Maffione, A. M., Marzola, M. C., Cittanti, C., Bartolomei, M., Panareo, S., Mansi, L., Lopci, E., Florimonte, L., & Castellani, M. (2023). The Role of Molecular Imaging in Patients with Brain Metastases: A Literature Review. Cancers, 15(7), 2184. https://doi.org/10.3390/cancers15072184










