Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases
Abstract
Simple Summary
Abstract
1. Introduction
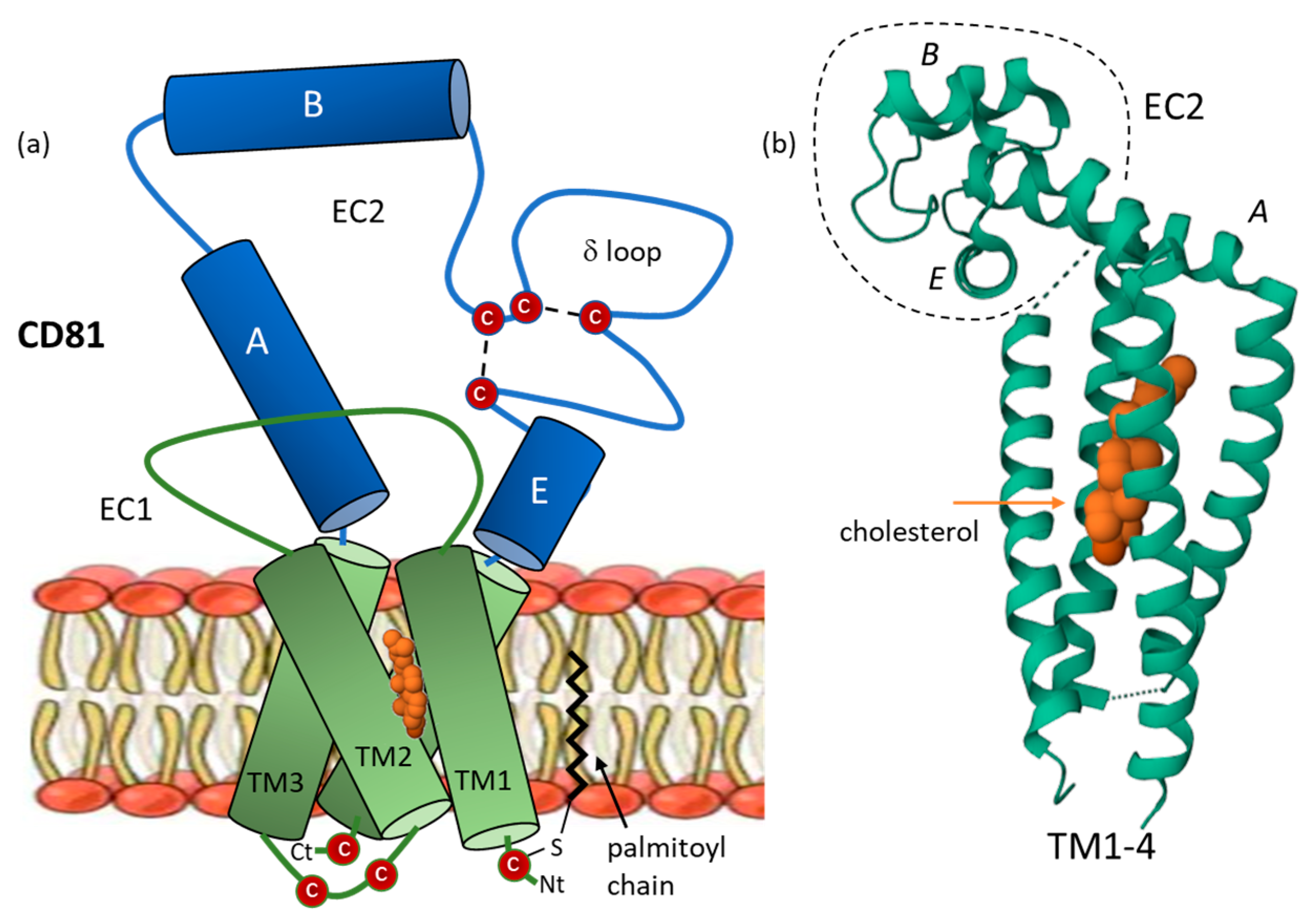
2. CD81 Structure and Dynamic Architecture
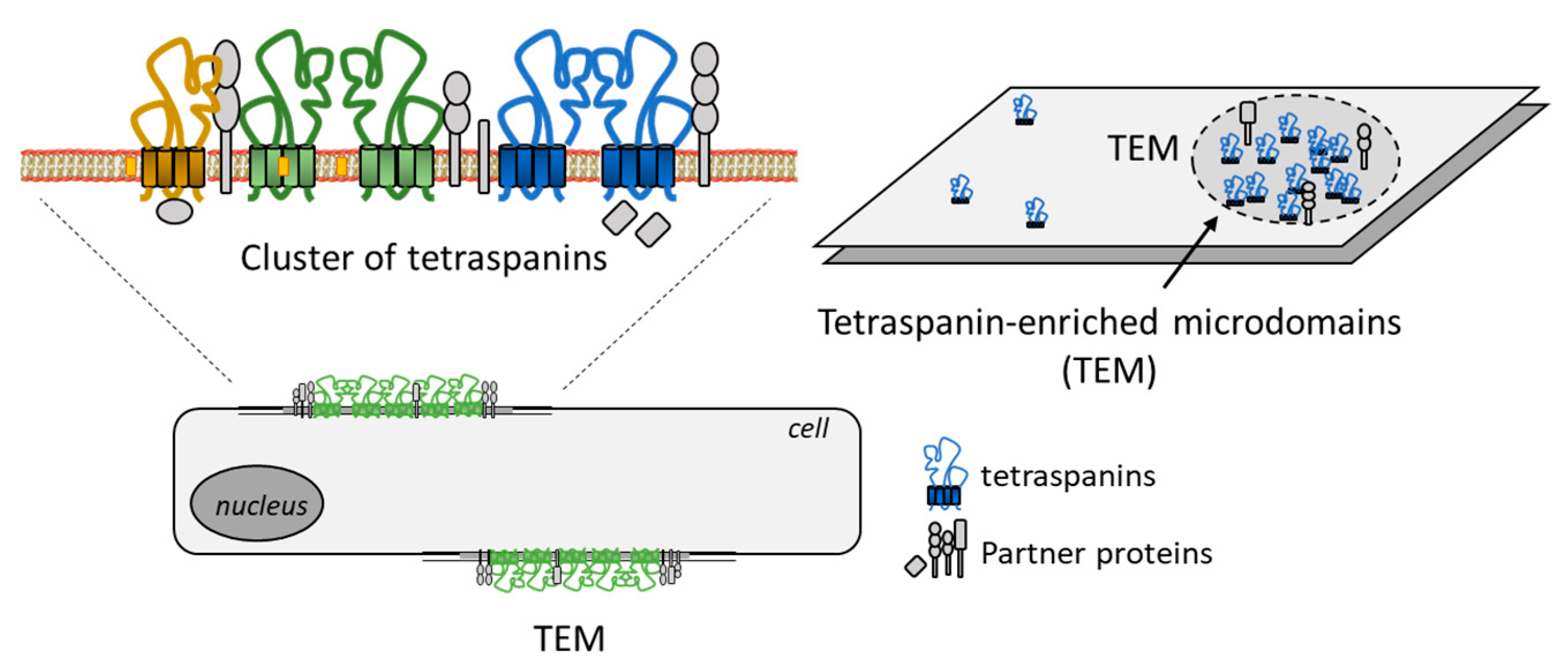
3. CD81 Biology, Trafficking and Signaling
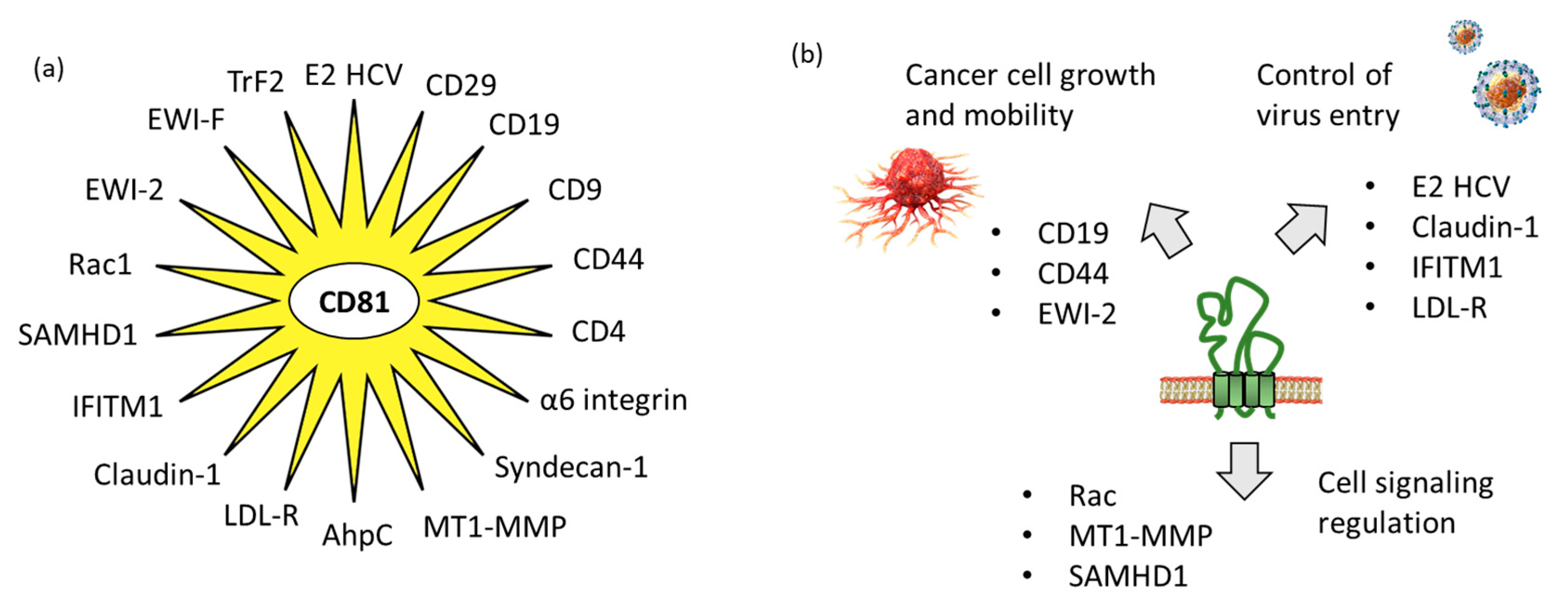
3.1. CD81 Protein Partners Implicated in Virus Uptake
3.2. CD81 Protein Partners Implicated in Tumor Growth and Dissemination
4. Regulation of CD81 Expression or Functions with Antibodies and Small Molecules
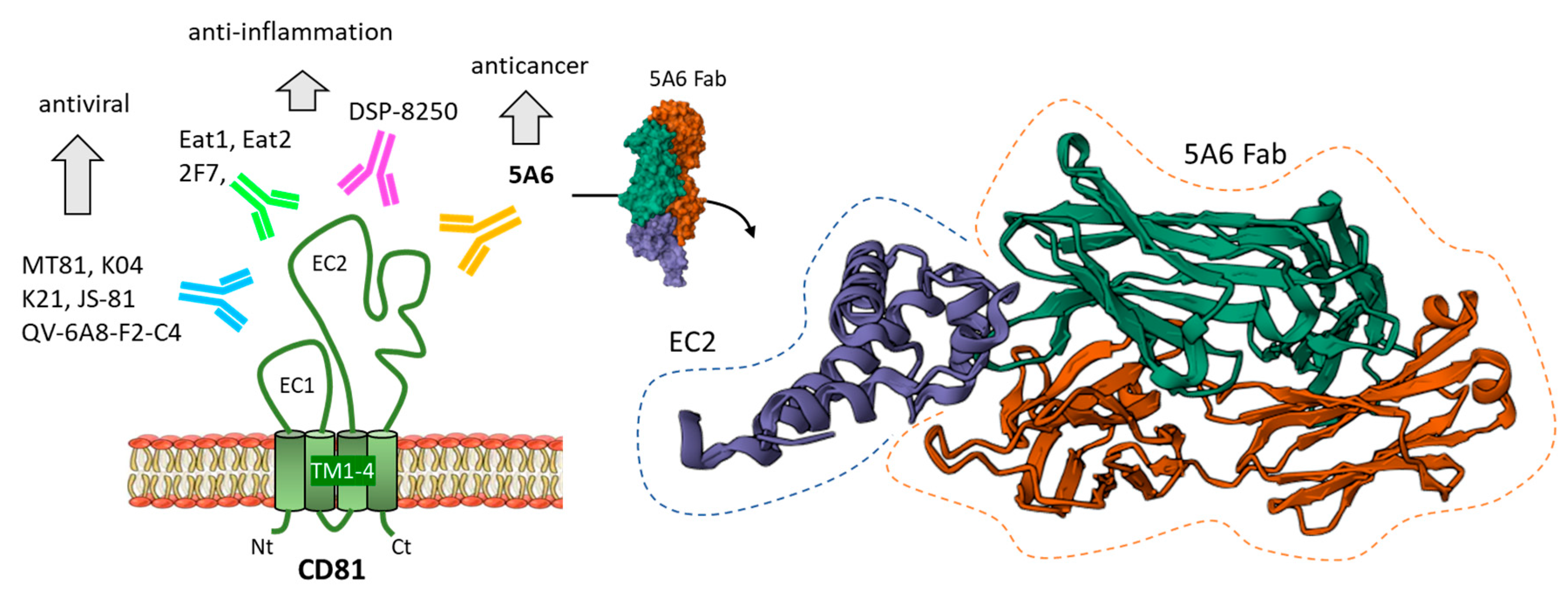
4.1. Anti-CD81 Antibodies
4.2. CD81 Small Molecule Binders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Yuan, S.; Dong, M.; Su, J.; Yu, C.; Shen, Y.; Xie, X.; Yu, Y.; Yu, X.; Chen, S.; et al. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics 2005, 86, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Becic, A.; Leifeld, J.; Shaukat, J.; Hollmann, M. Tetraspanins as Potential Modulators of Glutamatergic Synaptic Function. Front. Mol. Neurosci. 2022, 14, 801882. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Hochheimer, N. Tetraspanins. Curr. Biol. 2020, 30, R204–R206. [Google Scholar] [CrossRef] [PubMed]
- New, C.; Lee, Z.Y.; Tan, K.S.; Wong, A.H.; Wang, Y.; Tran, T. Tetraspanins: Host Factors in Viral Infections. Int. J. Mol. Sci. 2021, 22, 11609. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Méresse, S.; Kremer, L.; Daher, W. The roles of tetraspanins in bacterial infections. Cell. Microbiol. 2020, 22, e13260. [Google Scholar] [CrossRef]
- Titu, S.; Grapa, C.M.; Mocan, T.; Balacescu, O.; Irimie, A. Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications. Cancers 2021, 13, 5662. [Google Scholar] [CrossRef]
- Cai, S.; Deng, Y.; Peng, H.; Shen, J. Role of Tetraspanins in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 723341. [Google Scholar] [CrossRef]
- Deng, Y.; Cai, S.; Shen, J.; Peng, H. Tetraspanins: Novel Molecular Regulators of Gastric Cancer. Front. Oncol. 2021, 11, 702510. [Google Scholar] [CrossRef]
- Yang, Y.G.; Sari, I.N.; Zia, M.F.; Lee, S.R.; Song, S.J.; Kwon, H.Y. Tetraspanins: Spanning from solid tumors to hematologic malignancies. Exp. Hematol. 2016, 44, 322–328. [Google Scholar] [CrossRef]
- Floren, M.; Gillette, J.M. Acute myeloid leukemia: Therapy resistance and a potential role for tetraspanin membrane scaffolds. Int. J. Biochem. Cell. Biol. 2021, 137, 106029. [Google Scholar] [CrossRef]
- Yoshimura, T.; Miyoshi, H.; Shimono, J.; Nakashima, K.; Takeuchi, M.; Yanagida, E.; Yamada, K.; Shimasaki, Y.; Moritsubo, M.; Furuta, T.; et al. CD37 expression in follicular lymphoma. Ann. Hematol. 2022, 101, 1067–1075. [Google Scholar] [CrossRef]
- Peeters, R.; Cuenca-Escalona, J.; Zaal, E.A.; Hoekstra, A.T.; Balvert, A.C.G.; Vidal-Manrique, M.; Blomberg, N.; van Deventer, S.J.; Stienstra, R.; Jellusova, J.; et al. Fatty acid metabolism in aggressive B-cell lymphoma is inhibited by tetraspanin CD37. Nat. Commun. 2022, 13, 5371. [Google Scholar] [CrossRef]
- Bobrowicz, M.; Kubacz, M.; Slusarczyk, A.; Winiarska, M. CD37 in B Cell Derived Tumors-More than Just a Docking Point for Monoclonal Antibodies. Int. J. Mol. Sci. 2020, 21, 9531. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.H.; Amoussou, N.G.; Mai, H.L.; Logé, C.; Brouard, S. Tetraspanins: Useful multifunction proteins for the possible design and development of small-molecule therapeutic tools. Drug Discov. Today 2021, 26, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Hochheimer, N.; Sies, R.; Aschenbrenner, A.C.; Schneider, D.; Lang, T. Classes of non-conventional tetraspanins defined by alternative splicing. Sci. Rep. 2019, 9, 14075. [Google Scholar] [CrossRef] [PubMed]
- Haeuw, J.F.; Goetsch, L.; Bailly, C.; Corvaia, N. Tetraspanin CD151 as a target for antibody-based cancer immunotherapy. Biochem. Soc. Trans. 2011, 39, 553–558. [Google Scholar] [CrossRef]
- Kang, Z.; Luo, Y.; Xiao, E.; Li, Q.; Wang, L. CD151 and prostate cancer progression: A review of current literature. Asia Pac. J. Clin. Oncol. 2022; 1–5, online ahead of print. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Yang, S.; Pi, H.; Li, Z.; Yao, P.; Zhang, Q.; Wang, Q.; Shen, P.; Li, X.; et al. Proteomic Landscape of Exosomes Reveals the Functional Contributions of CD151 in Triple-Negative Breast Cancer. Mol. Cell. Proteomics. 2021, 20, 100121. [Google Scholar] [CrossRef]
- Homsi, Y.; Lang, T. The specificity of homomeric clustering of CD81 is mediated by its δ-loop. FEBS Open Bio. 2017, 7, 274–283. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Duault, C.; Kuo, C.C.; Rajapaksa, R.; Levy, R.; Levy, S. CD81 as a tumor target. Biochem. Soc. Trans. 2017, 45, 531–535. [Google Scholar] [CrossRef]
- Küppers, R. CD81 as target for B cell lymphomas. J. Exp. Med. 2019, 216, 1469–1470. [Google Scholar] [CrossRef]
- Espasa, A.; Tapia, G.; Vergara, S.; Raya, M.; Juncà, J.; Sorigue, M. Flow cytometric expression of CD71, CD81, CD44 and CD39 in B cell lymphoma. Scand. J. Clin. Lab. Invest. 2021, 81, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Quagliano, A.; Gopalakrishnapillai, A.; Kolb, E.A.; Barwe, S.P. CD81 knockout promotes chemosensitivity and disrupts in vivo homing and engraftment in acute lymphoblastic leukemia. Blood Adv. 2020, 4, 4393–4405. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.; Guihard, S.; Roumier, C.; Peyrouze, P.; Gonzales, F.; Berthon, C.; Quesnel, B.; Preudhomme, C.; Behal, H.; Duhamel, A.; et al. Tetraspanin CD81 is an adverse prognostic marker in acute myeloid leukemia. Oncotarget 2016, 7, 62377–62385. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Chelikani, P.; Reeves, P.J.; Zhang, S.; Khorana, H.G. High-level expression, single-step immunoaffinity purification and characterization of human tetraspanin membrane protein CD81. PLoS ONE 2008, 3, e2314. [Google Scholar] [CrossRef]
- Zimmerman, B.; Kelly, B.; McMillan, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 2016, 167, 1041–1051. [Google Scholar] [CrossRef]
- Seigneuret, M.; Delaguillaumie, A.; Lagaudrière-Gesbert, C.; Conjeaud, H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 2001, 276, 40055–40064. [Google Scholar] [CrossRef]
- Seigneuret, M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 2006, 90, 212–227. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Ponassi, M.; Galli, G.; Petracca, R.; Falugi, F.; Grandi, G.; Bolognesi, M. Subunit association and conformational flexibility in the head subdomain of human CD81 large extracellular loop. Biol. Chem. 2002, 383, 1447–1452. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Bordo, D.; Galli, G.; Petracca, R.; Falugi, F.; Abrignani, S.; Grandi, G.; Bolognesi, M. CD81 extracellular domain 3D structure: Insight into the tetraspanin superfamily structural motifs. EMBO J. 2001, 20, 12–18. [Google Scholar] [CrossRef]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef]
- van Deventer, S.J.; Dunlock, V.E.; van Spriel, A.B. Molecular interactions shaping the tetraspanin web. Biochem. Soc. Trans. 2017, 45, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Florin, L.; Lang, T. Tetraspanin Assemblies in Virus Infection. Front. Immunol. 2018, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yan, Y.; Tan, K.S.; Tan, S.S.L.; Seet, J.E.; Arumugam, T.V.; Chow, V.T.K.; Wang, Y.; Tran, T. CD151, a novel host factor of nuclear export signaling in influenza virus infection. J. Allergy Clin. Immunol. 2018, 141, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Benayas, B.; Sastre, I.; López-Martín, S.; Oo, A.; Kim, B.; Bullido, M.J.; Aldudo, J.; Yáñez-Mó, M. Tetraspanin CD81 regulates HSV-1 infection. Med. Microbiol. Immunol. 2020, 209, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Lasswitz, L.; Zapatero-Belinchón, F.J.; Moeller, R.; Hülskötter, K.; Laurent, T.; Carlson, L.A.; Goffinet, C.; Simmons, G.; Baumgärtner, W.; Gerold, G. The Tetraspanin CD81 Is a Host Factor for Chikungunya Virus Replication. mBio. 2022, 13, e0073122. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.N.; Gouin, E.; Rubinstein, E.; Boucheix, C.; Cossart, P.; Pizarro-Cerda, J. Tetraspanin CD81 is required for Listeria monocytogenes invasion. Infect. Immun. 2010, 78, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Martínez del Hoyo, G.; Ramírez-Huesca, M.; Levy, S.; Boucheix, C.; Rubinstein, E.; Minguito de la Escalera, M.; González-Cintado, L.; Ardavín, C.; Veiga, E.; Yáñez-Mó, M.; et al. CD81 controls immunity to Listeria infection through rac-dependent inhibition of proinflammatory mediator release and activation of cytotoxic T cells. J. Immunol. 2015, 194, 6090–6101. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Yalaoui, S.; Bartosch, B.; Cocquerel, L.; Franetich, J.F.; Boucheix, C.; Mazier, D.; Rubinstein, E.; Silvie, O. The Ig domain protein CD9P-1 down-regulates CD81 ability to support Plasmodium yoelii infection. J. Biol. Chem. 2009, 284, 31572–31578. [Google Scholar] [CrossRef]
- Palor, M.; Stejskal, L.; Mandal, P.; Lenman, A.; Alberione, M.P.; Kirui, J.; Moeller, R.; Ebner, S.; Meissner, F.; Gerold, G.; et al. Cholesterol sensing by CD81 is important for hepatitis C virus entry. J. Biol. Chem. 2020, 295, 16931–16948. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.R.; Greenberg, Z.J.; Zhou, F.; He, P.; Fan, L.; Liu, S.; Shen, G.; Egawa, T.; Gross, M.L.; et al. Open conformation of tetraspanins shapes interaction partner networks on cell membranes. EMBO J. 2020, 39, e105246. [Google Scholar] [CrossRef]
- Zuidscherwoude, M.; Göttfert, F.; Dunlock, V.M.; Figdor, C.G.; van den Bogaart, G.; van Spriel, A.B. The tetraspanin web revisited by super-resolution microscopy. Sci. Rep. 2015, 5, 12201. [Google Scholar] [CrossRef] [PubMed]
- Caparotta, M.; Masone, D. Cholesterol plays a decisive role in tetraspanin assemblies during bilayer deformations. Biosystems. 2021, 209, 104505. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, A.; Carter, R.H.; Brooks, S.; Bornmann, W.; Finn, R.; Dowd, C.S.; Pierce, S.K. B cell signaling is regulated by induced palmitoylation of CD81. J. Biol. Chem. 2004, 279, 31973–31982. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Luo, Y.; Cao, M.M.; Liu, Y.; Liu, X.Q.; Wang, W.; Wu, D.G.; Guan, M.; Xu, Q.Q.; Ren, H.; et al. Significance of palmitoylation of CD81 on its association with tetraspanin-enriched microdomains and mediating hepatitis C virus cell entry. Virology 2012, 429, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Ishimaru, H.; Watanabe, T.; Fujimuro, M. The Lysosome Pathway Degrades CD81 on the Cell Surface by Poly-ubiquitination and Clathrin-Mediated Endocytosis. Biol. Pharm. Bull. 2020, 43, 540–545. [Google Scholar] [CrossRef]
- Orinska, Z.; Hagemann, P.M.; Halova, I.; Draber, P. Tetraspanins in the regulation of mast cell function. Med. Microbiol. Immunol. 2020, 209, 531–543. [Google Scholar] [CrossRef]
- Lu, C.; Feng, Y.; Sun, X.; Li, N.; Kuang, D.; Wang, W.; Tong, P.; Han, Y.; Xia, X.; Dai, J. Tree shrew bone marrow-derived mesenchymal stem cells express CD81, OCLN, and miR-122, facilitating the entire hepatitis C virus life cycle. J. Med. Virol. 2020; online ahead of print. [Google Scholar] [CrossRef]
- Liao, L.; Wu, Z.; Chen, W.; Zhang, H.; Li, A.; Yan, Y.; Xie, Z.; Li, H.; Lin, W.; Ma, J.; et al. Anti-CD81 antibody blocks vertical transmission of avian leukosis virus subgroup J. Vet. Microbiol. 2022, 264, 109293. [Google Scholar] [CrossRef]
- Kumar, A.; Hossain, R.A.; Yost, S.A.; Bu, W.; Wang, Y.; Dearborn, A.D.; Grakoui, A.; Cohen, J.I.; Marcotrigiano, J. Structural insights into hepatitis C virus receptor binding and entry. Nature 2021, 598, 521–525. [Google Scholar] [CrossRef]
- Ayub, H.; Clare, M.; Milic, I.; Chmel, N.P.; Böning, H.; Devitt, A.; Krey, T.; Bill, R.M.; Rothnie, A.J. CD81 extracted in SMALP nanodiscs comprises two distinct protein populations within a lipid environment enriched with negatively charged headgroups. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183419. [Google Scholar] [CrossRef]
- Ströh, L.J.; Krey, T. HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development. Int. J. Mol. Sci. 2020, 21, 6781. [Google Scholar] [CrossRef]
- Bonander, N.; Jamshad, M.; Oberthür, D.; Clare, M.; Barwell, J.; Hu, K.; Farquhar, M.J.; Stamataki, Z.; Harris, H.J.; Dierks, K.; et al. Production, purification and characterization of recombinant, full-length human claudin-1. PLoS ONE 2013, 8, e64517. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Woodward, J.; Lau, D.T.; Barnes, A.; Joyce, M.; McFarlane, N.; McKeating, J.A.; Tyrrell, D.L.; Gale, M., Jr. IFITM1 is a tight junction protein that inhibits hepatitis C virus entry. Hepatology 2013, 57, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.K.; Helbig, K.J.; McCartney, E.M.; Eyre, N.S.; Bull, R.A.; Eltahla, A.; Lloyd, A.R.; Beard, M.R. The Interferon-induced Transmembrane Proteins, IFITM1, IFITM2, and IFITM3 Inhibit Hepatitis C Virus Entry. J. Biol. Chem. 2015, 290, 25946–25959. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Blanchet, M.; Seidah, N.G.; Labonté, P. Plasma Membrane Tetraspanin CD81 Complexes with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and Low-Density Lipoprotein Receptor (LDLR), and Its Levels Are Reduced by PCSK9. J. Biol. Chem. 2015, 290, 23385–23400. [Google Scholar] [CrossRef]
- Bridge, S.H.; Sheridan, D.A.; Felmlee, D.J.; Crossey, M.M.; Fenwick, F.I.; Lanyon, C.V.; Dubuc, G.; Seidah, N.G.; Davignon, J.; Thomas, H.C.; et al. PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: Evidence for genotype-specific regulation of lipoprotein metabolism. J. Hepatol. 2015, 62, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Susa, K.J.; Rawson, S.; Kruse, A.C.; Blacklow, S.C. Cryo-EM structure of the B cell co-receptor CD19 bound to the tetraspanin CD81. Science 2021, 371, 300–305. [Google Scholar] [CrossRef]
- Wang, H.X.; Sharma, C.; Knoblich, K.; Granter, S.R.; Hemler, M.E. EWI-2 negatively regulates TGF-β signaling leading to altered melanoma growth and metastasis. Cell. Res. 2015, 25, 370–385. [Google Scholar] [CrossRef]
- Kolesnikova, T.V.; Stipp, C.S.; Rao, R.M.; Lane, W.S.; Luscinskas, F.W.; Hemler, M.E. EWI-2 modulates lymphocyte integrin alpha4beta1 functions. Blood 2004, 103, 3013–3019. [Google Scholar] [CrossRef]
- Montpellier, C.; Tews, B.A.; Poitrimole, J.; Rocha-Perugini, V.; D’Arienzo, V.; Potel, J.; Zhang, X.A.; Rubinstein, E.; Dubuisson, J.; Cocquerel, L. Interacting regions of CD81 and two of its partners, EWI-2 and EWI-2wint, and their effect on hepatitis C virus infection. J. Biol. Chem. 2011, 286, 13954–13965. [Google Scholar] [CrossRef]
- Charrin, S.; Latil, M.; Soave, S.; Polesskaya, A.; Chrétien, F.; Boucheix, C.; Rubinstein, E. Normal muscle regeneration requires tight control of muscle cell fusion by tetraspanins CD9 and CD81. Nat. Commun. 2013, 4, 1674. [Google Scholar] [CrossRef]
- Ramos, E.K.; Tsai, C.F.; Jia, Y.; Cao, Y.; Manu, M.; Taftaf, R.; Hoffmann, A.D.; El-Shennawy, L.; Gritsenko, M.A.; Adorno-Cruz, V.; et al. Machine learning-assisted elucidation of CD81-CD44 interactions in promoting cancer stemness and extracellular vesicle integrity. Elife 2022, 11, e82669. [Google Scholar] [CrossRef] [PubMed]
- Jankovicova, J.; Frolikova, M.; Palenikova, V.; Valaskova, E.; Cerny, J.; Secova, P.; Bartokova, M.; Horovska, L.; Manaskova-Postlerova, P.; Antalikova, J.; et al. Expression and distribution of CD151 as a partner of alpha6 integrin in male germ cells. Sci. Rep. 2020, 10, 4374. [Google Scholar] [CrossRef] [PubMed]
- Hazawa, M.; Tomiyama, K.; Saotome-Nakamura, A.; Obara, C.; Yasuda, T.; Gotoh, T.; Tanaka, I.; Yakumaru, H.; Ishihara, H.; Tajima, K. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem. Biophys. Res. Commun. 2014, 446, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Peyrou, M.; Bourgoin, L.; Maeder, C.; Tchou, I.; Foti, M. CD4 dimerization requires two cysteines in the cytoplasmic domain of the molecule and occurs in microdomains distinct from lipid rafts. Mol. Immunol. 2010, 47, 2594–2603. [Google Scholar] [CrossRef]
- Frolikova, M.; Manaskova-Postlerova, P.; Cerny, J.; Jankovicova, J.; Simonik, O.; Pohlova, A.; Secova, P.; Antalikova, J.; Dvorakova-Hortova, K. CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization. Int. J. Mol. Sci. 2018, 19, 1236. [Google Scholar] [CrossRef]
- Rocha-Perugini, V.; Suárez, H.; Álvarez, S.; López-Martín, S.; Lenzi, G.M.; Vences-Catalán, F.; Levy, S.; Kim, B.; Muñoz-Fernández, M.A.; Sánchez-Madrid, F.; et al. CD81 association with SAMHD1 enhances HIV-1 reverse transcription by increasing dNTP levels. Nat. Microbiol. 2017, 2, 1513–1522. [Google Scholar] [CrossRef]
- Schröder, H.M.; Hoffmann, S.C.; Hecker, M.; Korff, T.; Ludwig, T. The tetraspanin network modulates MT1-MMP cell surface trafficking. Int. J. Biochem. Cell. Biol. 2013, 45, 1133–1144. [Google Scholar] [CrossRef]
- Grigorov, B.; Reungoat, E.; Gentil Dit Maurin, A.; Varbanov, M.; Blaising, J.; Michelet, M.; Manuel, R.; Parent, R.; Bartosch, B.; Zoulim, F.; et al. Hepatitis C virus infection propagates through interactions between Syndecan-1 and CD81 and impacts the hepatocyte glycocalyx. Cell. Microbiol. 2017, 19, e12711. [Google Scholar] [CrossRef]
- Karam, J.; Blanchet, F.P.; Vivès, É.; Boisguérin, P.; Boudehen, Y.M.; Kremer, L.; Daher, W. Mycobacterium abscessus alkyl hydroperoxide reductase C promotes cell invasion by binding to tetraspanin CD81. iScience 2023, 26, 106042. [Google Scholar] [CrossRef]
- Chen, J.; Enns, C.A. CD81 promotes both the degradation of transferrin receptor 2 (TfR2) and the Tfr2-mediated maintenance of hepcidin expression. J. Biol. Chem. 2015, 290, 7841–7850. [Google Scholar] [CrossRef]
- Khan, A.G.; Whidby, J.; Miller, M.T.; Scarborough, H.; Zatorski, A.V.; Cygan, A.; Price, A.A.; Yost, S.A.; Bohannon, C.D.; Jacob, J.; et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature 2014, 509, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Heo, T.H.; Lee, S.M.; Bartosch, B.; Cosset, F.L.; Kang, C.Y. Hepatitis C virus E2 links soluble human CD81 and SR-B1 protein. Virus Res. 2006, 121, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Harris, H.J.; Hu, K.; Drummer, H.E.; McKeating, J.A.; Mullins, J.G.; Balfe, P. In silico directed mutagenesis identifies the CD81/claudin-1 hepatitis C virus receptor interface. Cell. Microbiol. 2012, 14, 1892–1903. [Google Scholar] [CrossRef]
- Ramanathan, A.; Gusarova, V.; Stahl, N.; Gurnett-Bander, A.; Kyratsous, C.A. Alirocumab, a Therapeutic Human Antibody to PCSK9, Does Not Affect CD81 Levels or Hepatitis C Virus Entry and Replication into Hepatocytes. PLoS ONE 2016, 11, e0154498. [Google Scholar] [CrossRef]
- Sermer, D.; Elavalakanar, P.; Abramson, J.S.; Palomba, M.L.; Salles, G.; Arnason, J. Targeting CD19 for diffuse large B cell lymphoma in the era of CARs: Other modes of transportation. Blood Rev. 2023, 57, 101002. [Google Scholar] [CrossRef]
- Cohen, J.A.; Ghobadi, A. Axicabtagene ciloleucel for the treatment of relapsed or refractory follicular lymphoma. Expert Rev. Anticancer Ther. 2022, 22, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Susa, K.J.; Seegar, T.C.; Blacklow, S.C.; Kruse, A.C. A dynamic interaction between CD19 and the tetraspanin CD81 controls B cell co-receptor trafficking. Elife 2020, 9, e52337. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.P.; Gottschalk, S. Targeting CD19: The good, the bad, and CD81. Blood 2017, 129, 9–10. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Rajapaksa, R.; Srivastava, M.K.; Marabelle, A.; Kuo, C.C.; Levy, R.; Levy, S. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 2015, 75, 4517–4526. [Google Scholar] [CrossRef]
- Yang, L.; Liu, P.; Du, H.; Chen, R.; Zhou, B.; Li, Y.; Zhou, L.; Wang, X.; Liu, C.; Ding, Y.; et al. Novel CD81 Mutations in a Chinese Patient Led to IgA Nephropathy and Impaired BCR Signaling. J. Clin. Immunol. 2022, 42, 1672–1684. [Google Scholar] [CrossRef]
- Schultz, L.M.; Czerwinski, D.K.; Levy, R.; Levy, S. CD81 costimulation skews CAR transduction toward naive T cells. Proc. Natl. Acad. Sci. USA 2022, 119, e1910844119. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 2001, 276, 40545–40554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Hemler, M.E. Novel impact of EWI-2, CD9, and CD81 on TGF-β signaling in melanoma. Mol. Cell. Oncol. 2015, 2, e1030536. [Google Scholar] [CrossRef]
- Uretmen Kagiali, Z.C.; Sanal, E.; Karayel, Ö.; Polat, A.N.; Saatci, Ö.; Ersan, P.G.; Trappe, K.; Renard, B.Y.; Önder, T.T.; Tuncbag, N.; et al. Systems-level Analysis Reveals Multiple Modulators of Epithelial-mesenchymal Transition and Identifies DNAJB4 and CD81 as Novel Metastasis Inducers in Breast Cancer. Mol. Cell. Proteomics. 2019, 18, 1756–1771. [Google Scholar] [CrossRef]
- Sala-Valdés, M.; Ursa, A.; Charrin, S.; Rubinstein, E.; Hemler, M.E.; Sánchez-Madrid, F.; Yáñez-Mó, M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 2006, 281, 19665–19675. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, E.E.; Matheson, N.J.; Perlee, S.; Munson, P.B.; Symeonides, M.; Thali, M. EWI-2 Inhibits Cell-Cell Fusion at the HIV-1 Virological Presynapse. Viruses. 2019, 11, 1082. [Google Scholar] [CrossRef]
- Gray, L.R.; Turville, S.G.; Hitchen, T.L.; Cheng, W.J.; Ellett, A.M.; Salimi, H.; Roche, M.J.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS ONE 2014, 9, e90620. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Tang, X.; Liang, Y.; Jiao, Q.; Yu, B.; Dai, Z.; Yuan, X.; Li, J.; Yan, J.; et al. Stabilization of SAMHD1 by NONO is crucial for Ara-C resistance in AML. Cell. Death Dis. 2022, 13, 590. [Google Scholar] [CrossRef]
- Rothenburger, T.; Thomas, D.; Schreiber, Y.; Wratil, P.R.; Pflantz, T.; Knecht, K.; Digianantonio, K.; Temple, J.; Schneider, C.; Baldauf, H.M.; et al. Differences between intrinsic and acquired nucleoside analogue resistance in acute myeloid leukaemia cells. J. Exp. Clin. Cancer Res. 2021, 40, 317. [Google Scholar] [CrossRef]
- Oosterheert, W.; Xenaki, K.T.; Neviani, V.; Pos, W.; Doulkeridou, S.; Manshande, J.; Pearce, N.M.; Kroon-Batenburg, L.M.; Lutz, M.; van Bergen En Henegouwen, P.M.; et al. Implications for tetraspanin-enriched microdomain assembly based on structures of CD9 with EWI-F. Life Sci. Alliance. 2020, 3, e202000883. [Google Scholar] [CrossRef]
- Chambrion, C.; Le Naour, F. The tetraspanins CD9 and CD81 regulate CD9P1-induced effects on cell migration. PLoS ONE 2010, 5, e11219. [Google Scholar] [CrossRef] [PubMed]
- Colin, S.; Guilmain, W.; Creoff, E.; Schneider, C.; Steverlynck, C.; Bongaerts, M.; Legrand, E.; Vannier, J.P.; Muraine, M.; Vasse, M.; et al. A truncated form of CD9-partner 1 (CD9P-1), GS-168AT2, potently inhibits in vivo tumour-induced angiogenesis and tumour growth. Br. J. Cancer. 2011, 105, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Guilmain, W.; Colin, S.; Legrand, E.; Vannier, J.P.; Steverlynck, C.; Bongaerts, M.; Vasse, M.; Al-Mahmood, S. CD9P-1 expression correlates with the metastatic status of lung cancer, and a truncated form of CD9P-1, GS-168AT2, inhibits in vivo tumour growth. Br. J. Cancer. 2011, 104, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Vasse, M.; Colin, S.; Guilmain, W.; Creoff, E.; Muraine, M.; Vannier, J.P.; Al-Mahmood, S. [Tetraspanins: A new target for antiangiogenic therapy?]. Ann. Pharm. Fr. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Rajapaksa, R.; Kuo, C.C.; Miller, C.L.; Lee, A.; Ramani, V.C.; Jeffrey, S.S.; Levy, R.; Levy, S. Targeting the tetraspanin CD81 reduces cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018961118. [Google Scholar] [CrossRef]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef]
- Quast, T.; Eppler, F.; Semmling, V.; Schild, C.; Homsi, Y.; Levy, S.; Lang, T.; Kurts, C.; Kolanus, W. CD81 is essential for the formation of membrane protrusions and regulates Rac1-activation in adhesion-dependent immune cell migration. Blood 2011, 118, 1818–1827. [Google Scholar] [CrossRef]
- Tejera, E.; Rocha-Perugini, V.; López-Martín, S.; Pérez-Hernández, D.; Bachir, A.I.; Horwitz, A.R.; Vázquez, J.; Sánchez-Madrid, F.; Yáñez-Mo, M. CD81 regulates cell migration through its association with Rac GTPase. Mol. Biol. Cell. 2013, 24, 261–273. [Google Scholar] [CrossRef]
- Bailly, C.; Beignet, J.; Loirand, G.; Sauzeau, V. Rac1 as a therapeutic anticancer target: Promises and limitations. Biochem. Pharmacol. 2022, 203, 115180. [Google Scholar] [CrossRef]
- Antalíková, J.; Sečová, P.; Michalková, K.; Horovská, Ľ.; Páleníková, V.; Jankovičová, J. Expression of αV integrin and its potential partners in bull reproductive tissues, germ cells and spermatozoa. Int. J. Biol. Macromol. 2022, 209, 542–551. [Google Scholar] [CrossRef]
- Kummer, D.; Steinbacher, T.; Thölmann, S.; Schwietzer, M.F.; Hartmann, C.; Horenkamp, S.; Demuth, S.; Peddibhotla, S.S.D.; Brinkmann, F.; Kemper, B.; et al. A JAM-A-tetraspanin-αvβ5 integrin complex regulates contact inhibition of locomotion. J. Cell. Biol. 2022, 221, e202105147. [Google Scholar] [CrossRef] [PubMed]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lee, C.Y.; Changou, C.A.; Cedano-Prieto, D.M.; Takada, Y.K.; Takada, Y. The CD9, CD81, and CD151 EC2 domains bind to the classical RGD-binding site of integrin αvβ3. Biochem. J. 2017, 474, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gustafson-Wagner, E.; Stipp, C.S. The CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate α3β1 integrin-dependent tumor cell behaviors by overlapping but distinct mechanisms. PLoS ONE 2013, 8, e61834. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gómez, Á.; Cardeñes, B.; Díez-Sainz, E.; Lafuente, E.M.; Cabañas, C. Functional Integrin Regulation Through Interactions with Tetraspanin CD9. Methods Mol. Biol. 2021, 2217, 47–56. [Google Scholar]
- Bruening, J.; Lasswitz, L.; Banse, P.; Kahl, S.; Marinach, C.; Vondran, F.W.; Kaderali, L.; Silvie, O.; Pietschmann, T.; Meissner, F.; et al. Hepatitis C virus enters liver cells using the CD81 receptor complex proteins calpain-5 and CBLB. PLoS Pathog. 2018, 14, e1007111. [Google Scholar] [CrossRef]
- Salem, D.A.; Stetler-Stevenson, M. Clinical Flow-Cytometric Testing in Chronic Lymphocytic Leukemia. Methods Mol. Biol. 2019, 2032, 311–321. [Google Scholar]
- Ozdemir, Z.N.; Falay, M.; Parmaksiz, A.; Genc, E.; Beyler, O.; Gunes, A.K.; Ceran, F.; Dagdas, S.; Ozet, G. A novel differential diagnosis algorithm for chronic lymphocytic leukemia using immunophenotyping with flow cytometry. Hematol. Transfus. Cell. Ther. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Falay, M.; Serdar, M.A.; Dalgali, H.; Uçar, M.A.; Dagdaş, S.; Özet, G. Which Markers Should the used for Diagnostic Chronic Lymphocytic Leukemia Immunophenotyping Scoring System by Flow Cytometry? Clin. Lab. 2019, 65, 11. [Google Scholar] [CrossRef]
- Muzzafar, T.; Medeiros, L.J.; Wang, S.A.; Brahmandam, A.; Thomas, D.A.; Jorgensen, J.L. Aberrant underexpression of CD81 in precursor B-cell acute lymphoblastic leukemia: Utility in detection of minimal residual disease by flow cytometry. Am. J. Clin. Pathol. 2009, 132, 692–698. [Google Scholar] [CrossRef]
- Lishner, M.; Zismanov, V.; Tohami, T.; Tartakover-Matalon, S.; Elis, A.; Drucker, L. Tetraspanins affect myeloma cell fate via Akt signaling and FoxO activation. Cell. Signal. 2008, 20, 2309–2316. [Google Scholar] [CrossRef]
- Lin, K.K.; Rossi, L.; Boles, N.C.; Hall, B.E.; George, T.C.; Goodell, M.A. CD81 is essential for the re-entry of hematopoietic stem cells to quiescence following stress-induced proliferation via deactivation of the Akt pathway. PLoS Biol. 2011, 9, e1001148. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Qiao, L.; Kang, K.D.; Fan, J.; Wei, W.; Luo, G. Attachment and Postattachment Receptors Important for Hepatitis C Virus Infection and Cell-to-Cell Transmission. J. Virol. 2017, 91, e00280-17. [Google Scholar] [CrossRef] [PubMed]
- Mizoshiri, N.; Shirai, T.; Terauchi, R.; Tsuchida, S.; Mori, Y.; Hayashi, D.; Kishida, T.; Arai, Y.; Mazda, O.; Nakanishi, T.; et al. The tetraspanin CD81 mediates the growth and metastases of human osteosarcoma. Cell. Oncol. 2019, 42, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalán, F.; Kuo, C.C.; Sagi, Y.; Chen, H.; Kela-Madar, N.; van Zelm, M.C.; van Dongen, J.J.; Levy, S. A mutation in the human tetraspanin CD81 gene is expressed as a truncated protein but does not enable CD19 maturation and cell surface expression. J. Clin. Immunol. 2015, 35, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalán, F.; Rajapaksa, R.; Srivastava, M.K.; Marabelle, A.; Kuo, C.C.; Levy, R.; Levy, S. Tetraspanin CD81, a modulator of immune suppression in cancer and metastasis. Oncoimmunology 2015, 5, e1120399. [Google Scholar] [CrossRef]
- Aljowaie, R.M.; Almajhdi, F.N.; Ali, H.H.; El-Wetidy, M.S.; Shier, M.K. Inhibition of hepatitis C virus genotype 4 replication using siRNA targeted to the viral core region and the CD81 cellular receptor. Cell. Stress Chaperones. 2020, 25, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, Q.; Liu, H.; Hu, S.; Zhou, Y.; Bai, Y.; Zhang, J.; Pan, Y.; Shao, C. CD81 Enhances Radioresistance of Glioblastoma by Promoting Nuclear Translocation of Rad51. Cancers 2021, 13, 1998. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zheng, W.; Luo, J.; Yu, G.; Song, C.; Wu, Y.; Xu, J. Inhibiting uptake of extracellular vesicles derived from senescent bone marrow mesenchymal stem cells by muscle satellite cells attenuates sarcopenia. J. Orthop. Translat. 2022, 35, 23–36. [Google Scholar] [CrossRef]
- Takahashi, S.; Doss, C.; Levy, S.; Levy, R. TAPA-1, the target of an antiproliferative antibody, is associated on the cell surface with the Leu-13 antigen. J. Immunol. 1990, 145, 2207–2213. [Google Scholar] [CrossRef]
- VanCompernolle, S.E.; Levy, S.; Todd, S.C. Anti-CD81 activates LFA-1 on T cells and promotes T cell-B cell collaboration. Eur. J. Immunol. 2001, 31, 823–831. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Kuo, C.C.; Rajapaksa, R.; Duault, C.; Andor, N.; Czerwinski, D.K.; Levy, R.; Levy, S. CD81 is a novel immunotherapeutic target for B cell lymphoma. J. Exp. Med. 2019, 216, 1497–1508. [Google Scholar] [CrossRef]
- Hasezaki, T.; Yoshima, T.; Mattsson, M.; Särnefält, A.; Takubo, K. A monoclonal antibody recognizing a new epitope on CD81 inhibits T-cell migration without inducing cytokine production. J. Biochem. 2020, 167, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hasezaki, T.; Yoshima, T.; Mine, Y. Anti-CD81 antibodies reduce migration of activated T lymphocytes and attenuate mouse experimental colitis. Sci. Rep. 2020, 10, 6969. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, P.; Zhao, L.J.; Wu, S.M.; Zhu, S.Y.; Qi, Z.T. Identification and expression of human CD81 gene on murine NIH/3T3 cell membrane. J. Microbiol. Methods. 2003, 54, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, K.; Simeon, R.L.; Rice, C.M.; Chen, Z. A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc. Natl. Acad. Sci. USA 2010, 107, 3764–3769. [Google Scholar] [CrossRef]
- Fofana, I.; Xiao, F.; Thumann, C.; Turek, M.; Zona, L.; Tawar, R.G.; Grunert, F.; Thompson, J.; Zeisel, M.B.; Baumert, T.F. A novel monoclonal antvi-CD81 antibody produced by genetic immunization efficiently inhibits Hepatitis C virus cell-cell transmission. PLoS ONE 2013, 8, e64221. [Google Scholar] [CrossRef]
- Ji, C.; Liu, Y.; Pamulapati, C.; Bohini, S.; Fertig, G.; Schraeml, M.; Rubas, W.; Brandt, M.; Ries, S.; Ma, H.; et al. Prevention of hepatitis C virus infection and spread in human liver chimeric mice by an anti-CD81 monoclonal antibody. Hepatology 2015, 61, 1136–1144. [Google Scholar] [CrossRef]
- Vexler, V.; Yu, L.; Pamulapati, C.; Garrido, R.; Grimm, H.P.; Sriraman, P.; Bohini, S.; Schraeml, M.; Singh, U.; Brandt, M.; et al. Target-mediated drug disposition and prolonged liver accumulation of a novel humanized anti-CD81 monoclonal antibody in cynomolgus monkeys. MAbs 2013, 5, 776–786. [Google Scholar] [CrossRef]
- Grove, J.; Hu, K.; Farquhar, M.J.; Goodall, M.; Walker, L.; Jamshad, M.; Drummer, H.E.; Bill, R.M.; Balfe, P.; McKeating, J.A. A new panel of epitope mapped monoclonal antibodies recognising the prototypical tetraspanin CD81. Wellcome Open Res. 2017, 2, 82. [Google Scholar] [CrossRef]
- Ströh, L.J.; Nagarathinam, K.; Krey, T. Conformational Flexibility in the CD81-Binding Site of the Hepatitis C Virus Glycoprotein E2. Front. Immunol. 2018, 9, 1396. [Google Scholar] [CrossRef]
- Nelson, B.; Adams, J.; Kuglstatter, A.; Li, Z.; Harris, S.F.; Liu, Y.; Bohini, S.; Ma, H.; Klumpp, K.; Gao, J.; et al. Structure-Guided Combinatorial Engineering Facilitates Affinity and Specificity Optimization of Anti-CD81 Antibodies. J. Mol. Biol. 2018, 430, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Burkova, E.E.; Dmitrenok, P.S.; Bulgakov, D.V.; Vlassov, V.V.; Ryabchikova, E.I.; Nevinsky, G.A. Exosomes from human placenta purified by affinity chromatography on sepharose bearing immobilized antibodies against CD81 tetraspanin contain many peptides and small proteins. IUBMB Life. 2018, 70, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Pandrangi, S.; Kumari, S.; Gavara, M.M.; Badana, A.K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia Pac. J. Clin. Oncol. 2018, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Chia, W.C.; How, C.W.; Tor, Y.S.; Show, P.L.; Looi, Q.H.D.; Foo, J.B. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells. Mol. Biotechnol. 2021, 63, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.; Monk, P.N.; Moseley, G.W.; Partridge, L.J. Strategies for targeting tetraspanin proteins: Potential therapeutic applications in microbial infections. BioDrugs 2009, 23, 341–359. [Google Scholar] [CrossRef]
- Fernandez, L.; Malrieu, M.; Bénistant, C.; Dosset, P.; Rubinstein, E.; Odintsova, E.; Berditchevski, F.; Milhiet, P.E. CD82 and Gangliosides Tune CD81 Membrane Behavior. Int. J. Mol. Sci. 2021, 22, 8459. [Google Scholar] [CrossRef]
- Chuang, S.T.; Papp, H.; Kuczmog, A.; Eells, R.; Condor Capcha, J.M.; Shehadeh, L.A.; Jakab, F.; Buchwald, P. Methylene Blue Is a Nonspecific Protein-Protein Interaction Inhibitor with Potential for Repurposing as an Antiviral for COVID-19. Pharmaceuticals 2022, 15, 621. [Google Scholar] [CrossRef]
- Cao, J.; Liao, X.L.; Wu, S.M.; Zhao, P.; Zhao, L.J.; Wu, W.B.; Qi, Z.T. Selection of a phage-displayed peptide recognized by monoclonal antibody directed blocking the site of hepatitis C virus E2 for human CD81. J. Microbiol. Methods. 2007, 68, 601–604. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, P.; Miao, X.H.; Zhao, L.J.; Xue, L.J.; Qi, Z.T. Phage display selection on whole cells yields a small peptide specific for HCV receptor human CD81. Cell. Res. 2003, 13, 473–479. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Lin, S.; Liu, M. Synthesized peptide 710-725 from HCV subtype 1a E2 glycoprotein blocks HCV infection through competitive binding of CD81. Int. J. Mol. Med. 2016, 37, 836–842. [Google Scholar] [CrossRef]
- Rodríguez-Salazar, C.A.; Recalde-Reyes, D.P. Design of Inhibitory Peptides of the Interaction between the E2 Protein of the Hepatitis C Virus and the CD81 and CD209 receptors. Infectio 2021, 25, 241–249. [Google Scholar] [CrossRef]
- Takakusagi, Y.; Takakusagi, K.; Sakaguchi, K.; Sugawara, F. Phage display technology for target determination of small-molecule therapeutics: An update. Expert. Opin. Drug Discov. 2020, 15, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Khan, F.I.; Singh, T.; Elumalai, P.; Balakumar, C.; Premnath, D.; Lai, D.; Chuturgoon, A.A.; Saravanan, M. Green Synthesis, Experimental and Theoretical Studies to Discover Novel Binders of Exosomal Tetraspanin CD81 Protein. ACS Omega. 2020, 5, 17973–17982. [Google Scholar] [CrossRef]
- Drummer, H.E.; Wilson, K.A.; Poumbourios, P. Identification of the hepatitis C virus E2 glycoprotein binding site on the large extracellular loop of CD81. J. Virol. 2002, 76, 11143–11147. [Google Scholar] [CrossRef]
- Wagner, C.E.; Mohler, M.L.; Kang, G.S.; Miller, D.D.; Geisert, E.E.; Chang, Y.A.; Fleischer, E.B.; Shea, K.J. Synthesis of 1-boraadamantaneamine derivatives with selective astrocyte vs. C6 glioma antiproliferative activity. A novel class of anti-hepatitis C agents with potential to bind CD81. J. Med. Chem. 2003, 46, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Olaby, R.A.; Azzazy, H.M.; Harris, R.; Chromy, B.; Vielmetter, J.; Balhorn, R. Identification of ligands that target the HCV-E2 binding site on CD81. J. Comput. Aided Mol. Des. 2013, 27, 337–346. [Google Scholar] [CrossRef]
- Rajesh, S.; Sridhar, P.; Tews, B.A.; Fénéant, L.; Cocquerel, L.; Ward, D.G.; Berditchevski, F.; Overduin, M. Structural basis of ligand interactions of the large extracellular domain of tetraspanin CD81. J. Virol. 2012, 86, 9606–9616. [Google Scholar] [CrossRef]
- Qian, X.J.; Jin, Y.S.; Chen, H.S.; Xu, Q.Q.; Ren, H.; Zhu, S.Y.; Tang, H.L.; Wang, Y.; Zhao, P.; Qi, Z.T.; et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016, 97, 1134–1144. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Peng, Z.; Liu, D.; Si, L.; Wang, J.; Yuan, B.; Huang, J.; Proksch, P.; Lin, W. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg. Med. Chem. 2019, 27, 560–567. [Google Scholar] [CrossRef]
- Dey, D.; Biswas, P.; Paul, P.; Mahmud, S.; Ema, T.I.; Khan, A.A.; Ahmed, S.Z.; Hasan, M.M.; Saikat, A.S.M.; Fatema, B.; et al. Natural flavonoids effectively block the CD81 receptor of hepatocytes and inhibit HCV infection: A computational drug development approach. Mol. Divers. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Gottwein, J.M.; Scheel, T.K.; Jensen, T.B.; Lademann, J.B.; Prentoe, J.C.; Knudsen, M.L.; Hoegh, A.M.; Bukh, J. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: Role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 2009, 49, 364–377. [Google Scholar] [CrossRef]
- Welker, M.W.; von Wagner, M.; Ochs, D.; Zimmer, V.; Hofmann, W.P.; Piiper, A.; Hartmann, R.W.; Herrmann, E.; Zeuzem, S.; Kronenberger, B. Influence of amantadine on CD81 expression on lymphocytes in chronic hepatitis C. Dig. Liver Dis. 2010, 42, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Baldick, C.J.; Wichroski, M.J.; Pendri, A.; Walsh, A.W.; Fang, J.; Mazzucco, C.E.; Pokornowski, K.A.; Rose, R.E.; Eggers, B.J.; Hsu, M.; et al. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Pathog. 2010, 6, e1001086. [Google Scholar] [CrossRef] [PubMed]
- Al Olaby, R.R.; Cocquerel, L.; Zemla, A.; Saas, L.; Dubuisson, J.; Vielmetter, J.; Marcotrigiano, J.; Khan, A.G.; Vences Catalan, F.; Perryman, A.L.; et al. Identification of a novel drug lead that inhibits HCV infection and cell-to-cell transmission by targeting the HCV E2 glycoprotein. PLoS ONE 2014, 9, e111333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Park, C.; Luong, T.T.D.; Park, E.M.; Choi, D.H.; Han, K.M.; Mai, H.N.; Nguyen, H.C.; Lim, Y.S.; Hwang, S.B. 5-Oxo-1-[(2,3,6,7-tetramethoxy-9-phenanthrenyl)methyl]-L-proline Inhibits Hepatitis C Virus Entry. Sci. Rep. 2019, 9, 7288. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Ziegler, S.; Albrecht, B.; Kronenberger, B.; Kaul, A.; Bartenschlager, R.; Kattner, L.; Klein, C.D.; Hartmann, R.W. Identification of terfenadine as an inhibitor of human CD81-receptor HCV-E2 interaction: Synthesis and structure optimization. Molecules 2008, 13, 1081–1110. [Google Scholar] [CrossRef]
- Holzer, M.; Ziegler, S.; Neugebauer, A.; Kronenberger, B.; Klein, C.D.; Hartmann, R.W. Structural modifications of salicylates: Inhibitors of human CD81-receptor HCV-E2 interaction. Arch. Pharm. 2008, 341, 478–484. [Google Scholar] [CrossRef]
- Moura, A.F.; Lima, K.S.B.; Sousa, T.S.; Marinho-Filho, J.D.B.; Pessoa, C.; Silveira, E.R.; Pessoa, O.D.L.; Costa-Lotufo, L.V.; Moraes, M.O.; Araújo, A.J. In vitro antitumor effect of a lignan isolated from Combretum fruticosum, trachelogenin, in HCT-116 human colon cancer cells. Toxicol. Vitr. 2018, 47, 129–136. [Google Scholar] [CrossRef]
- Koech, P.K.; Jócsák, G.; Boldizsár, I.; Moldován, K.; Borbély, S.; Világi, I.; Dobolyi, A.; Varró, P. Anti-glutamatergic effects of three lignan compounds: Arctigenin, matairesinol and trachelogenin—An ex vivo study on rat brain slices. Planta Med. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Akella, M.; Malla, R. Molecular modeling and in vitro study on pyrocatechol as potential pharmacophore of CD151 inhibitor. J. Mol. Graph. Model. 2020, 100, 107681. [Google Scholar] [CrossRef]
- Kgk, D.; Kumari, S.; Shailender, G.; Malla, R.R. Marine natural compound cyclo(L-leucyl-L-prolyl) peptide inhibits migration of triple negative breast cancer cells by disrupting interaction of CD151 and EGFR signaling. Chem. Biol. Interact. 2020, 315, 108872. [Google Scholar] [CrossRef]
- Mekky, R.Y.; El-Ekiaby, N.; El Sobky, S.A.; Elemam, N.M.; Youness, R.A.; El-Sayed, M.; Hamza, M.T.; Esmat, G.; Abdelaziz, A.I. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Arch. Virol. 2019, 164, 1587–1595. [Google Scholar] [CrossRef]
- Mekky, R.Y.; El-Ekiaby, N.M.; Hamza, M.T.; Elemam, N.M.; El-Sayed, M.; Esmat, G.; Abdelaziz, A.I. Mir-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor. J. Infect. 2015, 70, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; van Spriel, A.B. Antitumor Immunity Is Controlled by Tetraspanin Proteins. Front. Immunol. 2018, 9, 1185. [Google Scholar] [CrossRef]
- Danilov, A.V.; Spurgeon, S.E.; Siddiqi, T.; Quinson, A.M.; Maier, D.; Smith, D.; Brown, J.R. A phase Ib, open label, dose escalation trial of the anti-CD37 monoclonal antibody, BI 836826, in combination with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia. Invest. New Drugs. 2021, 39, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Balzarotti, M.; Magagnoli, M.; Canales, M.Á.; Corradini, P.; Grande, C.; Sancho, J.M.; Zaja, F.; Quinson, A.M.; Belsack, V.; Maier, D.; et al. A phase Ib, open-label, dose-escalation trial of the anti-CD37 monoclonal antibody, BI 836826, in combination with gemcitabine and oxaliplatin in patients with relapsed/refractory diffuse large B-cell lymphoma. Invest. New Drugs. 2021, 39, 1028–1035. [Google Scholar] [CrossRef]
- Leung, K.T.; Zhang, C.; Chan, K.Y.Y.; Li, K.; Cheung, J.T.K.; Ng, M.H.L.; Zhang, X.B.; Sit, T.; Lee, W.Y.W.; Kang, W.; et al. CD9 blockade suppresses disease progression of high-risk pediatric B-cell precursor acute lymphoblastic leukemia and enhances chemosensitivity. Leukemia 2020, 34, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Rappa, G.; Fontana, S.; Karbanová, J.; Aalam, F.; Tai, D.; Li, Z.; Pucci, M.; Alessandro, R.; Morimoto, C.; et al. Anti-Human CD9 Fab Fragment Antibody Blocks the Extracellular Vesicle-Mediated Increase in Malignancy of Colon Cancer Cells. Cells 2022, 11, 2474. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.; Kumar Babasahib, S.; Prashantha Kumar, B.R.; Manjunathaiah Raghavendra, N. Biphenyl-based small molecule inhibitors: Novel cancer immunotherapeutic agents targeting PD-1/PD-L1 interaction. Bioorg. Med. Chem. 2022, 73, 117001. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; Cheng, K.; Bi, H.; Chen, J. Small molecule-based immunomodulators for cancer therapy. Acta Pharm. Sin. B. 2022, 12, 4287–4308. [Google Scholar] [CrossRef]
- Zwergel, C.; Fioravanti, R.; Mai, A. PD-L1 small-molecule modulators: A new hope in epigenetic-based multidrug cancer therapy? Drug Discov. Today 2023, 28, 103435. [Google Scholar] [CrossRef]

| Proteins | Types | Interaction and Effects | References |
|---|---|---|---|
| E2 HCV | Viral protein | The transmembrane E2 glycoprotein of HCV utilizes CD81 as a coreceptor for cell entry. | [50,51,52] |
| Claudin-1 | Tight junction protein | The interaction CD81-Claudin1 contributes to HCV infection. | [53] |
| IFITM1 | Tight junction protein | IFITM1 interacts with CD81 to limit HCV entry. | [54,55] |
| LDL-R | Membrane receptor | Interplay between CD81, LDL-R and PCSK9, to control HCV entry into hepatic cells. | [56,57] |
| CD19 | Membrane receptor | CD81 and CD19 are core subunits of the B cell co-receptor complex. CD81 controls CD19 export activity, via a dynamically regulated process upon B cell activation. | [58] |
| EWI-2 (IgSF8, PGRL, CD316) | Signaling protein | The CD81/EWI-2 interaction contributes to the tetraspanin web and plays role in cancer cell growth and motility, and in HCV entry. | [59,60,61] |
| EWI-F (FPRP, CD9P-1) | Signaling protein | Complexes formed between EWI-F and CD81 (and CD9) play a role in the fusion of myotubes, which are essential elements of muscle architecture. | [62] |
| CD44 | Adhesion molecule | The interaction between CD81 and CD44, through their extracellular regions, promotes tumor cell cluster formation and lung metastasis of triple negative breast cancer. | [63] |
| α6 integrin | Adhesion molecule | In male germ cells, CD81 interacts with α6 integrin subunit (which forms a dimer with β4 integrin). The complex plays a role in sperm maturation. | [64] |
| β1 integrin (CD29) | Adhesion molecule | Radiation was found to induce CD29/CD81 complex formation, thereby increasing the cellular uptake of exosomes. | [65] |
| Rac1 | Small GTPase | Interaction of Rac with the C-terminal cytoplasmic portion of CD81 to regulate cell motility. Also has a role in bacterial infection. | [38] |
| CD4 | Cell surface antigen | CD81 interacts with CD4 dimers concentrated in tetraspanin-enriched microdomains. | [66] |
| CD9 | Tetraspanin | Tetraspanins CD9 and CD81 are involved in tetraspanin web formation in sperm. Molecular modelling suggests protein-protein interactions during sperm-egg membrane fusion. | [67] |
| SAMHD1 | Enzyme | CD81 interacts with the deoxynucleoside triphosphate phosphohydrolase SAMHD1 and regulates its expression. The interaction promotes the proteasome-dependent degradation of SAMHD1. It is one of the metabolic regulators of HIV-1 replication. | [68] |
| MT1-MMP | Enzyme | Several tetraspanins, including CD81, associate with the membrane-type 1 matrix metalloproteinase (MT1-MMP) to regulate its cell surface localization and its function (notably its capacity to activate pro-MMP-2). | [69] |
| Syndecan-1 | Proteoglycan | Knockdown of Syndecan-1 and CD81 inhibits HCV infection, suggesting their cooperative action. A direct interaction between the two proteins has been evidenced (using a proximity ligation assay). | [70] |
| AhpC | Enzyme | The mycobacterial enzyme alkyl hydroperoxide reductase C (AhpC) interacts with CD81-LEL to promote uptake of the pathogen by host cells. | [71] |
| TfR2 | Membrane receptor | Transferrin receptor 2 (TfR2) is a binding partner for CD81. The interaction triggers RfR2 degradation by the ubiquitin E3 ligase GRAIL. | [72] |
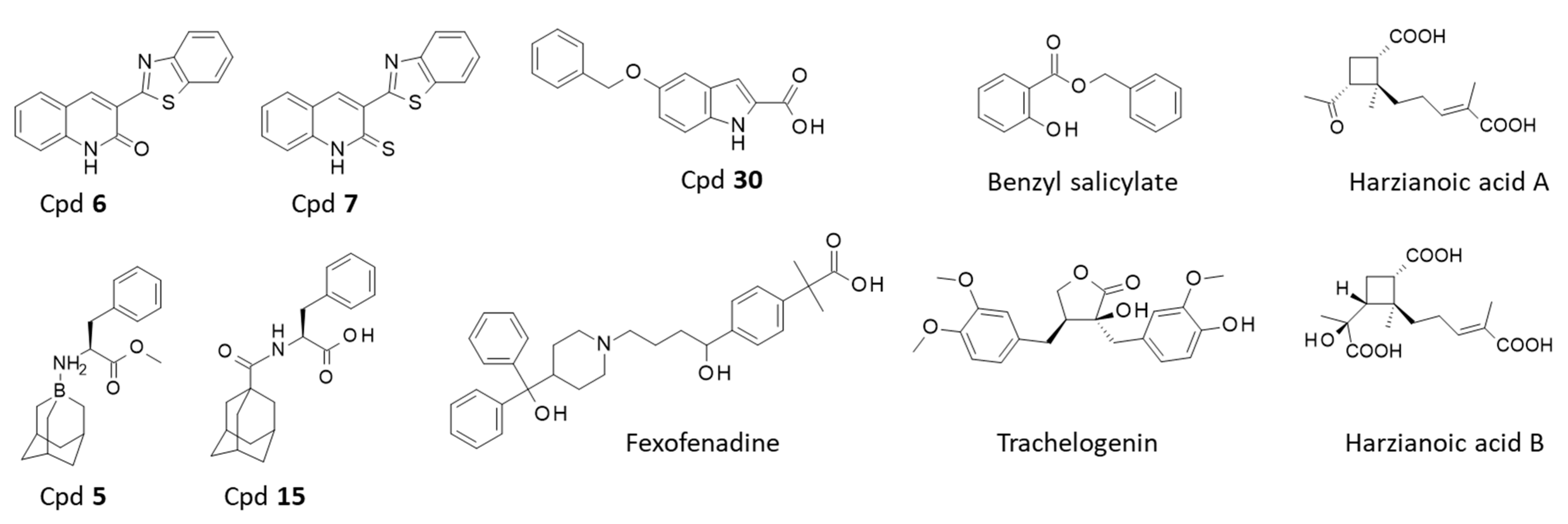
| Compounds 1 (Chemical Category) | CD81 Binding Information | References | |
|---|---|---|---|
| In Silico Data | Experimental Data | ||
| Cpd 6–7 (synthetic products) | Docking and molecular dynamic simulation predict binding of the compounds to the EC2 of CD81. | None | [146] |
| Cpd 5–15 (synthetic products) | Molecular modeling suggests binding of the compounds to EC2 of CD81. | None | [148] |
| Indole derivative 30 (synthetic products) | Binding site on EC2-CD81 identified by molecular modeling. | Surface plasmon resonance (SPR) confirmed binding of Cpd 30 to CD81 EC2. | [149] |
| Benzyl salicylate Fexofenadine (synthetic products) | None | Weak binding to EC2-CD80 characterized by NMR spectroscopy. | [150] |
| Trachelogenin (natural product) | Molecular docking used to identify the contact points between trachelogenin and CD81 EC2. | The NP reduces interaction between HCV E2 and CD81. Direct binding to CD81 EC2 confirmed using protein mutants. | [151] |
| Harzianoic acids A-B (natural products) | A docking analysis supported binding of the two NP to CD81 EC2. | Direct binding to CD81-EC2 evidenced by SPR. | [152] |
| Flavonoids: quercetin, puerarin, myricetin. (natural products) | Molecular docking and molecular dynamic simulation predict binding of the flavonoids to CD81 EC2. | None | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Thuru, X. Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases. Cancers 2023, 15, 2186. https://doi.org/10.3390/cancers15072186
Bailly C, Thuru X. Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases. Cancers. 2023; 15(7):2186. https://doi.org/10.3390/cancers15072186
Chicago/Turabian StyleBailly, Christian, and Xavier Thuru. 2023. "Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases" Cancers 15, no. 7: 2186. https://doi.org/10.3390/cancers15072186
APA StyleBailly, C., & Thuru, X. (2023). Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases. Cancers, 15(7), 2186. https://doi.org/10.3390/cancers15072186







