Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: Surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Terminology and Definition of AR
2.2. Surgical Indications for MIAR for HCC
2.3. Baseline Data Collection
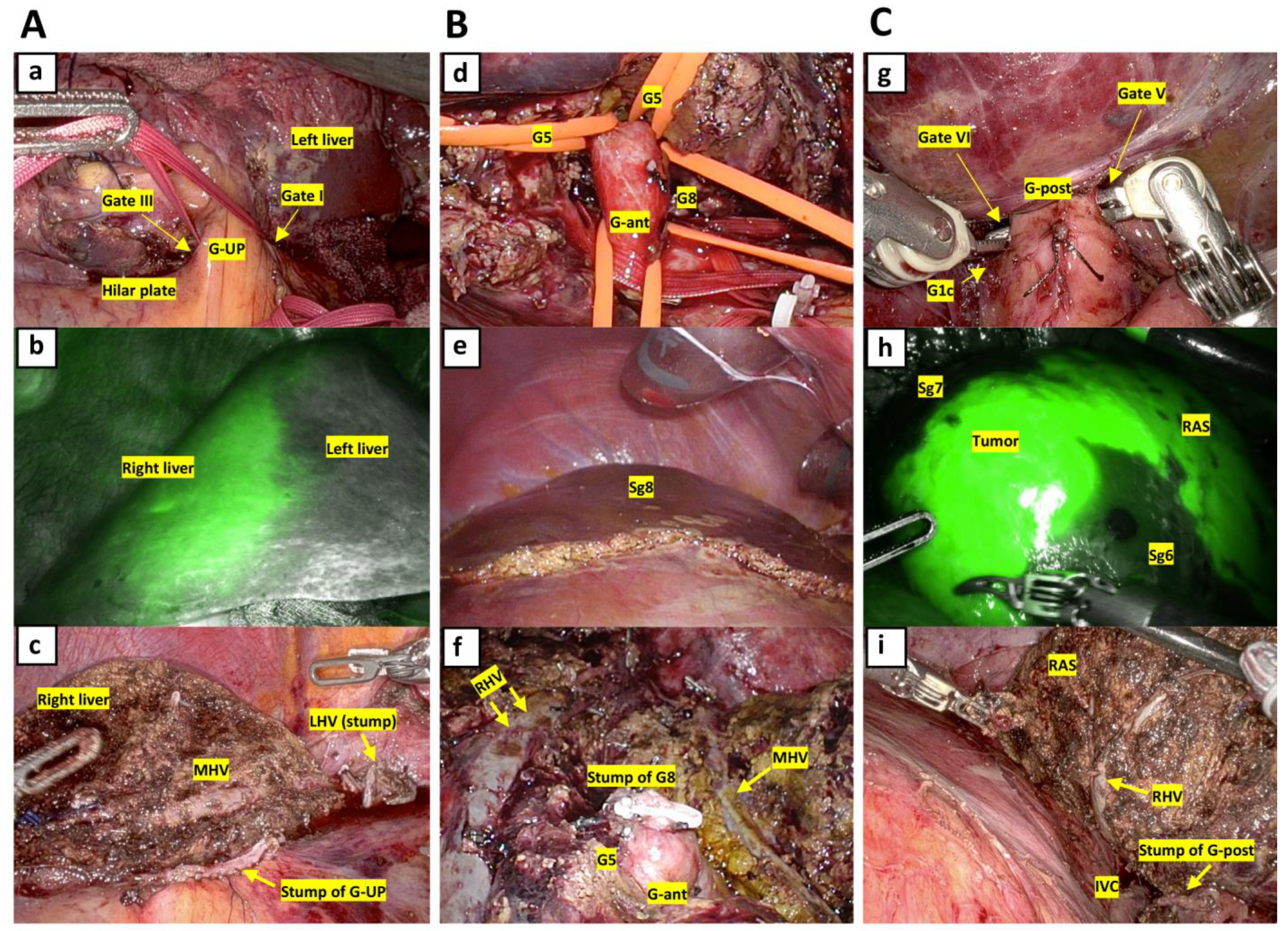
2.4. Surgical Techniques for AR
2.5. Perioperative Data
2.6. Statistical Analysis
3. Results
3.1. Perioperative Outcomes
3.1.1. Comparison between OAR and MIAR
Perioperative Outcomes
3.1.2. Comparison between Laparoscopic and Robotic AR for HCC
Patient and Tumor Baseline Data
Perioperative Outcomes
3.2. Long-Term Outcomes after AR for Newly Developed HCC
3.2.1. Comparison of Long-Term Outcomes between OAR and MIAR
Patient and Tumor Baseline Data
Long-Term Outcomes
3.2.2. Comparison of Long-Term Outcomes between Laparoscopic and Robotic AR
Patient and Tumor Baseline Data
Survival Data
Details of Postoperative Recurrence
Times of Surgery and Associated Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makuuchi, M.; Hasegawa, H.; Yamazaki, S. Ultrasonically guided subsegmentectomy. Surg. Gynecol. Obstet. 1985, 161, 346–350. [Google Scholar]
- Hasegawa, K.; Kokudo, N.; Imamura, H.; Matsuyama, Y.; Aoki, T.; Minagawa, M.; Sano, K.; Sugawara, Y.; Takayama, T.; Makuuchi, M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann. Surg. 2005, 242, 252–259. [Google Scholar] [CrossRef]
- Shindoh, J.; Makuuchi, M.; Matsuyama, Y.; Mise, Y.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J. Hepatol. 2016, 64, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Tomassini, F.; Berardi, G.; Mori, Y.; Shirata, C.; Abu Hilal, M.; Asbun, H.; Cherqui, D.; Gotohda, N.; Han, H.S.; et al. Glissonean approach for hepatic inflow control in minimally invasive anatomic liver resection: A systematic review. J. Hepatobiliary Pancreat. Sci. 2022, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Igarashi, K.; Li, C.J.; Ozaki, T.; Mishima, K.; Nakajima, K.; Honda, M.; Wakabayashi, G. Parenchymal sparing anatomical liver resections with full laparoscopic approach: Description of technique and short-term results. Ann. Surg. 2021, 273, 785–791. [Google Scholar] [CrossRef]
- Kato, Y.; Sugioka, A.; Kojima, M.; Kiguchi, G.; Tanahashi, Y.; Uchida, Y.; Yoshikawa, J.; Yasuda, A.; Nakajima, S.; Takahara, T.; et al. Laparoscopic isolated liver segmentectomy 8 for malignant tumors: Techniques and comparison of surgical results with the open approach using a propensity score-matched study. Langenbecks Arch. Surg. 2022, 407, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Lainas, P.; Carloni, A.; Caillard, C.; Champault, A.; Smadja, C.; Franco, D. Laparoscopic liver resection for hepatocellular carcinoma. Surg. Endosc. 2008, 22, 372–378. [Google Scholar]
- Lee, J.H.; Han, D.H.; Jang, D.S.; Choi, G.H.; Choi, J.S. Robotic extrahepatic Glissonean pedicle approach for anaomic liver resection in the right liver: Techniques and perioperative outcomes. Surg. Endosc. 2016, 30, 3882–3888. [Google Scholar] [CrossRef]
- Liao, K.; Yang, K.; Cao, L.; Lu, Y.; Zheng, B.; Li, X.; Wang, X.; Li, J.; Chen, J.; Zhenf, S. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomized controlled trial. Int. J. Surg. 2022, 102, 106652. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Belghiti, J.; Clavien, P.A.; Gadzijev, E.; Garden, J.O.; Lau, W.Y.; Makuuchi, M.; Strong, R.W. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Couinaud, C. Le Foie: Etudes Anatomiques et Chirurgicales; Masson: Paris, France, 1957; pp. 9–12. [Google Scholar]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sugioka, A.; Tanahashi, Y.; Kojima, M.; Nakajima, S.; Yasuda, A.; Yoshikawa, J.; Uyama, I. Standardization of isolated caudate lobectomy by extrahepatic Glissonean pedicle isolation and HV root-at first one-way resection based on Laennec’s capsule: Open and laparoscopic approaches. Surg. Gastroenterol. Oncol. 2020, 25, 89–92. [Google Scholar] [CrossRef]
- Makuuchi, M.; Kosuge, T.; Takayama, T.; Yamazaki, S.; Kakazu, T.; Miyagawa, S.; Kawasaki, S. Surgery for small liver cancers. Semin. Surg. Oncol. 1993, 9, 298–304. [Google Scholar] [CrossRef]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepatobiliary Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th ed.; Kokudo, N., Ed.; Kanehara: Tokyo, Japan, 2015; p. 26. [Google Scholar]
- Berardi, G.; Wakabayashi, G.; Igarashi, K.; Ozaki, T.; Toyota, N.; Tsuchiya, A.; Nishikawa, K. Full laparoscopic anatomical segment 8 resection for hepatocellular carcinoma using the Glissonian approach with indocyanine green dye fluorescence. Ann. Surg. Oncol. 2019, 26, 2577–2578. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, A.; Kato, Y.; Tanahashi, Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec’s capsule: Proposal of a novel comprehensive surgical anatomy of the liver. J. Hepatobiliary Pancreat. Sci. 2017, 24, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Sugioka, A.; Kato, Y.; Tanahashi, Y.; Yoshikawa, J.; Kiguchi, G.; Kojima, M.; Yasuda, A.; Nakajima, S.; Uyama, I. Standardization of anatomic liver resection based on Laennec’s capsule. Surg. Gastroenterol. Oncol. 2020, 25, 57–66. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Huang, X.; Ma, T.; Jin, H.; Lin, Y.; Yu, M.; Jian, Z. Postoperative complications affect long-term survival outcomes following hepatic resection for colorectal liver metastasis. World J. Surg. 2015, 39, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.C.; Dorcaratto, D.; Garces-Albir, M.; Munoz, E.; Arvizu, R.; Ortega, J.; Sabater, L. Impact of type and severity of postoperative complications on long-term outcomes after colorectal liver metastases resection. J. Surg. Oncol. 2020, 122, 212–225. [Google Scholar] [CrossRef]
- Kato, Y.; Sugioka, A.; Kojima, M.; Kiguchi, G.; Mii, S.; Uchida, Y.; Takahara, T.; Uyama, I. Initial experience with robotic liver resection: Audit of 120 consecutive cases at a single center and comparison with open and laparoscopic approaches. J. Hepatobiliary Pancreat. Sci. 2023, 30, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Dai, W.D.; Miao, X.Y.; Zhong, D.W.; Huang, S.F.; Wen, Y.; Xiong, S.Z. Anatomic resection of segment VIII of liver for hepatocellular carcinoma in cirrhotic patients based on an intrahepatic Glissonian approach. Surgery 2009, 146, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Ogiso, S.; Inoue, Y.; Shivathirthan, N.; Gayet, B. Laparoscopic parenchymal-sparing liver resections in the central segments: Feasible, safe, and effective. Surg. Endosc. 2015, 29, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepatobiliary Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef]
- Tozzi, F.; Berardi, G.; Vierstraete, M.; Kasai, M.; de Carvalho, L.A.; Vivarelli, M.; Montalti, R.; Troisi, R.I. Laparoscopic versus open approach for formal right and reft hepatectomy: A propensity score matching analysis. World J. Surg. 2018, 42, 2627–2634. [Google Scholar] [CrossRef]
- Untereiner, X.; Cagniet, A.; Memeo, R.; Cherkaoui, Z.; Piardi, T.; Severac, F.; Mutter, D.; Kianmanesh, R.; Wakabayashi, T.; Sommacale, D.; et al. Laparoscopic hepatectomy versus open hepatectomy for the management of hepatocellular carcinoma: A comparative study using a propensity score matching. World J. Surg. 2019, 43, 615–625. [Google Scholar] [CrossRef]
- Cheung, T.T.; Dai, W.C.; Tsang, S.H.; Chan, A.C.; Chok, K.S.; Chan, S.C.; Lo, C.M. Pure laparoscopic hepatectomy versus openhepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: A propensity analysis at a single center. Ann. Surg. 2016, 264, 612–620. [Google Scholar] [CrossRef]
- Lim, C.; Salloum, C.; Tudisco, A.; Ricci, C.; Osseis, M.; Napoli, N.; Lahat, E.; Boggi, U.; Azoulay, D. Short- and Long-term Outcomes after Robotic and Laparoscopic Liver Resection for Malignancies: A Propensity Score-Matched Study. World J. Surg. 2019, 43, 1594–1603. [Google Scholar] [CrossRef]
- Chong, C.C.; Fuks, D.; Lee, K.F.; Zhao, J.J.; Choi, G.H.; Sucandy, I.; Chiow, A.K.H.; Marino, M.V.; Gastaca, M.; Wang, X.; et al. Propensity score-matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg. 2022, 157, 436–444. [Google Scholar] [CrossRef]
- Yang, H.Y.; Choi, G.H.; Chin, K.H.; Choi, S.H.; Syn, N.L.; Chueng, T.T.; Chiow, A.K.H.; Sucandy, I.; Marino, M.V.; Prieto, M.; et al. Robotic and laparoscopic right anterior sectionectomy and central hepatectomy: Multicentre propensity score-matched analysis. Br. J. Surg. 2022, 109, 311–314. [Google Scholar] [CrossRef]
- Sucandy, I.; Rayman, S.; Lai, E.C.; Tang, C.N.; Chong, Y.; Efanov, M.; Fuks, D.; Choi, G.H.; Chong, C.C.; Chiow, A.K.H.; et al. Robotic versus laparoscopic left and extended left hepatectomy: An international multicenter study propensity score-matched analysis. Ann. Surg. Oncol. 2022, 29, 8398–8406. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Suda, K.; Shibasaki, S.; Nakamura, K.; Kadoya, S.; Kikuchi, K.; Inaba, K.; Uyama, I. Prognostic factors of minimally invasive surgery for gastric cancer: Does robotic gastrectomy bring oncological benefit? World J. Gastroenterol. 2021, 27, 6659–6672. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Okamura, Y.; Higaki, T.; Moriguchi, M.; Takayama, T. Effect of blood product transfusion on the prognosis on patients undergoing hepatectomy for hepatocellular carcinoma: A propensity score matching analysis. J. Gastroenterol. 2023, 58, 171–181. [Google Scholar] [CrossRef] [PubMed]



| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| OAR (N = 185) | MIAR (N = 142) | p | OAR (N = 91) | MIAR (N = 91) | p | |
| Age, years | 73 (31–91) | 71 (11–86) | 0.102 | 72 (43–91) | 72 (29–86) | 0.570 |
| Sex, M/F | 147/38 | 113/29 | 0.979 | 73/18 | 73/18 | 1.000 |
| BMI, kg/m2 | 23.0 (14.7–54.0) | 23.6 (15.2–36.3) | 0.013 | 23.1 (17.2–54.0) | 23.8 (16.0–36.3) | 0.456 |
| ASA score, I or II/≥III | 145/40 | 130/12 | 0.001 | 80/11 | 81/10 | 0.817 |
| Diabetes, n (%) | 71 (38.4) | 53 (37.3) | 0.846 | 38 (41.8) | 36 (39.6) | 0.763 |
| Total bilirubin, mg/dL | 0.7 (0.3–6.4) | 0.8 (0.2–1.7) | 0.097 | 0.7 (0.3–1.8) | 0.7 (0.2–1.7) | 0.782 |
| Prothrombin time, % | 98 (18–145) | 96 (28–129) | 0.386 | 97 (64–145) | 95 (63–129) | 0.319 |
| Platelet count, ×104/mm3 | 14.9 (1.2–47.2) | 15.5 (4.0–42.2) | 0.783 | 14.7 (1.2–47.2) | 15.0 (4.6–29.5) | 0.809 |
| ICGR15, % | 14.2 (0.6–52.6) | 11.1 (0.0–68.3) | 0.0002 | 14.1 (0.6–41.0) | 12.1 (0.0–68.3) | 0.072 |
| ≥13.0%, n (%) | 100 (55.3) | 49 (36.6) | 0.001 | 49 (53.9) | 42 (46.2) | 0.299 |
| Child-Pugh class, A/B | 177/8 | 141/1 | 0.047 | 86/5 | 90/1 | 0.097 |
| Etiology, HBV/HCV/NBNC | 37/70/78 | 35/48/59 | 0.561 | 19/30/42 | 20/31/40 | 0.956 |
| Cirrhosis (histology), n (%) | 58 (31.4) | 53 (37.3) | 0.258 | 31 (34.1) | 34 (37.4) | 0.643 |
| Tumor characteristics | ||||||
| Location, PS (%)/AL | 130 (70.3)/55 | 88 (62.0)/54 | 0.115 | 66 (72.5)/25 | 61 (67.0)/30 | 0.420 |
| Number | 1 (1–23) | 1 (1–6) | 0.053 | 1 (1–6) | 1 (1–6) | 0.254 |
| Single/Multiple | 122/63 | 107/35 | 0.066 | 70/21 | 63/28 | 0.242 |
| Size, cm | 5.0 (1.0–22.0) | 3.2 (0.7–16.0) | <0.0001 | 4.0 (1.0–17.7) | 3.8 (0.7–16.0) | 0.143 |
| ≥4.0 cm, n (%) | 114 (61.6) | 53 (37.3) | <0.0001 | 47 (51.7) | 44 (48.4) | 0.657 |
| Stage, I or II/≥III | 94/91 | 108/34 | <0.0001 | 63/28 | 59/32 | 0.528 |
| AFP, ng/mL | 15.1 (1.5–1,213,687.0) | 6.4 (1.0–149,880.0) | 0.0005 | 11.2 (1.5–636,200.0) | 6.9 (1.0–149,880.0) | 0.135 |
| DCP, mAU/mL | 287 (3–538,983) | 67 (10–47,453) | 0.0003 | 186 (10–159,600) | 74 (10–47,453) | 0.056 |
| Types of resection, n (%) | 0.004 | 0.976 | ||||
| Left lateral sectionectomy | 5 (2.7) | 8 (5.6) | 4 (4.4) | 4 (4.4) | ||
| Segmentectomy | 77 (41.6) | 83 (58.5) | 50 (55.0) | 47 (51.7) | ||
| Sectionectomy * | 56 (30.3) | 30 (21.1) | 22 (24.2) | 24 (26.4) | ||
| ≥Hemihepatectomy | 47 (25.4) | 21 (14.8) | 15 (16.5) | 16 (17.6) | ||
| Major Hx (≥3 segs), n (%) | 56 (30.3) | 23 (16.2) | 0.003 | 18 (19.8) | 17 (18.7) | 0.851 |
| Repeat Hx, n (%) | 21 (11.4) | 29 (20.4) | 0.024 | 13 (14.3) | 12 (13.2) | 0.830 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| OAR (N = 185) | MIAR (N = 142) | p | OAR (N = 91) | MIAR (N = 91) | p | |
| Operative time, min | 591 (498–781) | 637 (539–794) | 0.128 | 579 (474–731) | 643 (546–797) | 0.028 |
| Blood loss, g | 1083 (575–2006) | 244 (115–493) | <0.0001 | 955 (498–1753) | 279 (121–524) | <0.0001 |
| Transfusion*, n (%) | 98 (53.0) | 23 (16.2) | <0.0001 | 42 (47.3) | 16 (17.6) | <0.0001 |
| Pringle maneuver, n (%) | 27 (14.6) | 32 (22.5) | 0.064 | 16 (17.6) | 21 (23.1) | 0.357 |
| Open conversion, n (%) | NA | 4 (2.8) | NA | NA | 2 (2.2) | NA |
| Laboratory data | ||||||
| Max TB, mg/dL | 2.3 (1.6–3.4) | 1.5 (1.2–2.0) | <0.0001 | 2.2 (1.6–3.1) | 1.5 (1.2–1.9) | <0.0001 |
| Max AST, IU/L | 416 (291–808) | 598 (315–1026) | 0.005 | 438 (305–823) | 593 (348–1016) | 0.043 |
| Min PT, % | 63 (54–68) | 63 (58–71) | 0.093 | 64 (54–68) | 62 (56–71) | 0.810 |
| Max CRP, mg/dL | 10.40 (7.65–13.04) | 9.00 (6.25–13.01) | 0.028 | 11.10 (7.80–12.80) | 8.62 (6.32–12.60) | 0.017 |
| Morbidity (≤90 days), n (%) | ||||||
| Overall (≥CD-I) | 97 (52.4) | 50 (35.2) | 0.002 | 52 (57.1) | 33 (36.3) | 0.005 |
| Major (≥CD-IIIa) | 33 (17.8) | 12 (8.5) | 0.015 | 19 (20.9) | 4 (4.4) | 0.0008 |
| Bile leak or collection | 14 (7.6) | 6 (4.2) | 0.211 | 10 (11.0) | 1 (1.1) | 0.005 |
| Mortality, n (%) | ||||||
| ≤30 days | 2 (1.1) | 0 (0) | 0.214 | 1 (1.1) | 0 (0) | 0.316 |
| ≤90 days | 5 (2.7) | 0 (0) | 0.048 | 4 (4.4) | 0 (0) | 0.043 |
| R0 resection, n (%) | 179 (96.8) | 141 (99.3) | 0.116 | 88 (96.7) | 90 (98.9) | 0.312 |
| Length of hospital stay, days | 28 (20–40) | 15 (12–19) | <0.0001 | 29 (21–43) | 15 (13–19) | <0.0001 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Laparoscopic AR (N = 102) | Robotic AR (N = 40) | p | Laparoscopic AR (N = 31) | Robotic AR (N = 31) | p | |
| Age, years | 70 (11–86) | 72 (21–82) | 0.353 | 70 (36–83) | 72 (21–82) | 0.989 |
| Sex, M/F | 80/22 | 33/7 | 0.589 | 25/6 | 25/6 | 1.000 |
| BMI, kg/m2 | 23.6 (15.2–36.3) | 23.9 (17.9–30.3) | 0.895 | 23.0 (18.0–33.9) | 23.9 (17.9–30.3) | 0.186 |
| ASA score, I or II/≥III | 94/8 | 36/4 | 0.678 | 29/2 | 28/3 | 0.641 |
| Total bilirubin, mg/dL | 0.8 (0.2–1.7) | 0.7 (0.3–1.3) | 0.479 | 0.7 (0.2–1.6)) | 0.7 (0.3–1.3) | 0.854 |
| Prothrombin time, % | 96 (63–129) | 97 (28–127) | 0.701 | 96 (67–128) | 97 (83–127) | 0.849 |
| Platelet count, x104/mm3 | 15.7 (4.0–42.2) | 15.3 (7.6–23.5) | 0.665 | 15.5 (4.6–29.5) | 15.1 (9.1–23.5) | 0.961 |
| ICGR15, % | 11.1 (0.6–68.3) | 10.9 (0.0–30.8) | 0.284 | 10.5 (0.6–27.6) | 11.8 (0.0–30.8) | 0.554 |
| ≥13.0%, n (%) | 35 (36.5) | 14 (36.8) | 0.967 | 6 (22.2) | 13 (43.3) | 0.091 |
| Child-Pugh, A/B | 101/1 | 40/0 | 0.530 | 31/0 | 31/0 | 1.000 |
| Etiology, HBV/HCV/NBNC | 24/36/42 | 11/12/17 | 0.805 | 7/11/13 | 9/8/14 | 0.247 |
| Cirrhosis (histology), n (%) | 46 (45.1) | 7 (17.5) | 0.002 | 6 (19.4) | 7 (22.6) | 0.755 |
| Tumor characteristics | ||||||
| Location, PS (%)/AL | 65 (63.7)/37 | 23 (57.5)/17 | 0.492 | 20 (64.5)/11 | 16 (51.6)/15 | 0.303 |
| Number | 1 (1–4) | 1 (1–6) | 0.047 | 1 (1–3) | 1 (1–6) | 0.927 |
| Single/Multiple | 81/21 | 26/14 | 0.073 | 23/8 | 23/8 | 1.000 |
| Size, cm | 3.5 (0.7–16.0) | 2.7 (1.2–12.5) | 0.027 | 3.0 (1.5–16.0) | 3.0 (1.2–12.5) | 0.371 |
| ≥4.0 cm, n (%) | 44 (43.1) | 9 (22.5) | 0.022 | 12 (38.7) | 8 (25.8) | 0.277 |
| Stage, I or II/≥III | 81/21 | 27/1 | 0.135 | 23/8 | 21/10 | 0.576 |
| AFP, ng/mL | 8.2 (1.9–149,880.0) | 4.2 (1.0–5811.0) | 0.002 | 7.1 (2.0–2708.5) | 4.5 (1.0–5811.0) | 0.113 |
| DCP, mAU/mL | 75 (10–47,453) | 44 (11–30,899) | 0.071 | 68 (11–47,032) | 4.5 (1.0–5811.0) | 0.251 |
| Repeat Hx, n (%) | 13 (12.8) | 16 (40.0) | 0.0003 | 6 (19.4) | 7 (22.6) | 0.755 |
| Types of resection, n (%) | 0.775 | 0.271 | ||||
| Left lateral sectionectomy | 6 (5.9) | 2 (5.0) | 0 (0) | 2 (6.5) | ||
| Segmentectomy | 58 (56.9) | 25 (62.5) | 18 (58.1) | 20 (64.5) | ||
| Sectionectomy* | 21 (20.6) | 9 (22.5) | 10 (32.3) | 5 (16.1) | ||
| ≥Hemihepatectomy | 17 (16.7) | 4 (10.0) | 3 (9.7) | 4 (12.9) | ||
| Major Hx (≥3 segs), n (%) | 19 (18.6) | 4 (10.0) | 0.838 | 31 (100) | 4 (12.9) | 0.719 |
| Iwate criteria, level, n (%) | 0.549 | 0.327 | ||||
| Intermediate | 22 (21.6) | 12 (30.0) | 5 (16.1) | 10 (32.3) | ||
| Advanced | 51 (50.0) | 17 (42.5) | 18 (58.1) | 14 (45.2) | ||
| Expert | 29 (28.4) | 11 (27.5) | 8 (25.8) | 7 (22.6) | ||
| ≥Advanced, n (%) | 80 (78.4) | 28 (70.0) | 0.290 | 26 (83.9) | 21 (67.7) | 0.138 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Laparoscopic AR (N = 102) | Robotic AR (N = 40) | p | Laparoscopic AR(N = 31) | Robotic AR (N = 31) | p | |
| Operative time, min | 631 (525–774) | 667 (566–893) | 0.157 | 632 (569–732) | 642 (564–891) | 0.709 |
| Parenchymal dissection time, min | 240 (175–325) | 273 (177–340) | 0.548 | 248 (177–333) | 227 (139–367) | 0.906 |
| Blood loss, g | 245 (120–488) | 200 (98–635) | 0.890 | 227 (90–468) | 170 (98–598) | 0.989 |
| Transfusion*, n (%) | 16 (15.7) | 7 (17.5) | 0.792 | 3 (9.7) | 6 (19.4) | 0.279 |
| Pringle maneuver, n (%) | 18 (17.7) | 14 (35.0) | 0.026 | 5 (16.1) | 10 (32.3) | 0.138 |
| Open conversion, n (%) | 2 (2.0) | 2 (5.0) | 0.325 | 0 (0) | 1 (3.2) | 0.313 |
| Laboratory data | ||||||
| Max TB, mg/dL | 1.5 (1.2–1.9) | 1.5 (1.3–2.0) | 0.701 | 1.4 (1.1–1.6) | 1.5 (1.2–2.0) | 0.073 |
| Max AST, IU/L | 546 (296–913) | 767 (370–2,100) | 0.026 | 593 (315–903) | 707 (348–2,796) | 0.275 |
| Min PT, % | 64 (59–72) | 60 (49–71) | 0.032 | 62 (58–73) | 60 (51–72) | 0.135 |
| Max CRP, mg/dL | 8.68 (6.32–12.72) | 9.64 (5.97–12.18) | 0.396 | 8.44 (6.32–12.6) | 10.18 (5.45–14.11) | 0.477 |
| Morbidity (≤90 days), n (%) | ||||||
| Overall (≥CD-I) | 37 (36.3) | 13 (32.5) | 0.672 | 10 (32.3) | 9 (29.0) | 0.783 |
| Major (≥CD-IIIa) | 7 (6.9) | 5 (12.5) | 0.277 | 2 (6.5) | 5 (16.1) | 0.229 |
| Bile leak or collection | 5 (4.9) | 1 (2.5) | 0.522 | 2 (6.5) | 1 (3.2) | 0.554 |
| Mortality, n (%) | ||||||
| ≤30 days | 0 (0) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| ≤90 days | 0 (0) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| R0 resection, n (%) | 101 (99.0) | 40 (100) | 0.530 | 31 (100) | 31 (100) | 1.000 |
| Length of hospital stay, days | 15 (12–19) | 15 (11–18) | 0.416 | 14 (11–18) | 15 (11–18) | 0.965 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| OAR (N = 163) | MIAR (N = 113) | p | OAR (N = 76) | MIAR (N = 76) | p | |
| Age, years | 72 (31–91) | 71 (21–86) | 0.099 | 72 (43–86) | 72 (29–85) | 0.919 |
| Sex, M/F | 129/34 | 90/23 | 0.919 | 58/18 | 59/17 | 0.847 |
| BMI, kg/m2 | 23.1 (14.7–54.0) | 24.1 (16.0–36.3) | 0.011 | 23.1 (14.7–54.0) | 23.3 (16.0–36.3) | 0.235 |
| ASA score, I or II/≥III | 125/38 | 103/10 | 0.001 | 64/12 | 67/9 | 0.481 |
| Diabetes, n (%) | 66 (40.5) | 44 (38.9) | 0.796 | 29 (38.2) | 31 (40.8) | 0.740 |
| ICGR15, % | 14.2 (0.6–52.6) | 11.3 (0–39.4) | 0.0007 | 12.6 (1.2–41.0) | 12.1 (0–39.4) | 0.227 |
| ≥13.0%, n (%) | 89 (55.6) | 40 (37.0) | 0.003 | 35 (46.1) | 36 (47.4) | 0.871 |
| Etiology, HBV/HCV/NBNC | 32/56/75 | 23/37/53 | 0.961 | 16/27/33 | 14/25/37 | 0.803 |
| Cirrhosis (histology), n (%) | 49 (30.1) | 41 (36.3) | 0.278 | 31 (40.8) | 31 (40.8) | 1.000 |
| Tumor characteristics | ||||||
| Location, PS (%)/AL | 119 (73.0)/44 | 71 (62.8)/42 | 0.073 | 53 (69.7)/23 | 55 (72.4)/21 | 0.721 |
| Number | 1 (1–23) | 1 (1–4) | 0.049 | 1 (1–23) | 1 (1–4) | 0.766 |
| Single/Multiple | 109/54 | 86/27 | 0.098 | 57/19 | 55/21 | 0.713 |
| Size, cm | 5.5 (1.2–22.0) | 3.5 (0.7–16.0) | <0.0001 | 4.0 (1.2–22.0) | 4.0 (0.7–16.0) | 0.491 |
| ≥4.0 cm, n (%) | 110 (67.5) | 49 (43.4) | <0.0001 | 40 (52.6) | 43 (56.6) | 0.625 |
| Stage, I/II/III/IVA/IVB | 8/72/60/18/5 | 11/72/27/2/1 | 0.0005 | 6/42/24/3/1 | 8/42/23/2/1 | 0.973 |
| AFP, ng/mL | 15.9 (1.5–1,213,687.0) | 7.3 (1.4–149,880.0) | 0.010 | 13.7 (2.1–636,200.0) | 9.8 (2.0–149,880.0) | 0.340 |
| DCP, mAU/mL | 389 (3–538,983) | 78 (10–47,453) | 0.002 | 252 (3–538,983) | 148 (10–47,453) | 0.671 |
| Differentiation | 0.733 | 0.846 | ||||
| well | 6 | 6 | 4 | 3 | ||
| moderate | 150 | 102 | 70 | 69 | ||
| poor or sarcomatous | 4 | 2 | 1 | 2 | ||
| combined | 2 | 30 | 1 | 2 | ||
| necrosis | 1 | 0 | 0 | 0 | ||
| Types of resection, n (%) | 0.0003 | 0.905 | ||||
| Left lateral sectionectomy | 5 (3.1) | 7 (6.2) | 4 (5.3) | 3 (3.9) | ||
| Segmentectomy | 63 (38.7) | 66 (58.4) | 37 (48.7) | 40 (52.6) | ||
| Sectionectomy* | 52 (31.9) | 21 (18.6) | 17 (22.4) | 18 (23.7) | ||
| ≥Hemihepatectomy | 43 (26.4) | 19 (16.8) | 18 (23.7) | 15 (19.7) | ||
| Major Hx (≥3 segs), n (%) | 52 (31.9) | 21 (18.6) | 0.014 | 18 (23.7) | 16 (21.1) | 0.697 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Laparoscopic AR (N = 89) | Robotic AR (N = 24) | p | Laparoscopic AR (N = 22) | Robotic AR (N = 22) | p | |
| Major Hx (≥3 segs), n (%) | 17 (19.1) | 4 (16.7) | 0.786 | 3 (13.6) | 4 (18.2) | 0.680 |
| Age, years | 70 (29–86) | 72 (21–82) | 0.975 | 71 (53–83) | 72 (48–82) | 0.778 |
| Sex, M/F | 71/18 | 19/5 | 0.948 | 19/3 | 19/3 | 1.000 |
| BMI, kg/m2 | 24.0 (16.0–36.3) | 24.2 (17.9–30.3) | 0.744 | 23.5 (18.0–33.2) | 24.4 (17.9–30.3) | 0.411 |
| ASA score, I or II/≥III | 81/8 | 22/2 | 0.920 | 21/1 | 20/2 | 0.550 |
| Diabetes, n (%) | 37 (41.6) | 7 (29.2) | 0.269 | 9 (40.9) | 7 (31.8) | 0.531 |
| ICGR15, % | 11.3 (0.6–39.4) | 11.3 (0–17.5) | 0.329 | 10.5 (4.3–20.9) | 11.2 (0.0–16.0) | 0.865 |
| ≥13.0%, n (%) | 32 (37.7) | 8 (34.8) | 0.801 | 6 (28.6) | 7 (31.8) | 0.817 |
| Etiology, HBV/HCV/NBNC | 18/30/41 | 5/7/12 | 0.912 | 5/6/11 | 4/7/11 | 0.910 |
| Cirrhosis (histology), n (%) | 38 (42.7) | 3 (12.5) | 0.006 | 3 (13.6) | 3 (13.6) | 1.000 |
| Child-Pugh class, A/B | 88/1 | 24/0 | 0.602 | 22/0 | 22/0 | 1.000 |
| Tumor characteristics | ||||||
| Location, PS (%)/AL | 57 (64.0)/32 | 14 (58.3)/10 | 0.607 | 10 (45.5)/12 | 13 (59.1)/9 | 0.365 |
| Number | 1 (1–4) | 1 (1–4) | 0.517 | 1 (1–2) | 1 (1–4) | 0.936 |
| Single/Multiple | 69/20 | 17/7 | 0.495 | 17/5 | 17/5 | 1.000 |
| Size, cm | 3.5 (0.7–16.0) | 3.1 (1.5–12.5) | 0.111 | 3.3 (1.5–6.0) | 3.1 (1.5–6.0) | 0.814 |
| ≥4.0 cm, n (%) | 43 (48.3) | 6 (25.0) | 0.041 | 5 (22.7) | 5 (22.7) | 1.000 |
| Stage, I/II/III/IVA/IVB | 11/57/19/1/1 | 0/15/8/1/0 | 0.252 | 5/13/4/0 | 0/14/7/1 | 0.077 |
| AFP, ng/mL | 8.8 (2.0–14,980.0) | 6.0 (1.4–5,811.0) | 0.185 | 5.4 (2.0–2,708.5) | 6.0 (1.4–1,372.0) | 0.890 |
| DCP, mAU/mL | 95 (10–47,453) | 43 (14–20,843) | 0.073 | 40 (10–2,753) | 43 (14–20,843) | 0.576 |
| Differentiation | 0.471 | 0.178 | ||||
| well | 6 | 0 | 2 | 0 | ||
| moderate | 79 | 23 | 20 | 22 | ||
| poor or sarcomatous | 2 | 1 | 0 | 0 | ||
| combined | 2 | 0 | 0 | 0 | ||
| Types of resection, n (%) | 0.950 | 0.731 | ||||
| Left lateral sectionetomy | 6 (6.7) | 1(4.2) | 1 (4.6) | 0 (0) | ||
| Segmentectomy | 51 (57.3) | 15 (62.5) | 13 (59.1) | 14 (63.6) | ||
| Sectionectomy* | 17(19.1) | 4 (16.7) | 5 (22.7) | 4 (18.2) | ||
| ≥Hemihepatectomy | 15 (16.9) | 15 (16.9) | 3 (13.6) | 4 (18.2) | ||
| Recurrent Cases in the Unmatched Cohorts | Recurrent Cases in the Matched Cohorts | |||||
|---|---|---|---|---|---|---|
| OAR (N = 111) | MIAR (N = 51) | p | OAR (N = 50) | MIAR (N = 37) | p | |
| Patterns of recurrence, n (%) | 0.463 | 0.715 | ||||
| Intrahepatic-only | 84 (75.7) | 43 (84.3) | 37 (74.0) | 30 (81.1) | ||
| Extrahepatic-only | 7 (6.3) | 2 (3.9) | 3 (6.0) | 2 (5.4) | ||
| Intra- and extra-hepatic | 20 (18.0) | 6 (11.8) | 10 (20.0) | 5 (13.5) | ||
| Extrahepatic recurrence, n (%) | 27 (24.3) | 8 (15.7) | 0.215 | 13 (26.0) | 7 (18.9) | 0.438 |
| Lung | 13 (11.7) | 5 (9.8) | 0.720 | 6 (12.0) | 5 (13.5) | 0.834 |
| Bone | 11 (9.9) | 2 (3.9) | 0.193 | 6 (12.0) | 1 (2.7) | 0.115 |
| Lymph node | 9 (8.2) | 2 (3.9) | 0.325 | 5 (10.0) | 2 (5.4) | 0.436 |
| Hematogenous metastasis | 22 (19.8) | 6 (11.8) | 0.208 | 9 (18.0) | 5 (13.5) | 0.573 |
| Recurrence-free survival (mo), range | 7.6 (0.5–73.9) | 15.4 (1.4–86.3) | 0.007 | 9.4 (0.8–73.9) | 15.4 (1.4–86.3) | 0.125 |
| First recurrence <1 year, n (%) | 69 (62.2) | 19 (37.3) | 0.003 | 27 (54.0) | 13 (35.1) | 0.081 |
| First recurrence <2 years, n (%) | 87 (78.4) | 34 (66.7) | 0.111 | 38 (76.0) | 24 (64.9) | 0.257 |
| Pathologic tumor stage, n (%) | 0.008 | 0.696 | ||||
| I or II | 49 (44.1) | 34 (66.7) | 29 (58.0) | 23 (62.2) | ||
| ≥III | 62 (55.9) | 17 (33.3) | 21 (42.0) | 14 (37.8) | ||
| Pathologic differentiation, n (%) | 0.557 | 0.830 | ||||
| well | 3 (2.7) | 2 (3.9) | 2 (4.0) | 1 (2.7) | ||
| moderate | 104 (93.7) | 46 (88.5) | 46 (92.0) | 33 (89.2) | ||
| poor or sarcomatous | 2 (1.8) | 1 (1.9) | 1 (2.0) | 1 (2.7) | ||
| combined | 2 (1.8) | 3 (5.8) | 1 (2.0) | 2 (5.4) | ||
| Perioperative morbidity, n (%) | ||||||
| Any (C–D grade ≥I) | 57 (51.4) | 21 (41.2) | 0.229 | 25 (50.0) | 16 (43.2) | 0.533 |
| Major (C–D grade ≥III) | 18 (16.2) | 6 (11.8) | 0.459 | 5 (10.0) | 5 (13.5) | 0.612 |
| Bile leak/collection | 9 (8.1) | 3 (5.9) | 0.615 | 2 (4.0) | 2 (5.4) | 0.757 |
| Treatment for recurrent tumor, n (%) | ||||||
| Resection | ||||||
| Any organs | 35 (31.5) | 23 (45.1) | 0.094 | 16 (32.0) | 15 (40.5) | 0.411 |
| Liver (n/with liver recurrence) | 32/104 (30.8) | 23/49 (46.9) | 0.052 | 14/50 (29.8) | 15/35 (42.9) | 0.221 |
| Use of MTA* or immunotherapy** | 19 (17.7) | 9 (17.1) | 0.934 | 11 (22.0) | 9 (24.3) | 0.799 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y.; Sugioka, A.; Kojima, M.; Mii, S.; Uchida, Y.; Iwama, H.; Mizumoto, T.; Takahara, T.; Uyama, I. Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: Surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches. Cancers 2023, 15, 2219. https://doi.org/10.3390/cancers15082219
Kato Y, Sugioka A, Kojima M, Mii S, Uchida Y, Iwama H, Mizumoto T, Takahara T, Uyama I. Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: Surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches. Cancers. 2023; 15(8):2219. https://doi.org/10.3390/cancers15082219
Chicago/Turabian StyleKato, Yutaro, Atsushi Sugioka, Masayuki Kojima, Satoshi Mii, Yuichiro Uchida, Hideaki Iwama, Takuya Mizumoto, Takeshi Takahara, and Ichiro Uyama. 2023. "Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: Surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches" Cancers 15, no. 8: 2219. https://doi.org/10.3390/cancers15082219
APA StyleKato, Y., Sugioka, A., Kojima, M., Mii, S., Uchida, Y., Iwama, H., Mizumoto, T., Takahara, T., & Uyama, I. (2023). Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: Surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches. Cancers, 15(8), 2219. https://doi.org/10.3390/cancers15082219







