Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
- 1
- Hypertriglyceridemia (≥150 mg/dL) or treatment of hypertriglyceridemia;
- 2
- Low HDL cholesterol levels (<40 mg/dL in men or <50 mg/dL in women) or hypercholesterolemia drug administration;
- 3
- Elevated fasting glucose (≥100 mg/dL) or treatment of diabetes mellitus;
- 4
- Hypertension (≥130/85 mmHg) or treatment of hypertension.
- 1
- Normal BMI (BMI 20–24.9) vs. higher BMI (BMI ≥ 25);
- 2
- With MS vs. without MS;
- 3
- Gender (male vs. female).
2.2. Assessment of Serum Biomarkers
2.3. Statistical Analysis
3. Results
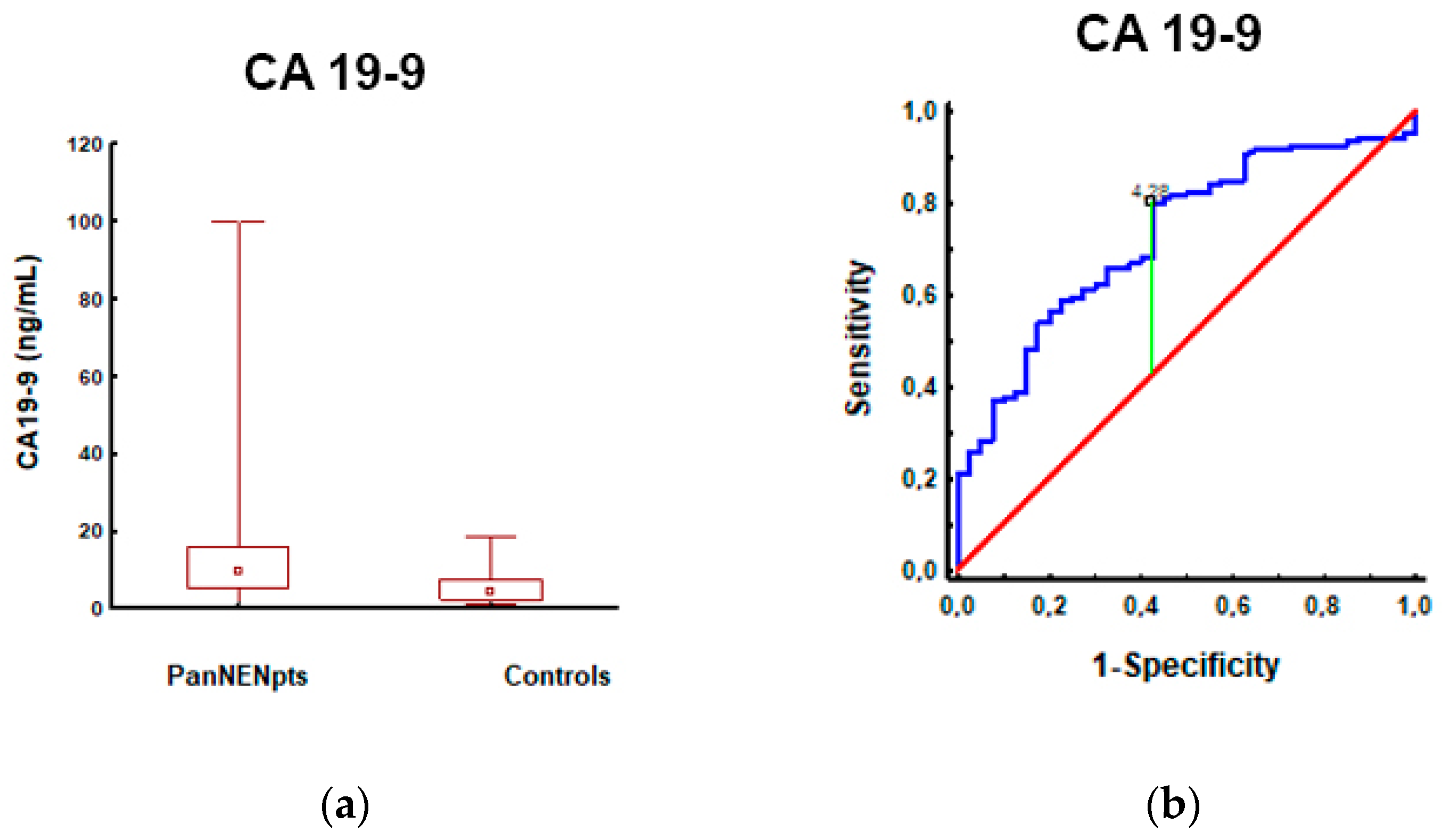
3.1. Pancreatic Neuroendocrine Neoplasm Patients vs. Controls
3.2. Patients with Pancreatic Neuroendocrine Tumors with Normal BMI vs. High BMI
3.3. Patients with Pancreatic Neuroendocrine Neoplasms (PanNENs) with Metabolic Syndrome (MS) vs. PanNENs without MS
3.4. Patients with Pancreatic Neuroendocrine Tumors: Male vs. Female
3.5. Spearman’s Correlation
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic neuroendocrine neoplasms—Update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Howe, R.; Merchant, J.; Conrad, N.; Keutgen, C.; Hallet, X.; Drebin, J.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou-Zerdan, M.; Allam, S.; Bou-Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetes Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; López-Saavedra, A.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.D.L.; Sánchez-Margalet, V. Leptin, both bad and good actor in cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Siemińska, L.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Warakomski, J.; Kos-Kudła, B. Serum concentrations of adipokines in men with prostate cancer and benign prostate hyperplasia. Endokrynol. Pol. 2018, 69, 120–127. [Google Scholar] [CrossRef]
- Warakomski, J.; Romuk, E.; Jarząb, B.; Krajewska, J.; Siemińska, L. Concentrations of selected adipokines, interleukin-6 and Vitamin D in patients with papillary thyroid carcinoma in respect to thyroid cancer stages. Int. J. Endocrinol. 2018, 11, 4921803. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9—Tumor marker: Past, present, and future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–454. [Google Scholar] [CrossRef]
- Muntjewerff, E.M.; Dunkel, G.; Nicolasen, M.J.T.; Mahata, S.K.; Van Den Bogaart, G. Catestatin as a target for treatment of inflammatory diseases. Front. Immunol. 2018, 9, 2199. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Mularczyk, M.; Marycz, K.; Bourebaba, L. Catestatin peptide of chromogranin A as a potential new target for several risk factors management in the course of metabolic syndrome. Biomed. Pharmacother. 2021, 134, 111–113. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188326. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumors of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Bałdys-Waligórska, A.; Nowak, A. Neuroendocrine neoplasms of the digestive system—Current classification and terminology. Nowotwory 2021, 71, 26–37. [Google Scholar] [CrossRef]
- Raposo, L. Metabolic syndrome in Poland: The WOBASZ II study. Pol. Arch. Intern. Med. 2021, 131, 501–502. [Google Scholar] [CrossRef]
- Natalicchio, A.; Faggiano, A.; Zatelli, M.C.; Argentiero, A.; D’Oronzo, S.; Marrano, N.; Beretta, G.D.; Acquati, S.; Adinolfi, V.; Di Bartolo, P.; et al. Metabolic disorders and gastroenteropancreatic-neuroendocrine tumors (GEP-NENs): How do they influence each other? An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit. Rev. Oncol. Hematol. 2022, 169, 103572. [Google Scholar] [CrossRef]
- Font-Burgada, J.; Sun, B.; Karin, M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016, 23, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Kompella, P.; Vasquez, K.M. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol. Carcinog. 2019, 58, 1531–1550. [Google Scholar] [CrossRef]
- Karczewski, J.; Begier, B.; Rafał, K.; Edyta, S. Obesity and the Risk of Gastrointestinal Cancers. Dig. Dis. Sci. 2019, 64, 2740–2749. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci-Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef]
- Gukovsky, I.; Li, N.; Todoric, J.; Gukovskaya, A.; Karin, M. Inflammation, autophagy, and obesity: Common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Byers, T.; Sedjo, R.L. Body fatness as a cause of cancer: Epidemiologic clues to biologic mechanisms. Endocr.-Relat. Cancer 2015, 22, 125–134. [Google Scholar] [CrossRef]
- Duvillié, B.; Kourdoughli, R.; Druillennec, S.; Eychène, A.; Pouponnot, C. Interplay Between Diabetes and Pancreatic Ductal Adenocarcinoma and Insulinoma: The Role of Aging, GeNENic Factors, and Obesity. Front. Endocrinol. 2020, 11, 563267. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G.; La Vecchia, C.; Boccia, S.; Rindi, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 68–81. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Bamlet, W.R.; McWilliams, R.R.; Hobday, T.J.; Burch, P.A.; Rabe, K.G.; Petersen, G.M. Risk factors for pancreatic neuroendocrine tumors a clinic-based case-control study. Pancreas 2014, 43, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Cong, L.; Zhao, Y.; Zhang, T.; Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.H.; Levi, Z.; Twig, G.; Kark, J.D.; Leiba, A.; Derazne, E.; Liphshiz, I.; Keinan-Boker, L.; Eisenstein, S.; Afek, A. Risk factors associated with gastroenteropancreatic neuroendocrine tumors in a cohort of 2.3 million Israeli adolescents. Int. J. Cancer 2018, 143, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Feola, T.; Puliani, G.; Sesti, F.; Modica, R.; Centello, R.; Minotta, R.; Cannavale, G.; Di Meglio, S.; Di Vito, V.; Lauretta, R.; et al. Risk factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A three-centric case-control study. J. Endocrinol. Investig. 2022, 45, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Santos, A.C.; Castro, C.; Raposo, L.; Pereira, S.S.; Torres, I.; Henrique, R.; Cardoso, H.; Monteiro, M.P. Visceral obesity and metabolic syndrome are associated with well-differentiated gastroenteropancreatic neuroendocrine tumors. Cancers 2018, 10, 293. [Google Scholar] [CrossRef]
- Santos, A.P.; Castro, C.; Antunes, L.; Henrique, R.; Cardoso, M.H.; Monteiro, M.P. Disseminated Well-Differentiated Gastro-Entero-Pancreatic Tumors Are Associated with Metabolic Syndrome. J. Clin. Med. 2019, 8, 1479. [Google Scholar] [CrossRef] [PubMed]
- Oweira, H.; Mazotta, A.; Mehrabi, A.; Reissfelder, C.; Rahbari, N.; Betzler, A.; Elhadedy, H.; Soubrane, O.; Chaouch, M.A. Using a Reinforced Stapler Decreases the Incidence of Postoperative Pancreatic Fistula After Distal Pancreatectomy: A Systematic Review and Meta-Analysis. World J. Surg. 2022, 46, 1969–1979. [Google Scholar] [CrossRef]
- Gavin, K.M. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. N. Am. 2022, 49, 215–228. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, N.S.; Song, Y.L.; Bai, C.M.; Wang, Q.; Zhao, Y.P.; Xiao, Y.; Yu, S.; Li, M.; Chen, Y.J. Plasma levels of acylated ghrelin in patients with insulinoma and expression of ghrelin and its receptor in insulinomas. Endocrine 2020, 68, 448–457. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Modica, R.; Altieri, B.; Pugliese, G.; Minotta, R.; Faggiano, A.; Colao, A.; Savastano, S. Cardio-Metabolic Indices and Metabolic Syndrome as Predictors of Clinical Severity of Gastroenteropancreatic Neuroendocrine Tumors. Front. Endocrinol. 2021, 12, 649496. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Modica, R.; Laudisio, D.; Aprano, S.; Faggiano, A.; Colao, A.; Savastano, S. Chronotype: What role in the context of gastroenteropancreatic neuroendocrine tumors? J. Transl. Med. 2021, 19, 324. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Newton, C.C.; Silverman, D.T.; Pollak, M.; Nogueira, L.M.; Weinstein, S.J.; Albanes, D.; Männistö, S.; Jacobs, E.J. Circulating leptin and risk of pancreatic cancer: A pooled analysis from 3 cohorts. Am. J. Epidemiol. 2015, 182, 187–197. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Zhang, L.L.; Xu, Y.; Chen, X.; Zhang, B.; Li, L.X.; Li, S.; Shang, D. Circulating leptin and adiponectin levels in patients with pancreatic cancer. Chin. Med. J. 2021, 134, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, H.; Moon, A. Sex differences in cancer: Epidemiology, genetics, and therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Tevfik-Dorak, M.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 28, 1–11. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Quackenbush, J.; DeMeo, D.L. Genome-Wide Sex and Gender Differences in Cancer. Front. Oncol. 2020, 10, 597788. [Google Scholar] [CrossRef]


| Variables | PanNEN n = 106 | Controls n = 40 |
|---|---|---|
| Sex | ||
| Women | 59 (56%) | 31 (77.5%) |
| Men | 47 (44%) | 9 (22.5%) |
| Age, years: mean (range) | 52.59 (19–79) | 48.53 (22–76) |
| Women | 53.15 (21–79) | 47.45 (22–76) |
| Men | 52.02 (19–77) | 52.22 (23–70) |
| Age [years] | ||
| <55 | 54 (51%) | 22 (55%) |
| ≥55 | 52 (49%) | 18 (45%) |
| Metabolic syndrome | N/A | |
| No | 79 (75%) | |
| Yes | 27 (25%) | |
| BMI [kg/m2] | N/A | |
| <25 | 51 (48%) | |
| ≥25 | 55 (52%) | |
| Hyperglycemia | N/A | |
| No | 57 (54%) | |
| Yes | 49 (46%) | |
| IFG | 15 (14%) | |
| IGT | 11 (10%) | |
| DM 2 | 23 (22%) | |
| Hyperlipidemia | N/A | |
| Hypertriglyceridemia | 20 (19%) | |
| Hypercholesterolemia | 51 (48%) | |
| Hypertension | N/A | |
| No | 72 (68%) | |
| Yes | 34 (32%) |
| BMI < 25; n = 51 (mean ± SD; Median) | BMI ≥ 25; n = 55 (mean ± SD; Median) | p | |
|---|---|---|---|
| Age (years) | 49.57 ± 13.83; 52.40 | 55.51 ± 13.00; 57.77 | NS |
| Weight (kg) | 61.64 ± 10.32; 61.00 | 80.98 ± 11.63; 81.00 | <0.001 |
| Height (cm) | 168.73 ± 10.29; 167.00 | 169.58 ± 8.53; 170.00 | NS |
| BMI (kg/m2) | 21.57 ± 2.37; 22.40 | 28.11 ± 3.18; 27.34 | <0.001 |
| Cholesterol (mg/dL) | 183.61 ± 56.02; 178.00 | 194.62 ± 42.11; 192.00 | NS |
| TGs (mg/dL) | 100.84 ± 62.81; 82.00 | 116.40 ± 56.44; 98.00 | NS |
| Glucose (mg/dL) | 90.22 ± 17.84; 90.20 | 99.63 ± 26.93; 96.00 | NS |
| Leptin (ng/mL) | 9.21 ± 14.70; 6.33 | 16.03 ± 13.60; 9.36 | <0.001 |
| CA19-9 (U/mL) | 10.67 ± 8.64; 7.87 | 14.96 ± 18.42; 11.09 | NS |
| CEA (µg/L) | 1.52 ± 1.28; 1.06 | 1.76 ± 1.85; 1.21 | NS |
| CgA (ug/L) | 266.44 ± 597.89; 52.90 | 87.10 ± 139.60; 42.19 | NS |
| Parameter | R Spearman | p |
|---|---|---|
| Age (years) | 0.31 | 0.001 |
| Weight (kg) | 0.83 | <0.001 |
| Height (cm) | 0.06 | NS |
| Cholesterol (mg/dL) | 0.21 | NS |
| TGs (mg/dL) | 0.26 | <0.01 |
| Glucose (mg/dL) | 0.23 | NS |
| Leptin (ng/mL) | 0.49 | <0.001 |
| CA19-9 (U/mL) | 0.03 | NS |
| CEA (µg/L) | 0.02 | NS |
| CgA (ug/L) | −0.14 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiek, V.; Bocian-Jastrzębska, A.; Kos-Kudła, B. Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome. Cancers 2023, 15, 2348. https://doi.org/10.3390/cancers15082348
Rosiek V, Bocian-Jastrzębska A, Kos-Kudła B. Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome. Cancers. 2023; 15(8):2348. https://doi.org/10.3390/cancers15082348
Chicago/Turabian StyleRosiek, Violetta, Agnes Bocian-Jastrzębska, and Beata Kos-Kudła. 2023. "Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome" Cancers 15, no. 8: 2348. https://doi.org/10.3390/cancers15082348
APA StyleRosiek, V., Bocian-Jastrzębska, A., & Kos-Kudła, B. (2023). Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome. Cancers, 15(8), 2348. https://doi.org/10.3390/cancers15082348






