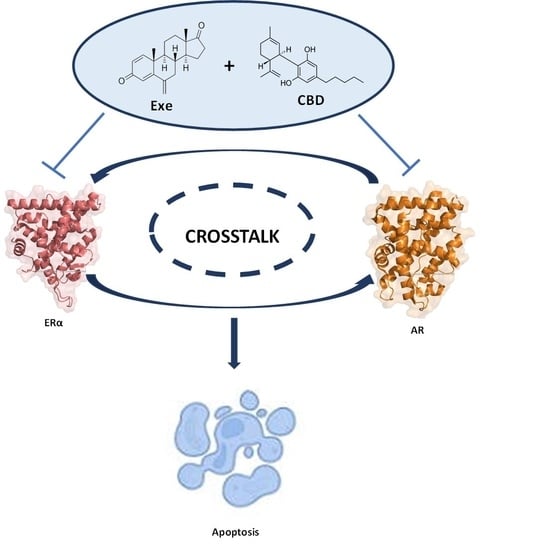
Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability
2.3. Analysis of Apoptosis
2.4. Western Blot Analysis
2.5. RNA Extraction and qPCR Analysis
2.6. siRNA Transfection
2.7. ER and AR Transactivation Assays
2.8. Statistical Analysis
3. Results
3.1. Effects of CBD When Combined with AIs on Viability of Non-Tumorous Cells and Breast Cancer Cells
3.2. Effects of CBD Plus AIs on Apoptotic Cell Death
3.3. Involvement of Aromatase in the Effects Induced by CBD Plus AIs
3.4. Involvement of ERα on the Effects Induced by CBD Plus AIs
3.5. Involvement of AR in the Effects Induced by CBD Plus AIs
3.6. Crosstalk between ERα and AR
3.7. Involvement of AKT and ERK1/2 Signaling Pathways in the Effects Observed for CBD Plus AIs
3.8. Involvement of the Hormonal Environment on the Effects Induced by CBD Plus Exe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Breast Cancer Facts & Figure 2019 and Figure 2020; American Cancer Society Inc.: Atlanta, GA, USA, 2019. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Awan, A.; Esfahani, K. Endocrine therapy for breast cancer in the primary care setting. Curr. Oncol. 2018, 25, 285–291. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)(dagger). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Ferreira Almeida, C.; Oliveira, A.; João Ramos, M.; Fernandes, P.A.; Teixeira, N.; Amaral, C. Estrogen receptor-positive (ER(+)) breast cancer treatment: Are multi-target compounds the next promising approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar] [CrossRef]
- Augusto, T.V.; Correia-da-Silva, G.; Rodrigues, C.M.P.; Teixeira, N.; Amaral, C. Acquired resistance to aromatase inhibitors: Where we stand! Endocr. Relat. Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef]
- Amaral, C.; Borges, M.; Melo, S.; da Silva, E.T.; Correia-da-Silva, G.; Teixeira, N. Apoptosis and autophagy in breast cancer cells following exemestane treatment. PLoS ONE 2012, 7, e42398. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Almeida, C.F.; Teixeira, N.; Correia-da-Silva, G. Differential biological effects of aromatase inhibitors: Apoptosis, autophagy, senescence and modulation of the hormonal status in breast cancer cells. Mol. Cell Endocrinol. 2021, 537, 111426. [Google Scholar] [CrossRef]
- Itoh, T.; Karlsberg, K.; Kijima, I.; Yuan, Y.C.; Smith, D.; Ye, J.; Chen, S. Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: A microarray approach. Mol. Cancer Res. 2005, 3, 203–218. [Google Scholar] [CrossRef]
- Thiantanawat, A.; Long, B.J.; Brodie, A.M. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res. 2003, 63, 8037–8050. [Google Scholar]
- Amaral, C.; Augusto, T.V.; Almada, M.; Cunha, S.C.; Correia-da-Silva, G.; Teixeira, N. The potential clinical benefit of targeting androgen receptor (AR) in estrogen-receptor positive breast cancer cells treated with Exemestane. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165661. [Google Scholar] [CrossRef]
- Olson, E. Combination Therapies in Advanced, Hormone Receptor-Positive Breast Cancer. J. Adv. Pract. Oncol. 2018, 9, 43–54. [Google Scholar]
- Roberto, M.; Astone, A.; Botticelli, A.; Carbognin, L.; Cassano, A.; D'Auria, G.; Fabbri, A.; Fabi, A.; Gamucci, T.; Krasniqi, E.; et al. CDK4/6 Inhibitor Treatments in Patients with Hormone Receptor Positive, Her2 Negative Advanced Breast Cancer: Potential Molecular Mechanisms, Clinical Implications and Future Perspectives. Cancers 2021, 13, 332. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Saatci, O.; Huynh-Dam, K.T.; Sahin, O. Endocrine resistance in breast cancer: From molecular mechanisms to therapeutic strategies. J. Mol. Med. 2021, 99, 1691–1710. [Google Scholar] [CrossRef]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Portman, N.; Alexandrou, S.; Carson, E.; Wang, S.; Lim, E.; Caldon, C.E. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr. Relat. Cancer 2019, 26, R15–R30. [Google Scholar] [CrossRef]
- Papadimitriou, M.C.; Pazaiti, A.; Iliakopoulos, K.; Markouli, M.; Michalaki, V.; Papadimitriou, C.A. Resistance to CDK4/6 inhibition: Mechanisms and strategies to overcome a therapeutic problem in the treatment of hormone receptor-positive metastatic breast cancer. Biochim. Biophys. Acta Mol. Cell Res 2022, 1869, 119346. [Google Scholar] [CrossRef]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An Update of Current Cannabis-Based Pharmaceuticals in Pain Medicine. Pain Ther. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Bramness, J.G.; Dom, G.; Gual, A.; Mann, K.; Wurst, F.M. A Survey on the Medical Use of Cannabis in Europe: A Position Paper. Eur. Addict. Res. 2018, 24, 201–205. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Alves, P.A.C.; Teixeira, N.; Correia-da-Silva, G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Giovannoni, G.; Thompson, A.J. The therapeutic potential of cannabis. Lancet. Neurol. 2003, 2, 291–298. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S163–S171. [Google Scholar] [CrossRef]
- Grimaldi, C.; Capasso, A. The endocannabinoid system in the cancer therapy: An overview. Curr. Med. Chem. 2011, 18, 1575–1583. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Perez-Gomez, E.; Guzman, M.; Sanchez, C. Cannabinoids: A new hope for breast cancer therapy? Cancer Treat. Rev. 2012, 38, 911–918. [Google Scholar] [CrossRef]
- Almeida, C.F.; Teixeira, N.; Correia-da-Silva, G.; Amaral, C. Cannabinoids in Breast Cancer: Differential Susceptibility According to Subtype. Molecules 2021, 27, 156. [Google Scholar] [CrossRef]
- Velasco, G.; Sanchez, C.; Guzman, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef]
- Sledzinski, P.; Zeyland, J.; Slomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Fraguas-Sanchez, A.I.; Martin-Sabroso, C.; Torres-Suarez, A.I. Insights into the effects of the endocannabinoid system in cancer: A review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef]
- Das, S.; Kaul, K.; Mishra, S.; Charan, M.; Ganju, R.K. Cannabinoid Signaling in Cancer. Adv. Exp. Med. Biol. 2019, 1162, 51–61. [Google Scholar] [CrossRef]
- Morin-Buote, J.; Ennour-Idrissi, K.; Poirier, É.; Lemieux, J.; Furrer, D.; Burguin, A.; Durocher, F.; Diorio, C. Association of Breast Tumour Expression of Cannabinoid Receptors CBR1 and CBR2 with Prognostic Factors and Survival in Breast Cancer Patients. J. Pers. Med. 2021, 11, 852. [Google Scholar] [CrossRef]
- Kiskova, T.; Mungenast, F.; Suvakova, M.; Jager, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1673. [Google Scholar] [CrossRef]
- Dobovisek, L.; Krstanovic, F.; Borstnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef]
- Amaral, C.; Trouille, F.M.; Almeida, C.F.; Correia-da-Silva, G.; Teixeira, N. Unveiling the mechanism of action behind the anti-cancer properties of cannabinoids in ER(+) breast cancer cells: Impact on aromatase and steroid receptors. J. Steroid Biochem. Mol. Biol. 2021, 210, 105876. [Google Scholar] [CrossRef]
- Almada, M.; Amaral, C.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N. Cannabidiol (CBD) but not tetrahydrocannabinol (THC) dysregulate in vitro decidualization of human endometrial stromal cells by disruption of estrogen signaling. Reprod. Toxicol. 2020, 93, 75–82. [Google Scholar] [CrossRef]
- Almada, M.; Oliveira, A.; Amaral, C.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.; Correia-da-Silva, G.; Teixeira, N. Anandamide targets aromatase: A breakthrough on human decidualization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158512. [Google Scholar] [CrossRef]
- Berthois, Y.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Phenol red in tissue culture media is a weak estrogen: Implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. USA 1986, 83, 2496–2500. [Google Scholar] [CrossRef]
- Amaral, C.; Augusto, T.V.; Tavares-da-Silva, E.; Roleira, F.M.F.; Correia-da-Silva, G.; Teixeira, N. Hormone-dependent breast cancer: Targeting autophagy and PI3K overcomes Exemestane-acquired resistance. J. Steroid Biochem. Mol. Biol. 2018, 183, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Teixeira, N.; Oliveira, A.; Augusto, T.V.; Correia-da-Silva, G.; Ramos, M.J.; Fernandes, P.A.; Amaral, C. Discovery of a multi-target compound for estrogen receptor-positive (ER(+)) breast cancer: Involvement of aromatase and ERs. Biochimie 2021, 181, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Correia-da-Silva, G.; Almeida, C.F.; Valente, M.J.; Varela, C.; Tavares-da-Silva, E.; Vinggaard, A.M.; Teixeira, N.; Roleira, F.M.F. An Exemestane Derivative, Oxymestane-D1, as a New Multi-Target Steroidal Aromatase Inhibitor for Estrogen Receptor-Positive (ER(+)) Breast Cancer: Effects on Sensitive and Resistant Cell Lines. Molecules 2023, 28, 789. [Google Scholar] [CrossRef]
- Kurokawa, H.; Nishio, K.; Fukumoto, H.; Tomonari, A.; Suzuki, T.; Saijo, N. Alteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cells. Oncol. Rep. 1999, 6, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Varela, C.; Azevedo, M.; da Silva, E.T.; Roleira, F.M.; Chen, S.; Correia-da-Silva, G.; Teixeira, N. Effects of steroidal aromatase inhibitors on sensitive and resistant breast cancer cells: Aromatase inhibition and autophagy. J. Steroid Biochem. Mol. Biol. 2013, 135, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S. Aromatase destabilizer: Novel action of exemestane, a food and drug administration-approved aromatase inhibitor. Cancer Res. 2006, 66, 10281–10286. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.L.; Mauricio, J.; Sobral, A.F.; Costa, S.C.; Roleira, F.M.F.; Tavares-da-Silva, E.J.; Correia-da-Silva, G.; Teixeira, N. Anti-tumor efficacy of new 7alpha-substituted androstanes as aromatase inhibitors in hormone-sensitive and resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 218–228. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Varela, C.L.; Bernardo, F.; da Silva, E.T.; Roleira, F.F.M.; Costa, S.; Teixeira, N.; Correia-da-Silva, G. Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors. J. Steroid. Biochem. Mol. Biol. 2019, 195, 105486. [Google Scholar] [CrossRef]
- Inoue, A.; Omoto, Y.; Yamaguchi, Y.; Kiyama, R.; Hayashi, S.I. Transcription factor EGR3 is involved in the estrogen-signaling pathway in breast cancer cells. J. Mol. Endocrinol. 2004, 32, 649–661. [Google Scholar] [CrossRef]
- Wang, X.; Masri, S.; Phung, S.; Chen, S. The role of amphiregulin in exemestane-resistant breast cancer cells: Evidence of an autocrine loop. Cancer Res. 2008, 68, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W. The History and Mechanism of Action of Fulvestrant. Clin. Breast Cancer 2005, 6, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.L.; Shioda, K.; Coser, K.R.; Rivizzigno, D.; McSweeney, K.R.; Shioda, T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor α protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS ONE 2013, 8, e60889. [Google Scholar] [CrossRef]
- Long, X.; Nephew, K.P. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J. Biol. Chem. 2006, 281, 9607–9615. [Google Scholar] [CrossRef]
- Prather, P.L.; FrancisDevaraj, F.; Dates, C.R.; Greer, A.K.; Bratton, S.M.; Ford, B.M.; Franks, L.N.; Radominska-Pandya, A. CB1 and CB2 receptors are novel molecular targets for Tamoxifen and 4OH-Tamoxifen. Biochem. Biophys. Res. Commun. 2013, 441, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Song, Z.H. CB2 cannabinoid receptor is a novel target for third-generation selective estrogen receptor modulators bazedoxifene and lasofoxifene. Biochem. Biophys. Res. Commun. 2014, 443, 144–149. [Google Scholar] [CrossRef]
- Franks, L.N.; Ford, B.M.; Prather, P.L. Selective Estrogen Receptor Modulators: Cannabinoid Receptor Inverse Agonists with Differential CB1 and CB2 Selectivity. Front. Pharm. 2016, 7, 503. [Google Scholar] [CrossRef]
- Mandal, R.; Raab, M.; Matthess, Y.; Becker, S.; Knecht, R.; Strebhardt, K. pERK 1/2 inhibit Caspase-8 induced apoptosis in cancer cells by phosphorylating it in a cell cycle specific manner. Mol. Oncol. 2014, 8, 232–249. [Google Scholar] [CrossRef]









| Symbol | Primers | Annealing Temperature |
|---|---|---|
| AREG | Forward: 5′-TGTCGCTCTTGATACTCGGC -3′ Reverse: 5′-ATGGTTCACGCTTCCCAGAG -3′ | 56 °C |
| EGR3 | Forward: 5′-GACTCCCCTTCCAACTGGTG-3′ Reverse: 5′- GGATACATGGCCTCCACGTC-3′ | 56 °C |
| TFF1 | Forward: 5′-GTGGTTTTCCTGGTGTCACG-3′ Reverse: 5′-AGGATAGAAGCACCAGGGGA-3′ | 55 °C |
| β-Actin | Forward: 5′-TACAGCTTCACCACCACAGC-3′ Reverse: 5′- AAGGAAGGCTGGAAGAGAGC-3′ | 55 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.F.; Teixeira, N.; Valente, M.J.; Vinggaard, A.M.; Correia-da-Silva, G.; Amaral, C. Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors. Cancers 2023, 15, 2517. https://doi.org/10.3390/cancers15092517
Almeida CF, Teixeira N, Valente MJ, Vinggaard AM, Correia-da-Silva G, Amaral C. Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors. Cancers. 2023; 15(9):2517. https://doi.org/10.3390/cancers15092517
Chicago/Turabian StyleAlmeida, Cristina Ferreira, Natércia Teixeira, Maria João Valente, Anne Marie Vinggaard, Georgina Correia-da-Silva, and Cristina Amaral. 2023. "Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors" Cancers 15, no. 9: 2517. https://doi.org/10.3390/cancers15092517
APA StyleAlmeida, C. F., Teixeira, N., Valente, M. J., Vinggaard, A. M., Correia-da-Silva, G., & Amaral, C. (2023). Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors. Cancers, 15(9), 2517. https://doi.org/10.3390/cancers15092517