Tumor Organoid and Spheroid Models for Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. 2D Cancer Models and Importance of TME
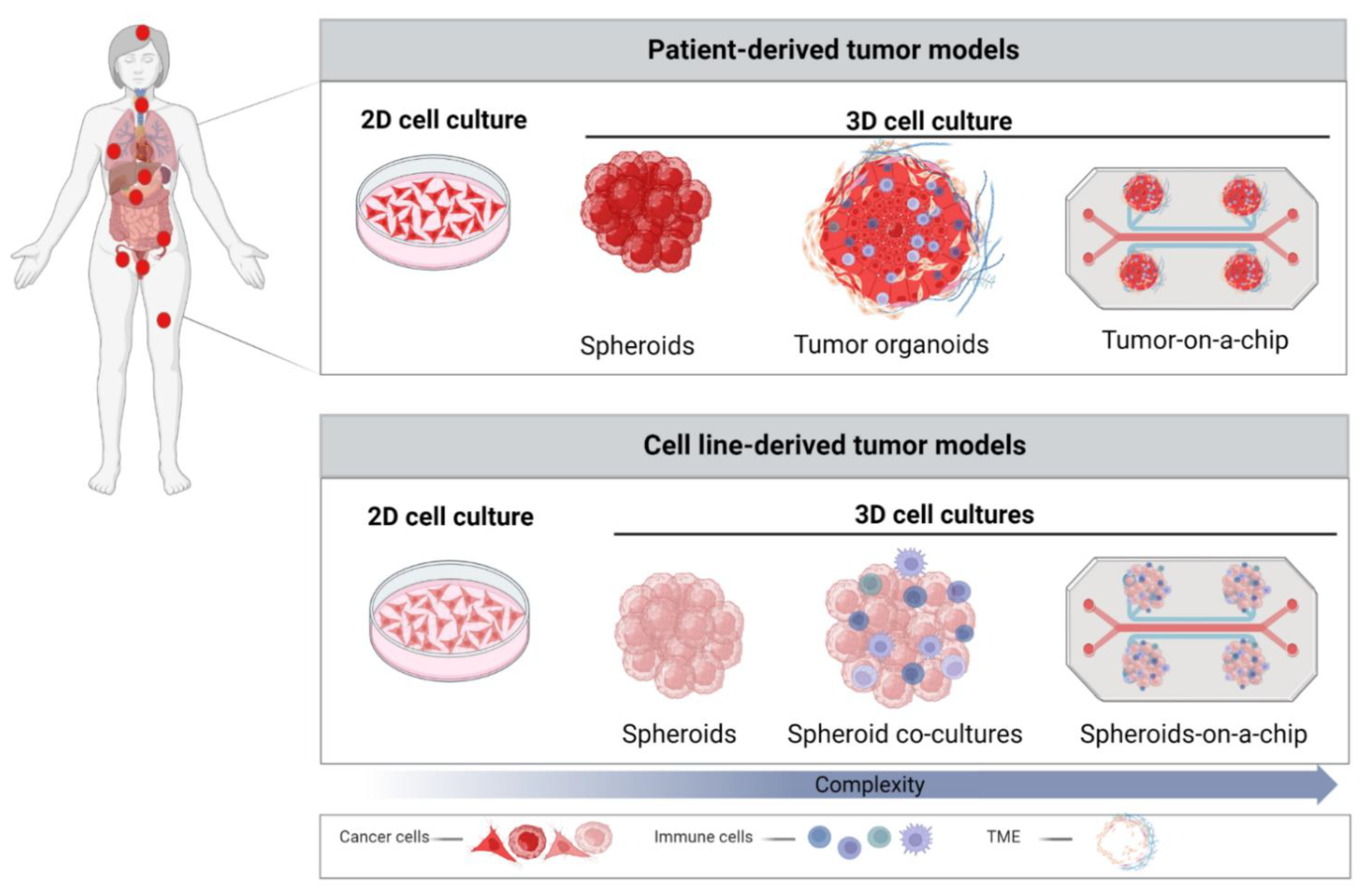
3. Role of 3D Models in Cancer Research
3.1. Spheroids
3.2. Patient-Derived Organoids (PDOs)
| Cervical Cancer In Vitro Models | Cell Origin | Application | Advantage | Limitation |
|---|---|---|---|---|
 | CC cell lines CaSki HeLa SiHa C-33-A | Disease modeling [90] Drug screening [91] Immunotherapy [91] Anti-HPV vaccine [92] | Simple and low-cost maintenance Well-established Simple analysis Long-term cultures Reproducible High-throughput potential | Lack of cell–cell and cell–ECM interaction Lack of natural structures Altered cellular functions Higher sensitivity to drugs Unpredictable for clinical trials |
 | CC cell lines CaSki HeLa SiHa | Disease modeling [52,90,93,94,95] Drug response [96,97,98,99] Immunotherapy [100,101,102] Anti-HPV vaccine [92] | Simple maintenance Moderate costs Cell–cell interaction Preserved cellular functions Mimicking tumor structure Flexible to increase the complexity High-throughput potential | Lack of cell–ECM interactions Lack of heterogeneity Variation in uniformity and reproducibility Challenging analysis |
 | Cancer tissue (SCC, AdCC, neuroendocrine CC) | Disease modeling [88,89,103,104] Personalized therapy [24,89,103] NCT04278326 | Natural tumor cell functions Mimicking tumor structure and TME More predictable drug responses High-throughput potential Biobanks establishment Personalized therapy | Difficult to maintain Patient-dependent variation ECM matrix variations High costs Challenging analysis Ethical concern |
4. 3D Cervical Cancer Spheroids and Organoids in Translational Research
4.1. Drug Discovery
4.2. Pre-Clinical Testing of Immunotherapies
4.3. 3D Cultures as a Platform to Test Combinatorial Treatment Strategies against Cervical Cancer
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- RKI. Krebs in Deutschland für 2015/2016; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical CancerGuideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 2 with Recommendations on Psycho-oncology, Rehabilitation, Follow-up, Recurrence, Palliative Therapy and Healthc. Geburtshilfe Frauenheilkd. 2022, 82, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical CancerGuideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 1 with Recommendations on Epidemiology, Screening, Diagnostics and Therapy. Geburtshilfe Frauenheilkd. 2022, 82, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Hirte, H.W.; Clark, D.A.; Mazurka, J.; O’Connell, G.; Rusthoven, J. A rapid and simple method for the purification of tumor cells from ascitic fluid of ovarian carcinoma. Gynecol. Oncol. 1992, 44, 223–226. [Google Scholar] [CrossRef]
- Shepherd, T.G.; Thériault, B.L.; Campbell, E.J.; Nachtigal, M.W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protoc. 2006, 1, 2643–2649. [Google Scholar] [CrossRef]
- Bongso, A.; Gajra, B.; Lian, N.P.; Wong, P.C.; Soon-Chye, N.; Ratnam, S. Establishment of human endometrial cell cultures. Hum. Reprod. 1988, 3, 705–713. [Google Scholar] [CrossRef]
- Karst, A.M.; Drapkin, R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat. Protoc. 2012, 7, 1755–1764. [Google Scholar] [CrossRef]
- Inada, K.; Hayashi, S.; Iguchi, T.; Sato, T. Establishment of a Primary Culture Model of Mouse Uterine and Vaginal Stroma for Studying In Vitro Estrogen Effects. Exp. Biol. Med. 2006, 231, 303–310. [Google Scholar] [CrossRef]
- Stanley, M.A.; Parkinson, E.K. Growth requirements of human cervical epithelial cells in culture. Int. J. Cancer 1979, 24, 407–414. [Google Scholar] [CrossRef]
- Fan, T.; Li, X.; Li, Y.; Zhi, Y.; Rong, S.; Cheng, G.; Zhang, X. An improved method for primary culture of normal cervical epithelial cells and establishment of cell model in vitro with HPV-16 E6 gene by lentivirus. J. Cell. Physiol. 2018, 233, 2773–2780. [Google Scholar] [CrossRef]
- Zuñiga Martinez, M.D.L.; López Mendoza, C.M.; Tenorio Salazar, J.; García Carrancá, A.M.; Cerbón Cervantes, M.A.; Alcántara-Quintana, L.E. Establishment, authenticity, and characterization of cervical cancer cell lines. Mol. Cell. Oncol. 2022, 9, 2078628. [Google Scholar] [CrossRef] [PubMed]
- Skok, K.; Gradišnik, L.; Maver, U.; Kozar, N.; Sobočan, M.; Takač, I.; Arko, D.; Kavalar, R. Gynaecological cancers and their cell lines. J. Cell. Mol. Med. 2021, 25, 3680–3698. [Google Scholar] [CrossRef] [PubMed]
- Ghoshdastider, U.; Rohatgi, N.; Naeini, M.M.; Baruah, P.; Revkov, E.; Guo, Y.A.; Rizzetto, S.; Wong, A.M.L.; Solai, S.; Nguyen, T.T.; et al. Pan-cancer analysis of ligand-receptor cross-talk in the tumor microenvironment. Cancer Res. 2021, 81, 1802–1812. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- NCI. NCI Dictionary of Cancer Terms; National Institutes of Health: Bethesda, MD, USA, 2018.
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E.A. Tumor acidity as evolutionary spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef]
- Pickl, M.; Ries, C.H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009, 28, 461–468. [Google Scholar] [CrossRef]
- El Feky, S.; Megahed, M.; Abd El Moneim, N.; Zaher, E.; Khamis, S.; Ali, L. Cytotoxic, chemosensitizing and radiosensitizing effects of curcumin based on thioredoxin system inhibition in breast cancer cells: 2D vs. 3D cell culture system. Exp. Ther. Med. 2021, 21, 506. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells cultured in 2D vs 3D reveals differences in AKT/mTOR/S6-kinase signaling and drug response. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and In Vivo by Integrin Blocking Antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Brady, L.; Gil da Costa, R.M.; Coleman, I.M.; Matson, C.K.; Risk, M.C.; Coleman, R.T.; Nelson, P.S. A comparison of prostate cancer cell transcriptomes in 2D monoculture vs 3D xenografts identify consistent gene expression alterations associated with tumor microenvironments. Prostate 2020, 80, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishie, R.; Ueda, S.; Miyamoto, S.; Hashida, S.; Konishi, H.; Terada, S.; Kogata, Y.; Sasaki, H.; Tsunetoh, S.; et al. Patient-Derived Xenograft Models in Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9369. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Bachran, C.; Stanke, J.; Elezkurtaj, S.; Kaufmann, A.M.; Fuchs, H.; Loddenkemper, C.; Schneider, A.; Cichon, G. Creation and characterization of a xenograft model for human cervical cancer. Gynecol. Oncol. 2010, 118, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Kim, S.; Choi, Y.-L.; Cho, Y.J.; Oh, E.; Choi, J.-J.; Jung, K.; Song, J.-Y.; Ahn, S.E.; Kim, B.-G.; et al. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219–36230. [Google Scholar] [CrossRef] [PubMed]
- Larmour, L.I.; Cousins, F.L.; Teague, J.A.; Deane, J.A.; Jobling, T.W.; Gargett, C.E. A patient derived xenograft model of cervical cancer and cervical dysplasia. PLoS ONE 2018, 13, e0206539. [Google Scholar] [CrossRef]
- Chaudary, N.; Pintilie, M.; Hedley, D.; Fyles, A.W.; Milosevic, M.; Clarke, B.; Hill, R.P.; Mackay, H. Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation. Cancer 2012, 118, 3105–3115. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Zhang, Y.; Zhang, N.; Maawy, A.; Mii, S.; Yamamoto, M.; Uehara, F.; Miwa, S.; Yano, S.; Murakami, T.; et al. Establishment of a Patient-Derived Orthotopic Xenograft (PDOX) Model of HER-2-Positive Cervical Cancer Expressing the Clinical Metastatic Pattern. PLoS ONE 2015, 10, e0117417. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, K.; Walton, Z.; Wang, Y.; Ebi, H.; Shimamura, T.; Liu, Y.; Tupper, T.; Ouyang, J.; Li, J.; et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012, 483, 613–617. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Multicellular spheroids. J. Cancer Res. Clin. Oncol. 1987, 113, 101–122. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, R.L.; Gey, G.O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J. Natl. Cancer Inst. 1956, 16, 1375–1403. [Google Scholar] [PubMed]
- Bissell, M.J. The Differentiated State of Normal and Malignant Cells or How to Define a “Normal” Cell in Culture. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1981; pp. 27–100. [Google Scholar]
- Emerman, J.T.; Burwen, S.J.; Pitelka, D.R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 1979, 11, 109–119. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas2. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Groebe, K.; Mueller-Klieser, W. Distributions of oxygen, nutrient, and metabolic waste concentrations in multicellular spheroids and their dependence on spheroid parameters. Eur. Biophys. J. 1991, 19, 169–181. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Sivakumar, H.; Devarasetty, M.; Kram, D.E.; Strowd, R.E.; Skardal, A. Multi-Cell Type Glioblastoma Tumor Spheroids for Evaluating Sub-Population-Specific Drug Response. Front. Bioeng. Biotechnol. 2020, 8, 538663. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Naseri, M.; Karimi-Busheri, F.; Atyabi, F.; Mirsharif, E.S.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021, 21, 204. [Google Scholar] [CrossRef]
- Pant, T.; Gaikwad, G.; Jain, D.; Dandekar, P.; Jain, R. Establishment and characterization of lung co-culture spheroids for paclitaxel loaded Eudragit® RL 100 nanoparticle evaluation. Biotechnol. Prog. 2021, 37, e3203. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Li, X.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Establishment and Characterization of a Recurrent Osteosarcoma Cell Line: OSA 1777. J. Orthop. Res. 2020, 38, 902–910. [Google Scholar] [CrossRef]
- Cavaco, M.; Fraga, P.; Valle, J.; Andreu, D.; Castanho, M.A.R.B.; Neves, V. Development of Breast Cancer Spheroids to Evaluate Cytotoxic Response to an Anticancer Peptide. Pharmaceutics 2021, 13, 1863. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug resistance evaluation in novel 3D in vitro model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef] [PubMed]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Asra Ahmad, Z.; Annabel Dass, S.; Shamsuddin, S.; Mohana Kumaran, N.; Balakrishnan, V. Growth and Invasion of 3D Spheroid Tumor of HeLa and CasKi Cervical Cancer Cells. Oncologie 2021, 23, 279–291. [Google Scholar] [CrossRef]
- López, J.; Poitevin, A.; Mendoza-Martínez, V.; Pérez-Plasencia, C.; García-Carrancá, A. Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer 2012, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Shibata, K.; Takahashi, J.; Watanabe, M.; Nakata, S.; Nakamura, K.; Yamaguchi, T. Glycolytic oscillations in HeLa cervical cancer cell spheroids. FEBS J. 2022, 289, 5551–5570. [Google Scholar] [CrossRef]
- Daum, A.-K.; Dittmann, J.; Jansen, L.; Peters, S.; Dahmen, U.; Heger, J.I.; Hoppe-Seyler, F.; Gille, A.; Clement, J.H.; Runnebaum, I.B.; et al. ITIH5 shows tumor suppressive properties in cervical cancer cells grown as multicellular tumor spheroids. Am. J. Transl. Res. 2021, 13, 10298–10314. [Google Scholar]
- Gottfried, E.; Kunz-Schughart, L.A.; Andreesen, R.; Kreutz, M. Brave Little World: Spheroids as an in vitro Model to Study Tumor-Immune-Cell Interactions. Cell Cycle 2006, 5, 691–695. [Google Scholar] [CrossRef]
- Courau, T.; Bonnereau, J.; Chicoteau, J.; Bottois, H.; Remark, R.; Assante Miranda, L.; Toubert, A.; Blery, M.; Aparicio, T.; Allez, M.; et al. Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J. Immunother. Cancer 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.E. Invited review Multicell spheroids as a model for cell kinetic studies. Cell Prolif. 1990, 23, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Kaur, G.; Doroshow, J.H.; Teicher, B.A. Format (2D vs 3D) and media effect target expression and response of patient-derived and standard NSCLC lines to EGFR inhibitors. Cancer Treat. Res. Commun. 2021, 29, 100463. [Google Scholar] [CrossRef]
- Tamaki, M.; McDonald, W.; Amberger, V.R.; Moore, E.; Del Maestro, R.F. Implantation of C6 astrocytoma spheroid into collagen type I gels: Invasive, proliferative, and enzymatic characterizations. J. Neurosurg. 1997, 87, 602–609. [Google Scholar] [CrossRef]
- Gunay, G.; Kirit, H.A.; Kamatar, A.; Baghdasaryan, O.; Hamsici, S.; Acar, H. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol. Oncol. 2020, 159, 563–572. [Google Scholar] [CrossRef]
- Vinci, M.; Box, C.; Eccles, S.A. Three-dimensional (3D) tumor spheroid invasion assay. J. Vis. Exp. 2015, 99, e52686. [Google Scholar] [CrossRef]
- Timmins, N.; Dietmair, S.; Nielsen, L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 2004, 7, 97–103. [Google Scholar] [CrossRef]
- Jadhav, U.; Chigurupati, S.; Lakka, S.S.; Mohanam, S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int. J. Oncol. 2004, 25, 1407–1414. [Google Scholar] [CrossRef]
- Upreti, M.; Jamshidi-Parsian, A.; Koonce, N.A.; Webber, J.S.; Sharma, S.K.; Asea, A.A.A.; Mader, M.J.; Griffin, R.J. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl. Oncol. 2011, 4, 365-IN3. [Google Scholar] [CrossRef] [PubMed]
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In vitro 3D Systems to Model Tumor Angiogenesis and Interactions With Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 594903. [Google Scholar] [CrossRef] [PubMed]
- KORFF, T.; KIMMINA, S.; MARTINY-BARON, G.; AUGUSTIN, H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, S.M.; Welch-Reardon, K.M.; Waterman, M.L.; Hughes, C.C.W.; George, S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 2014, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Krishnapriya, S.; Sidhanth, C.; Manasa, P.; Sneha, S.; Bindhya, S.; Nagare, R.P.; Ramachandran, B.; Vishwanathan, P.; Murhekar, K.; Shirley, S.; et al. Cancer stem cells contribute to angiogenesis and lymphangiogenesis in serous adenocarcinoma of the ovary. Angiogenesis 2019, 22, 441–455. [Google Scholar] [CrossRef]
- Kwak, T.J.; Lee, E. In vitro modeling of solid tumor interactions with perfused blood vessels. Sci. Rep. 2020, 10, 20142. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Rosenbluth, J.M.; Schackmann, R.C.J.; Gray, G.K.; Selfors, L.M.; Li, C.M.-C.; Boedicker, M.; Kuiken, H.J.; Richardson, A.; Brock, J.; Garber, J.; et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 2020, 11, 1711. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Iaquinta, P.J.; Drost, J.; Gracanin, A.; van Boxtel, R.; Wongvipat, J.; Dowling, C.M.; Gao, D.; Begthel, H.; Sachs, N.; et al. Identification of Multipotent Luminal Progenitor Cells in Human Prostate Organoid Cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.N.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell– and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Grassi, L.; Alfonsi, R.; Francescangeli, F.; Signore, M.; De Angelis, M.L.; Addario, A.; Costantini, M.; Flex, E.; Ciolfi, A.; Pizzi, S.; et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019, 10, 201. [Google Scholar] [CrossRef]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef]
- Hill, S.J.; Decker, B.; Roberts, E.A.; Horowitz, N.S.; Muto, M.G.; Worley, M.J.; Feltmate, C.M.; Nucci, M.R.; Swisher, E.M.; Nguyen, H.; et al. Prediction of DNA Repair Inhibitor Response in Short-Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov. 2018, 8, 1404–1421. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [PubMed]
- Campaner, E.; Zannini, A.; Santorsola, M.; Bonazza, D.; Bottin, C.; Cancila, V.; Tripodo, C.; Bortul, M.; Zanconati, F.; Schoeftner, S.; et al. Breast Cancer Organoids Model Patient-Specific Response to Drug Treatment. Cancers 2020, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Piccotti, F.; Allevi, R.; Truffi, M.; Sorrentino, L.; Russo, L.; Agozzino, M.; Signati, L.; Bonizzi, A.; Villani, L.; et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online 2019, 21, 12. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Ebisawa, K.; Odaka, A.; Sugiyama, T.; Itami, M.; Hippo, Y. Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma. Cancer Sci. 2019, 110, 2992–3005. [Google Scholar] [CrossRef]
- Lõhmussaar, K.; Oka, R.; Espejo Valle-Inclan, J.; Smits, M.H.H.; Wardak, H.; Korving, J.; Begthel, H.; Proost, N.; van de Ven, M.; Kranenburg, O.W.; et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 2021, 28, 1380–1396.e6. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.S.; Oh, J.H.; Choi, E.; Kim, S.; Kim, H.; Nam, E.J. Preclinical investigation of patient-derived cervical cancer organoids for precision medicine. J. Gynecol. Oncol. 2023, 34, e35. [Google Scholar] [CrossRef]
- Wan, P.K.-T.; Leung, T.H.-Y.; Siu, M.K.-Y.; Mo, X.-T.; Tang, H.W.-M.; Chan, K.K.-L.; Cheung, A.N.-Y.; Ngan, H.Y.-S. HPV-induced Nurr1 promotes cancer aggressiveness, self-renewal, and radioresistance via ERK and AKT signaling in cervical cancer. Cancer Lett. 2021, 497, 14–27. [Google Scholar] [CrossRef]
- González-Torres, A.; Bañuelos-Villegas, E.G.; Martínez-Acuña, N.; Sulpice, E.; Gidrol, X.; Alvarez-Salas, L.M. MYPT1 is targeted by miR-145 inhibiting viability, migration and invasion in 2D and 3D HeLa cultures. Biochem. Biophys. Res. Commun. 2018, 507, 348–354. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, W.; Liu, Q.; Guan, X.; Hu, J. Dendritic cells treated with HPV16mE7 in a three-dimensional model promote the secretion of IL-12p70 and IFN-γ. Exp. Mol. Pathol. 2011, 91, 325–330. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 35001. [Google Scholar] [CrossRef]
- Thippabhotla, S.; Zhong, C.; He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef]
- De Gregorio, V.; La Rocca, A.; Urciuolo, F.; Annunziata, C.; Tornesello, M.L.; Buonaguro, F.M.; Netti, P.A.; Imparato, G. Modeling the epithelial-mesenchymal transition process in a 3D organotypic cervical neoplasia. Acta Biomater. 2020, 116, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, O.P.; González-Torres, A.; Álvarez-Salas, L.M.; Hernández-Sánchez, H.; García-Pérez, B.E.; del Rocío Thompson-Bonilla, M.; Jaramillo-Flores, M.E. Effect of naringenin and its combination with cisplatin in cell death, proliferation and invasion of cervical cancer spheroids. RSC Adv. 2021, 11, 129–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014, 139, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Seo, O.W.; Kim, M.; Hulme, J.; An, S.S.A. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. Onco Targets Ther. 2016, 9, 7207–7218. [Google Scholar] [CrossRef]
- Casagrande, N.; De Paoli, M.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical evaluation of a new liposomal formulation of cisplatin, lipoplatin, to treat cisplatin-resistant cervical cancer. Gynecol. Oncol. 2013, 131, 744–752. [Google Scholar] [CrossRef]
- Giannattasio, A.; Weil, S.; Kloess, S.; Ansari, N.; Stelzer, E.H.K.; Cerwenka, A.; Steinle, A.; Koehl, U.; Koch, J. Cytotoxicity and infiltration of human NK cells in in vivo-like tumor spheroids. BMC Cancer 2015, 15, 351. [Google Scholar] [CrossRef]
- Yuti, P.; Wutti-in, Y.; Sawasdee, N.; Kongkhla, K.; Phanthaphol, N.; Choomee, K.; Chieochansin, T.; Panya, A.; Junking, M.; Yenchitsomanus, P.; et al. Anti-CD19 chimeric antigen receptor T cells secreting anti-PD-L1 single-chain variable fragment attenuate PD-L1 mediated T cell inhibition. Int. Immunopharmacol. 2022, 113, 109442. [Google Scholar] [CrossRef]
- Park, D.; Son, K.; Hwang, Y.; Ko, J.; Lee, Y.; Doh, J.; Jeon, N.L. High-Throughput Microfluidic 3D Cytotoxicity Assay for Cancer Immunotherapy (CACI-IMPACT Platform). Front. Immunol. 2019, 10, 1133. [Google Scholar] [CrossRef]
- Maru, Y.; Kawata, A.; Taguchi, A.; Ishii, Y.; Baba, S.; Mori, M.; Nagamatsu, T.; Oda, K.; Kukimoto, I.; Osuga, Y.; et al. Establishment and Molecular Phenotyping of Organoids from the Squamocolumnar Junction Region of the Uterine Cervix. Cancers 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Nakamura, K.; Takeda, T.; Chiwaki, F.; Banno, K.; Aoki, D.; Takeshita, F.; Sasaki, H. Aurora kinase blockade drives de novo addiction of cervical squamous cell carcinoma to druggable EGFR signalling. Oncogene 2022, 41, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, B.; Dong, X.; Zhou, Y.; He, Y.; Zhang, T.; Bao, L. Enhancement of cisplatin-induced cytotoxicity against cervical cancer spheroid cells by targeting long non-coding RNAs. Pathol. Res. Pract. 2019, 215, 152653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rashmi, R.; Inkman, M.; Jayachandran, K.; Ruiz, F.; Waters, M.R.; Grigsby, P.W.; Markovina, S.; Schwarz, J.K. Integrating imaging and RNA-seq improves outcome prediction in cervical cancer. J. Clin. Investig. 2021, 131, e139232. [Google Scholar] [CrossRef]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef]
- Rozzo, C.; Chiesa, V.; Caridi, G.; Pagnan, G.; Ponzoni, M. Induction of apoptosis in human neuroblastoma cells by abrogation of integrin-mediated cell adhesion. Int. J. Cancer 1997, 70, 688–698. [Google Scholar] [CrossRef]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell Adhesion Mediated Drug Resistance (CAM-DR): Role of Integrins and Resistance to Apoptosis in Human Myeloma Cell Lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Li, W.; Chen, W.; He, Q.; Zhang, X.; Tang, J.; Wang, Y.; Liu, B.; Liu, J. 3D bioprinted tumor model with extracellular matrix enhanced bioinks for nanoparticle evaluation. Biofabrication 2022, 14, 025002. [Google Scholar] [CrossRef]
- Pirola, L.; Ciesielski, O.; Balcerczyk, A. The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting. Cancers 2018, 10, 268. [Google Scholar] [CrossRef]
- Heredia-Mendez, A.J.; Sánchez-Sánchez, G.; López-Camarillo, C. Reprogramming of the Genome-Wide DNA Methylation Landscape in Three-Dimensional Cancer Cell Cultures. Cancers 2023, 15, 1991. [Google Scholar] [CrossRef]
- Amatangelo, M.; Garipov, A.; Li, H.; Conejo-Garcia, J.R.; Speicher, D.; Zhang, R. Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition. Cell Cycle 2013, 12, 2113–2119. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Sheraton, M.V.; Chiew, G.G.Y.; Melnikov, V.; Tan, E.Y.; Luo, K.Q.; Verma, N.; Sloot, P.M.A. Emergence of spatio-temporal variations in chemotherapeutic drug efficacy: In-vitro and in-Silico 3D tumour spheroid studies. BMC Cancer 2020, 20, 1201. [Google Scholar] [CrossRef]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular Tumor Spheroids for Evaluation of Cytotoxicity and Tumor Growth Inhibitory Effects of Nanomedicines In Vitro: A Comparison of Docetaxel-Loaded Block Copolymer Micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Patel, N.R.; Torchilin, V.P. Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG–PE micelles in ovarian cancer cell spheroid model. J. Control. Release 2012, 164, 95–102. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Nocelo, M.; Abuín, C.; López-López, R.; de la Fuente, M. Development and characterization of a three-dimensional co-culture model of tumor T cell infiltration. Biofabrication 2016, 8, 025002. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Cany, J.; van den Brand, D.; Oudenampsen, M.; Brock, R.; Torensma, R.; Bekkers, R.L.; Jansen, J.H.; Massuger, L.F.; Dolstra, H. Umbilical cord blood CD34+ progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rgnull mice. Oncoimmunology 2017, 6, e1320630. [Google Scholar] [CrossRef]
- Lanuza, P.M.; Vigueras, A.; Olivan, S.; Prats, A.C.; Costas, S.; Llamazares, G.; Sanchez-Martinez, D.; Ayuso, J.M.; Fernandez, L.; Ochoa, I.; et al. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. Oncoimmunology 2018, 7, e1395123. [Google Scholar] [CrossRef]
- Sherman, H.; Gitschier, H.J.; Rossi, A.E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Xenia Elena, B.; Nicoleta Gales, L.; Florina Zgura, A.; Iliescu, L.; Maricela Anghel, R.; Haineala, B. Assessment of Immune Status in Dynamics for Patients with Cancer Undergoing Immunotherapy. J. Oncol. 2021, 2021, 6698969. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Ros, W.; Delord, J.-P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chester, C.; Melero, I.; Kohrt, H. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann. Oncol. 2016, 27, 1190–1198. [Google Scholar] [CrossRef]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Röder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Takeshita, R.; Mukaida, R.; Takatori, E.; Nagasawa, T.; Omi, H.; Sugiyama, T. Safe administration of bevacizumab combination chemotherapy for the patients with recurrent cervical cancer after pelvic radiotherapy: Two case reports. Mol. Clin. Oncol. 2018, 9, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Salani, R.; Brady, W.E.; Lankes, H.A.; Cohn, D.E.; Mutch, D.G.; Mannel, R.S.; Bell-McGuinn, K.M.; Di Silvestro, P.A.; Jelovac, D.; et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology Study (NCT#01281852). Ann. Oncol. 2017, 28, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Wattanapanitch, M.; Thongsin, N.; Phanthaphol, N.; Chiawpanit, C.; Thuwajit, C.; Yenchitsomanus, P.-T.; Panya, A. Doxorubicin sensitizes breast cancer cells to natural killer cells in connection with increased Fas receptors. Int. J. Mol. Med. 2022, 49, 40. [Google Scholar] [CrossRef]
- De Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lõhmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Gopal, S.; Kwon, S.-J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 893. [Google Scholar] [CrossRef]
- Kusakabe, M.; Taguchi, A.; Tanikawa, M.; Hoshi, D.; Tsuchimochi, S.; Qian, X.; Toyohara, Y.; Kawata, A.; Wagatsuma, R.; Yamaguchi, K.; et al. Application of organoid culture from HPV18-positive small cell carcinoma of the uterine cervix for precision medicine. Cancer Med. 2023, 12, 8476–8489. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of Organoid Culture in Cancer Research. Cancer Med. 2023; early view. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, Z.; Li, Q.; Yang, S.; Jiang, L.; Yang, Y.; Li, Y.; Gu, Z. Organoids revealed: Morphological analysis of the profound next generation in-vitro model with artificial intelligence. Bio Des. Manuf. 2023, 1–21. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, N.; Sun, X.; Li, Q.; Zeng, Y.; Chen, F.; Sun, S.; Xu, J.; Zhang, J.; Ye, H.; et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials 2021, 272, 120770. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Creighton, C.J.; Zhang, Y.; Chen, F.; Thrall, M.J.; Kim, M.P. Ex Vivo Four-Dimensional Lung Cancer Model Mimics Metastasis. Ann. Thorac. Surg. 2015, 99, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, R.; Zhang, J.; Zhao, Y.; Qiao, S.; Crouzier, T.; Yan, H.; Tian, W. A novel 4D cell culture mimicking stomach peristalsis altered gastric cancer spheroids growth and malignance. Biofabrication 2021, 13, 035034. [Google Scholar] [CrossRef]
- Murphy, R.J.; Browning, A.P.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. Designing and interpreting 4D tumour spheroid experiments. Commun. Biol. 2022, 5, 91. [Google Scholar] [CrossRef]
- Wadman, M. FDA no longer has to require animal testing for new drugs. Science 2023, 379, 127–128. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutle, I.; Polten, R.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers 2023, 15, 2518. https://doi.org/10.3390/cancers15092518
Kutle I, Polten R, Hachenberg J, Klapdor R, Morgan M, Schambach A. Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers. 2023; 15(9):2518. https://doi.org/10.3390/cancers15092518
Chicago/Turabian StyleKutle, Ivana, Robert Polten, Jens Hachenberg, Rüdiger Klapdor, Michael Morgan, and Axel Schambach. 2023. "Tumor Organoid and Spheroid Models for Cervical Cancer" Cancers 15, no. 9: 2518. https://doi.org/10.3390/cancers15092518
APA StyleKutle, I., Polten, R., Hachenberg, J., Klapdor, R., Morgan, M., & Schambach, A. (2023). Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers, 15(9), 2518. https://doi.org/10.3390/cancers15092518







