Robust Beam Selection Based on Water Equivalent Thickness Analysis in Passive Scattering Carbon-Ion Radiotherapy for Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and CT-Image Acquisition
2.2. Original Treatment Plan
2.3. WET Analysis
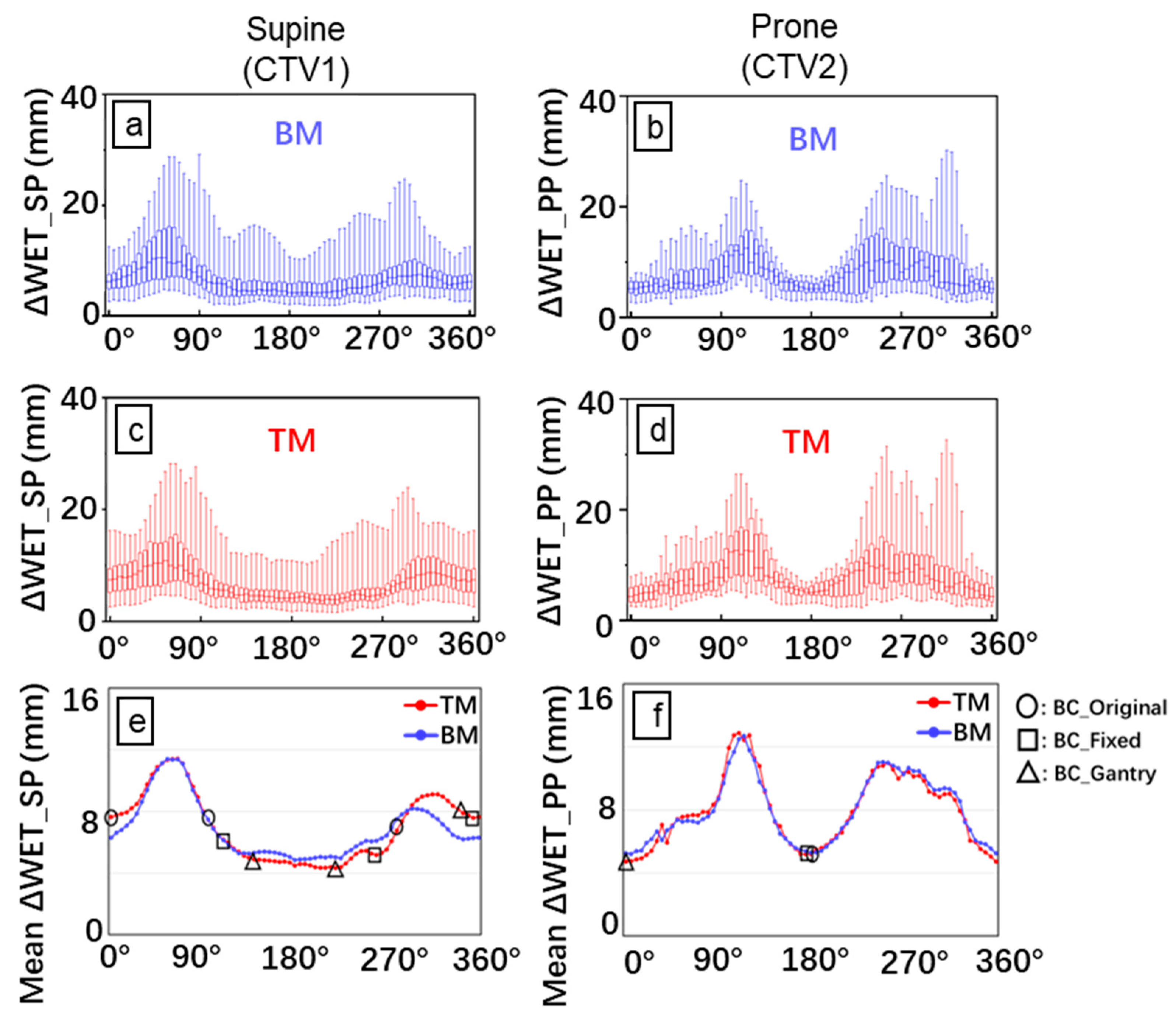
- To identify beam paths passing through the CTV in all selected beam angles (0° to 360° in steps of 5°), raytracing was performed for each CT_plan and CT_daily [16,17,18,19,20]; the ray separation interval was equal to the voxel size (i.e., 2 mm in the SI direction and 1.07 mm in the coronal plane) [see Figure S1(I)];
- CT_daily was aligned rigidly to the corresponding CT_plan using BM or TM, wherein the CT_plan of the supine and prone positions were aligned to the corresponding CT_daily;
- The total WET value from the body surface to the distal end of a CTV in a given beam path was the summation of the WET of all voxels along the path. The WET of a voxel was calculated as the product of the intersection length of the path within the respective voxel and the stopping power ratio evaluated from the CT value. The WET values were calculated in the CT_plan (WET_plan) and CT_daily (WET_daily) after BM and TM, respectively [Figure S1(II)];
- The paths that simultaneously passed the CTV_plan and CTV_daily were identified. The WET change of each interested path was calculated as the WET_plan minus the corresponding WET_daily [Figure S1(III)];
- ΔWET was averaged for the absolute WET change of all identified paths [Figure S1(IV)].
2.4. ΔWET Based Beam Configuration and Planning
2.5. Daily Dose Evaluation
2.6. Accumulated Dose Evaluation
2.7. Statistical Analysis
3. Results
3.1. ΔWET Analysis
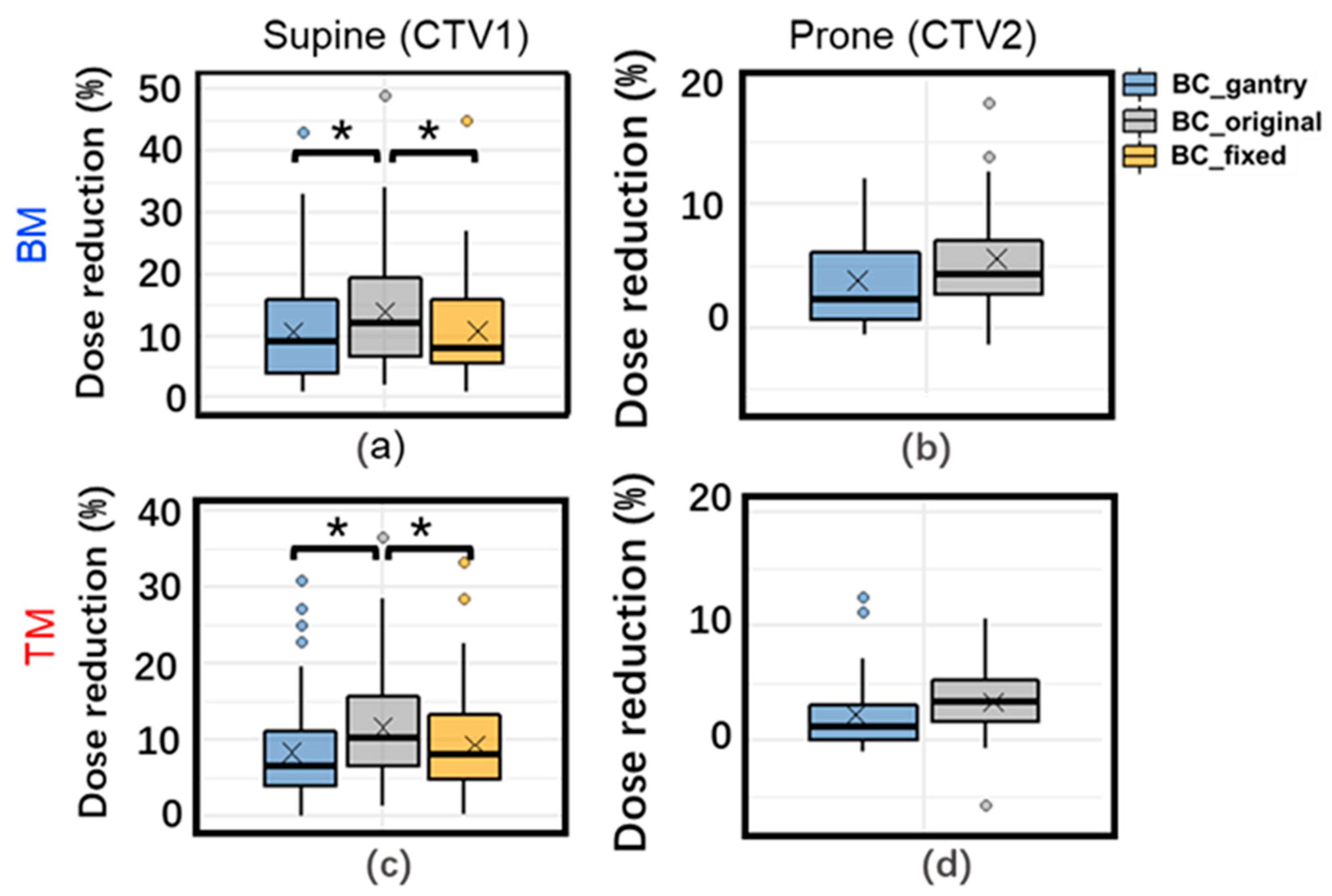
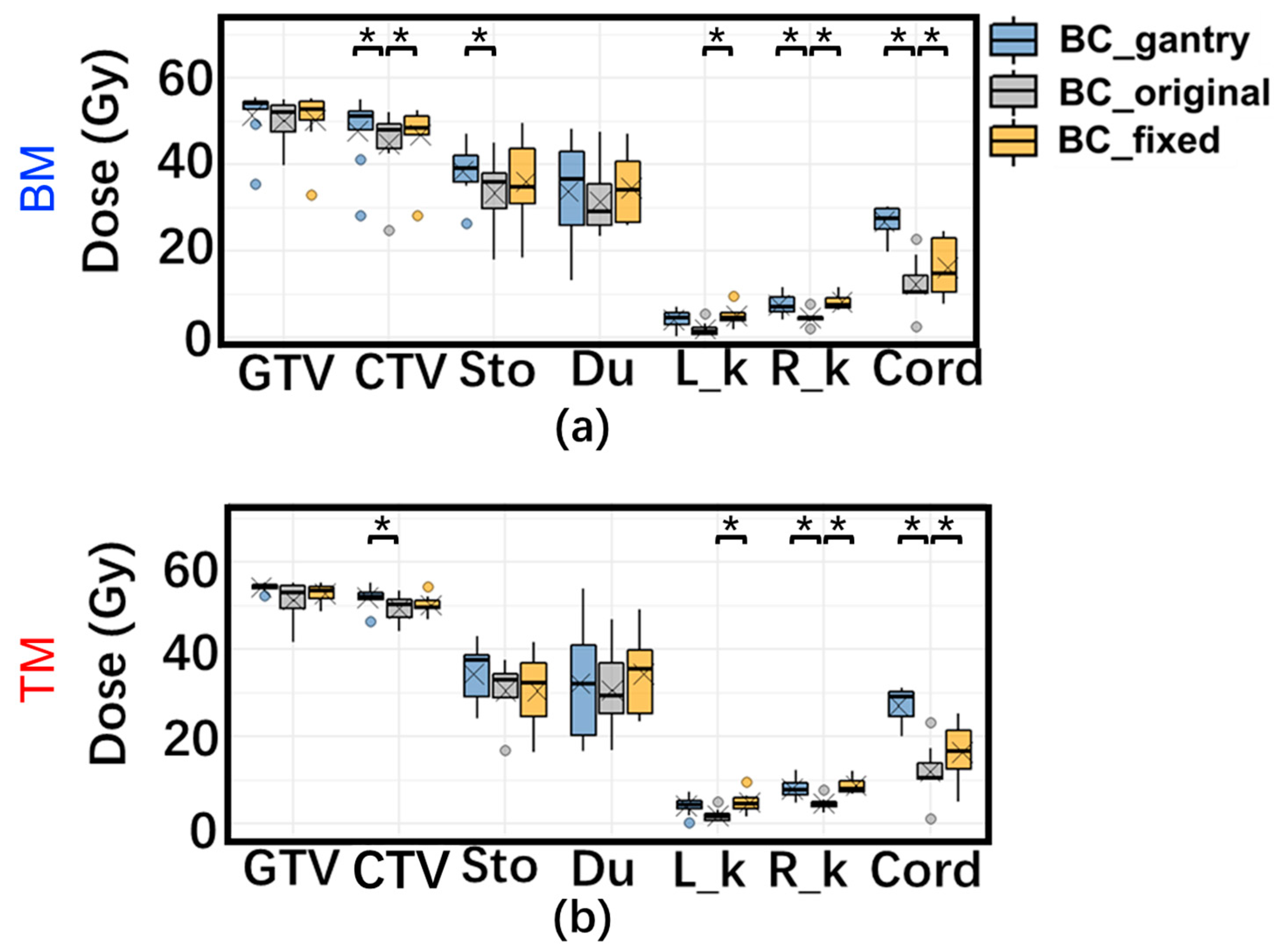
3.2. Daily Dose Variation
3.3. Accumulated Dose Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassler, N.; Kantemiris, I.; Karaiskos, P.; Engelke, J.; Holzscheiter, M.H.; Petersen, J.B. Comparison of optimized single and multifield irradiation plans of antiproton, proton and carbon ion beams. Radiother. Oncol. 2010, 95, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, K.; Cui, X.; Hirakawa, H.; Fujimori, A.; Kamijo, T.; Yamada, S.; Yokosuka, O.; Kamada, T. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother. Oncol. 2012, 105, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kawashiro, S.; Yamada, S.; Okamoto, M.; Ohno, T.; Nakano, T.; Shinoto, M.; Shioyama, Y.; Nemoto, K.; Isozaki, Y.; Tsuji, H.; et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. 2018, 101, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Asano, T.; et al. Carbon Ion Radiation Therapy with Concurrent Gemcitabine for Patients with Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. 2015, 95, 498–504. [Google Scholar] [CrossRef]
- Vitolo, V.; Cobianchi, L.; Brugnatelli, S.; Barcellini, A.; Peloso, A.; Facoetti, A.; Vanoli, A.; Delfanti, S.; Preda, L.; Molinelli, S.; et al. Preoperative chemotherapy and carbon ions therapy for treatment of resectable and borderline resectable pancreatic adenocarcinoma: A prospective, phase II, multicentre, single-arm study. BMC Cancer 2019, 19, 922. [Google Scholar] [CrossRef]
- Houweling, A.C.; Fukata, K.; Kubota, Y.; Shimada, H.; Rasch, C.R.; Ohno, T.; Bel, A.; van der Horst, A. The impact of interfractional anatomical changes on the accumulated dose in carbon ion therapy of pancreatic cancer patients. Radiother. Oncol. 2016, 119, 319–325. [Google Scholar] [CrossRef]
- Kubota, Y.; Okamoto, M.; Shiba, S.; Okazaki, S.; Matsui, T.; Li, Y.; Itabashi, Y.; Sakai, M.; Kubo, N.; Tsuda, K.; et al. Robustness of daily dose for each beam angle and accumulated dose for inter-fractional anatomical changes in passive carbon-ion radiotherapy for pancreatic cancer: Bone matching versus tumor matching. Radiother. Oncol. 2021, 157, 85–92. [Google Scholar] [CrossRef]
- Kubota, Y.; Katoh, H.; Shibuya, K.; Shiba, S.; Abe, S.; Sakai, M.; Yuasa, D.; Tsuda, K.; Ohno, T.; Nakano, T. Comparison between bone matching and marker matching for evaluation of intra- and inter-fractional changes in accumulated dose of carbon ion radiotherapy for hepatocellular carcinoma. Radiother. Oncol. 2019, 137, 77–82. [Google Scholar] [CrossRef]
- Li, Y.; Kubota, Y.; Kubo, N.; Mizukami, T.; Sakai, M.; Kawamura, H.; Irie, D.; Okano, N.; Tsuda, K.; Matsumura, A.; et al. Dose assessment for patients with stage I non-small cell lung cancer receiving passive scattering carbon-ion radiotherapy using daily computed tomographic images: A prospective study. Radiother. Oncol. 2020, 144, 224–230. [Google Scholar] [CrossRef]
- Kumagai, M.; Hara, R.; Mori, S.; Yanagi, T.; Asakura, H.; Kishimoto, R.; Kato, H.; Yamada, S.; Kandatsu, S.; Kamada, T. Impact of Intrafractional Bowel Gas Movement on Carbon Ion Beam Dose Distribution in Pancreatic Radiotherapy. Int. J. Radiat. Oncol. 2009, 73, 1276–1281. [Google Scholar] [CrossRef]
- Liu, F.; Erickson, B.; Peng, C.; Li, X.A. Characterization and Management of Interfractional Anatomic Changes for Pancreatic Cancer Radiotherapy. Int. J. Radiat. Oncol. 2012, 83, e423–e429. [Google Scholar] [CrossRef]
- Van der Horst, A.; Wognum, S.; Fajardo, R.D.; De Jong, R.; Van Hooft, J.E.; Fockens, P.; Van Tienhoven, G.; Bel, A. Interfractional position variation of pancreatic tumors quantified using intratumoral fiducial markers and daily cone beam computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Kubota, Y.; Sakai, M.; Ohno, T. Robust Angle Selection in Particle Therapy. Front. Oncol. 2021, 11, 715025. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Lu, H.-M.; Wolfgang, J.A.; Choi, N.C.; Chen, G.T.Y. Effects of interfractional anatomical changes on water-equivalent pathlength in charged-particle radiotherapy of lung cancer. J. Radiat. Res. 2009, 50, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Matney, J.E.; Park, P.C.; Li, H.; Court, L.E.; Zhu, X.R.; Dong, L.; Liu, W.; Mohan, R. Perturbation of water-equivalent thickness as a surrogate for respiratory motion in proton therapy. J. Appl. Clin. Med Phys. 2016, 17, 368–378. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Liao, L.; Li, H.; Zhu, R.; Park, P.C.; Sahoo, N.; Gillin, M.; Li, Y.; Chang, J.Y.; et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys. 2016, 43, 1111–1118. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, M.W.; Li, Z. A Beam-Angle-Selection Method to Improve Inter-Fraction Motion Robustness for Lung Tumor Irradiation with Passive Proton Scattering. Technol. Cancer Res. Treat. 2020, 19, 1533033820948052. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.-K.; Sharp, G.; Busse, P.; Winey, B. Beam angle optimization using angular dependency of range variation assessed via water equivalent path length (WEPL) calculation for head and neck proton therapy. Phys. Medica 2020, 69, 19–27. [Google Scholar] [CrossRef]
- Casares-Magaz, O.; Toftegaard, J.; Muren, L.P.; Kallehauge, J.F.; Bassler, N.; Poulsen, P.R.; Petersen, J.B. A method for selection of beam angles robust to intra-fractional motion in proton therapy of lung cancer. Acta Oncologica. 2014, 53, 1058–1063. [Google Scholar] [CrossRef]
- Gorgisyan, J.; Perrin, R.; Lomax, A.J.; Persson, G.F.; Josipovic, M.; Engelholm, S.A.; Weber, D.C.; Rosenschold, P.M.A. Impact of beam angle choice on pencil beam scanning breath-hold proton therapy for lung lesions. Acta Oncol. 2017, 56, 853–859. [Google Scholar] [CrossRef]
- Cao, W.; Lim, G.J.; Lee, A.; Li, Y.; Liu, W.; Zhu, X.R.; Zhang, X. Uncertainty incorporated beam angle optimization for IMPT treatment planning. Med Phys. 2012, 39, 5248–5256. [Google Scholar] [CrossRef] [PubMed]
- Dreher, C.; Habermehl, D.; Ecker, S.; Brons, S.; El-Shafie, R.; Jäkel, O.; Debus, J.; Combs, S.E. Optimization of carbon ion and proton treatment plans using the raster-scanning technique for patients with unresectable pancreatic cancer. Radiat. Oncol. 2015, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Kanai, T.; Yamada, S.; Yusa, K.; Tashiro, M.; Shimada, H.; Torikai, K.; Yoshida, Y.; Kitada, Y.; Katoh, H.; et al. Carbon Ion Radiotherapy at the Gunma University Heavy Ion Medical Center: New Facility Set-up. Cancers 2011, 3, 4046–4060. [Google Scholar] [CrossRef]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-Ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. 1999, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kubota, Y.; Okamoto, M.; Shiba, S.; Okazaki, S.; Matsui, T.; Komatsu, S.; Ohno, T. Determination of Deformable Image Registration Algorithms for Accumulating Dose in Carbon-ion Radiotherapy for Pancreatic Cancer. Anticancer. Res. 2021, 41, 835–843. [Google Scholar] [CrossRef]
- Batista, V.; Richter, D.; Combs, S.E.; Jäkel, O. Planning strategies for inter-fractional robustness in pancreatic patients treated with scanned carbon therapy. Radiat. Oncol. 2017, 12, 94. [Google Scholar] [CrossRef]
- Miki, K.; Fukahori, M.; Kumagai, M.; Yamada, S.; Mori, S. Effect of patient positioning on carbon-ion therapy planned dose distribution to pancreatic tumors and organs at risk. Phys. Medica 2016, 33, 38–46. [Google Scholar] [CrossRef]
- Li, Y.; Kubota, Y.; Okamoto, M.; Shiba, S.; Okazaki, S.; Matsui, T.; Tashiro, M.; Nakano, T.; Ohno, T. Adaptive planning based on single beam optimization in passive scattering carbon ion radiotherapy for patients with pancreatic cancer. Radiat. Oncol. 2021, 16, 111. [Google Scholar] [CrossRef]
- Jia, S.; Chen, J.; Ma, N.; Zhao, J.; Mao, J.; Jiang, G.; Lu, J.; Wu, K. Adaptive carbon ion radiotherapy for locally advanced non-small cell lung cancer: Organ-sparing potential and target coverage. Med Phys. 2022, 49, 3980–3989. [Google Scholar] [CrossRef]
- Kawashima, M.; Tashiro, M.; Varnava, M.; Shiba, S.; Matsui, T.; Okazaki, S.; Li, Y.; Komatsu, S.; Kawamura, H.; Okamoto, M.; et al. An adaptive planning strategy in carbon ion therapy of pancreatic cancer involving beam angle selection. Phys. Imaging Radiat. Oncol. 2022, 21, 35–41. [Google Scholar] [CrossRef]
- Steitz, J.; Naumann, P.; Ulrich, S.; Haefner, M.F.; Sterzing, F.; Oelfke, U.; Bangert, M. Worst case optimization for interfractional motion mitigation in carbon ion therapy of pancreatic cancer. Radiat. Oncol. 2016, 11, 134. [Google Scholar] [CrossRef]
- Molinelli, S.; Vai, A.; Russo, S.; Loap, P.; Meschini, G.; Paganelli, C.; Barcellini, A.; Vitolo, V.; Orlandi, E.; Ciocca, M. The role of multiple anatomical scenarios in plan optimization for carbon ion radiotherapy of pancreatic cancer. Radiother. Oncol. 2022, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Katoh, H.; Minohara, S.; Fujii, H.; Miyasaka, Y.; Takayama, Y.; Imura, K.; Kusunoki, T.; Miyakawa, S.; Kamada, T.; et al. Robust treatment planning in scanned carbon-ion radiotherapy for pancreatic cancer: Clinical verification using in-room computed tomography images. Front. Oncol. 2022, 12, 974728. [Google Scholar] [CrossRef]
- Niebuhr, N.I.; Splinter, M.; Bostel, T.; Seco, J.; Hentschke, C.M.; Floca, R.O.; Hörner-Rieber, J.; Alber, M.; Huber, P.; Nicolay, N.H.; et al. Biologically consistent dose accumulation using daily patient imaging. Radiat. Oncol. 2021, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Mori, S.; Shinoto, M.; Nakayama, Y.; Kamada, T.; Yamada, S. Comparison of carbon-ion passive and scanning irradiation for pancreatic cancer. Radiother. Oncol. 2016, 119, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.; Richter, D.; Chaudhri, N.; Naumann, P.; Herfarth, K.; Jäkel, O. Significance of intra-fractional motion for pancreatic patients treated with charged particles. Radiat. Oncol. 2018, 120, 13. [Google Scholar] [CrossRef] [PubMed]


| Patient Number | Age | Sex | Target | CTV_Plan Volume (cm3) | CTV_Daily Volume (Mean ± SD) (cm3) | |
|---|---|---|---|---|---|---|
| 1 | 50 | F | CTV1: | 94.0 | 99.3 | ±4.8 |
| CTV2: | 51.1 | 53.0 | ±1.9 | |||
| 2 | 76 | M | CTV1: | 158.1 | 156.7 | ±5.3 |
| CTV2: | 63.6 | 57.0 | ±3.9 | |||
| 3 | 83 | F | CTV1: | 170.3 | 173.9 | ±6.2 |
| CTV2: | 89.4 | 76.1 | ±3.1 | |||
| 4 | 81 | M | CTV1: | 202.0 | 176.4 | ±15.2 |
| CTV2: | 113.4 | 83.4 | ±4.7 | |||
| 5 | 51 | F | CTV1: | 126.8 | 114.6 | ±9.5 |
| CTV2: | 54.5 | 48.7 | ±4.8 | |||
| 6 | 61 | F | CTV1: | 127.6 | 126.5 | ±7.0 |
| CTV2: | 70.3 | 55.8 | ±7.6 | |||
| 7 | 78 | F | CTV1: | 131.3 | 129.0 | ±5.5 |
| CTV2: | 45.8 | 52.9 | ±1.9 | |||
| 8 | 74 | M | CTV1: | 165.8 | 168.3 | ±7.0 |
| CTV2: | 99.3 | 87.6 | ±1.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Sakai, M.; Li, Y.; Kubota, Y.; Okamoto, M.; Shiba, S.; Okazaki, S.; Matsui, T.; Ohno, T. Robust Beam Selection Based on Water Equivalent Thickness Analysis in Passive Scattering Carbon-Ion Radiotherapy for Pancreatic Cancer. Cancers 2023, 15, 2520. https://doi.org/10.3390/cancers15092520
Zhou Y, Sakai M, Li Y, Kubota Y, Okamoto M, Shiba S, Okazaki S, Matsui T, Ohno T. Robust Beam Selection Based on Water Equivalent Thickness Analysis in Passive Scattering Carbon-Ion Radiotherapy for Pancreatic Cancer. Cancers. 2023; 15(9):2520. https://doi.org/10.3390/cancers15092520
Chicago/Turabian StyleZhou, Yuan, Makoto Sakai, Yang Li, Yoshiki Kubota, Masahiko Okamoto, Shintaro Shiba, Shohei Okazaki, Toshiaki Matsui, and Tatsuya Ohno. 2023. "Robust Beam Selection Based on Water Equivalent Thickness Analysis in Passive Scattering Carbon-Ion Radiotherapy for Pancreatic Cancer" Cancers 15, no. 9: 2520. https://doi.org/10.3390/cancers15092520
APA StyleZhou, Y., Sakai, M., Li, Y., Kubota, Y., Okamoto, M., Shiba, S., Okazaki, S., Matsui, T., & Ohno, T. (2023). Robust Beam Selection Based on Water Equivalent Thickness Analysis in Passive Scattering Carbon-Ion Radiotherapy for Pancreatic Cancer. Cancers, 15(9), 2520. https://doi.org/10.3390/cancers15092520







