Abstract
Simple Summary
The survival rate for pediatric cancer has increased over the past few decades, short- and long-term complications have been detected and studied, and oral complications have emerged as an important topic of research. Here, we aimed to highlight the importance of oral manifestations that may only become apparent years or even decades after cancer treatment. Childhood cancer survivors presented a higher risk of having dental alterations than control counterparts. Additional analyses reveal possible sex-based differences that should be explored in future studies. These results collectively highlight the importance of oral healthcare and the prevention of disease in childhood cancer survivors.
Abstract
The survival rate for pediatric cancer has increased over the past few decades, short- and long-term complications have been detected and studied, and oral complications have emerged as an important topic of research. Here, we aimed to highlight the importance of oral manifestations that may only become apparent years or even decades after cancer treatment. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis. We searched articles using PubMed via the MEDLINE, Web of Science, and LILACS databases until October 2023. Overall, 35 observational studies were included, and the results estimated a pooled prevalence of the following dental anomalies: discoloration, 53%; crown-root malformations and agenesis, 36%; enamel hypoplasia, 32%; root development alterations, 29%; unerupted teeth, 24%; microdontia, 16%; hypodontia, 13%; and macrodontia, 7%. Most childhood cancer survivors have at least one dental sequela. Childhood cancer survivors presented a higher risk of having dental alterations than control counterparts. Additional analyses reveal possible sex-based differences that should be explored in future studies. These results collectively highlight the importance of oral healthcare and the prevention of disease in childhood cancer survivors.
1. Introduction
Childhood cancer is a leading cause of death, with an estimated 400,000 children and adolescents between the ages of 0 and 19 years diagnosed with cancer [,]. Because they are generally not prevented or detected by screening, accurate and timely diagnosis is essential to promote clinical success and high survival rates []. The treatment options for pediatric malignancies include chemotherapy, radiation therapy, surgery, and multimodal approaches [,].
The survival rates for children with cancer have increased; nevertheless, up to 40% of children present complications later due to cancer treatment []. Short- and long-term complications have been identified, and oral complications are an important research topic []. In addition, children are three-times more likely than adults to experience developmental complications, exacerbating the impact of searching for this topic []. Some oral manifestations may occur early during treatment or years or decades after cancer treatment. Short-term adverse effects may include dental caries, mucositis, bleeding, taste alterations, secondary infections, periodontal disease, trismus and osteoradionecrosis [,]. Long-term complications were not described until the 1970s because the post-treatment observation period was still short [,]. More recently, combined anticancer treatments have been identified as being responsible for late oral effects, including craniofacial and dental developmental defects and salivary gland dysfunction, especially when performed at a young age [,,,].
This systematic review aimed to summarize the findings of estimating the prevalence of oral short- and long-term adverse effects in pediatric cancer survivors during and after oncologic treatment. We aimed to provide information that will allow for the reinforcement of the role of pediatric oncologists for possible dental abnormalities that have a negative impact on the quality of life of both patients and families.
2. Materials and Methods
2.1. Protocol and Registration
All authors established the protocol, registered it at the National Institute for Health Research PROSPERO platform (ID Number: CRD42022336369), and reported it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [] (Supplementary Table S1).
2.2. Focused Questions and Eligibility Criteria
We developed a protocol to answer two PICO questions:
- “What is the prevalence of late oral health adverse effects in childhood cancer survivors with a history of chemotherapy and radiotherapy”?
- “Are children who undergo cancer therapy more likely to have late oral health adverse effects when compared with healthy controls counterparts”?
Late oral health adverse effects were defined as late sequelae of oncological treatment-related toxicities to dentofacial structures.
The respective statements were as follows: pediatric patients with malignant cancer diagnosed between the ages of 3 and 18 years (P, Participants); patients who had undergone a therapeutic combination of radiotherapy/chemotherapy or not by the age of 18 years and were in the primary/mixed/permanent dentition were included (I, Intervention); the presence or absence of a control group was not a limitation (C, Control); estimated prevalence of the late effects of the oral complications (mucositis, candidiasis, ulcers) and dental structures (microdontia, hypodontia, hypoplasia, malformed teeth, impaired root growth, interrupted root growth, V-shaped roots, taurodontism, premature apical closure, and tooth agenesis) (O, Outcome).
Randomized clinical trials, controlled clinical trials, cohort studies (prospective or retrospective design), and cross-sectional studies were eligible for inclusion. The exclusion criteria were as follows: (1) duplicate studies; (2) abstracts, commentaries, reviews, letters to the editor, consensus, opinions, case studies, and case series; (3) unpublished information; (4) lack of appropriate clinical measures; (5) secondary analysis of data sourced from a previous study; and (6) inclusion of animal studies. There were no restrictions on the year or language of publication.
2.3. Data Search Strategy and Study Selection
We searched PubMed through MEDLINE, Web of Science, and LILACS for all relevant articles published until October 2023. Grey literature was also searched for using OpenGrey (http://www.opengrey.eu/, accessed on 20 November 2023). The following search terms were used: (1) (chemotherap* OR radiotherap* OR cancer); (2) (child* OR adolescent* OR pediat* OR paediat*); (3) (caries OR decay OR xerostomia OR root stunting OR periodont* OR gum OR gingiv*) NOT adult*. Two independent reviewers (J.P.L. and L.B.L.) performed the search and included studies.
Two independent examiners performed, in duplicate, the assessment of titles and/or abstracts of retrieved studies independently (J.P.L. and L.B.L.). For measurement reproducibility, inter-examiner reliability following full-text assessment was calculated using kappa statistics. Any disagreements were resolved by discussion with a third author (M. M.).
2.4. Data Extraction Process and Data Items
Data extraction was performed by two reviewers independently and in duplicate (J.P.L. and L.B.L.). Any paper deemed potentially eligible by one of the reviewers was independently reviewed. All disagreements were resolved by discussion with a third reviewer (VM). The following information was collected: general description, research characteristics, methodology, and outcome measures. The following standard information was extracted from each eligible study: the first author’s name, year of publication, country and place of sampling, study period, sample size (male/female), case definition setting, observation setting, sampling strategy, cancer type, treatment (chemotherapy and/or radiotherapy), adverse oral health effects, study funding, and risk of bias.
2.5. Risk of Bias (RoB) Assessment
The methodological quality of the eligible studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist []. This tool allowed for analysis in eight domains, presented in the form of questions as follows: (1) Were the criteria for inclusion in the sample clearly defined? (2) Were the study participants and settings described in detail? (3) Was exposure measured in a valid and reliable way? (4) Were objective and standard criteria used for the measurement of the condition? (5) Were confounding factors identified? (6) Were the strategies to deal with the confounding factors stated? (7) Were the outcomes measured in a valid and reliable manner? (8) Was appropriate statistical analysis used? Each item was scored as Y (i.e., yes)—reported and adequate, N (i.e., no)—not reported, and U (i.e., unclear)—reported inadequately. Any disagreements between examiners were resolved through discussion with a third author. Only studies with all items scored with “Y” were considered to be of high quality, studies with at least one item “N” were of low quality, and, finally, for those which presented at least one “U” item and all the others “Y” were of unclear quality. The Risk-Of-Bias VISualization (ROBVIS) tool was used to analyze the risk of bias [].
2.6. Summary Measures and Synthesis of Results
Standard spreadsheet software (Microsoft Excel for Mac, version 16.50. Microsoft, Redmond, WA, USA) was used for data extraction. Frequencies and percentages were used to describe categorical variables, whereas continuous variables were reported as mean ± standard deviation (SD) and range. Random-effects meta-analysis and forest plots of prevalence were calculated in R version 3.4.1 (R Studio Team 2018) using the ‘meta’ package [], through the DerSimonian–Laird random-effects meta-analysis. A meta-analysis was performed to calculate dental anomalies in pediatric cancer survivors. A risk ratio (RR) with a 95% confidence interval (CI) was used to describe the dental disharmonies of cancer survivors compared to healthy children. The RR was pooled using a random-effects model in R version 3.4.1 (R Studio Team 2018), using the ‘readxl’ package and pairwise random-effects meta-analysis, and p-values less than 0.05 were considered statistically significant. The chi-square (χ2) test was used to calculate overall homogeneity, and substantial heterogeneity was considered when I2 statistics exceeded 50% []. To explore potential sources of heterogeneity, we performed a subgroup analysis according to the methodological quality of the included studies and the female/male ratio. Publication bias was considered when the meta-analysis included at least 10 studies [].
3. Results
3.1. Study Selection
The online search strategy identified 3601 potentially relevant publications. After removing duplicates, 3029 articles were assessed against the eligibility criteria, and 2950 were excluded after title and/or abstract review. Of the 79 articles assessed for eligibility for full-paper review, 44 were excluded, with the respective reasons for exclusion detailed in Supplementary Table S2. As a result, a final number of 35 observational studies were included for qualitative synthesis; a PRISMA diagram is shown in Figure 1. The inter-examiner reliability of the full-text screening was considered very high (kappa score = 0.915, 95% CI: 0.895–0.925).
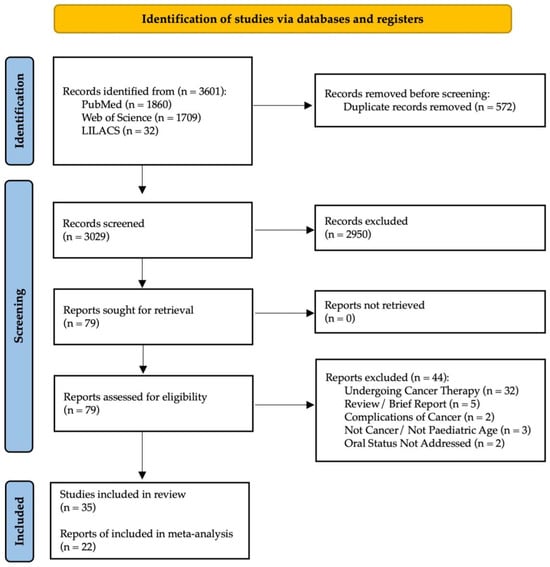
Figure 1.
PRISMA flowchart of studies.
3.2. Studies’ Characteristics
Overall, a total of 3761 participants from all 35 included studies were included in this systematic review, 2625 childhood cancer survivors (889 females and 1122 males, 10 did not report sex) and 1136 healthy children (209 females and 243 males, 11 did not report sex) (Table 1). All studies addressed long-term adverse oral health effects in childhood cancer survivors, although 14 studies did not present a control group [,,,,,,,,,,,].
Regarding the type of study, 15 were cohort studies [,,,,,,,,,,,,,,], 13 case–control studies [,,,,,,,,,,,,], and 7 cross-sectional studies [,,,,,,].
Several points were considered in the case definition setting. Some studies addressed multiple topics: 24 assessed caries incidence [,,,,,,,,,,,,,,,,,,,,,,,], 18 assessed dental abnormalities such as root stunting and microdontia [,,,,,,,,,,,,,,,,], and 15 stressed the developmental defects of enamel [,,,,,,,,,,,,,]. The other 13 studies mentioned plaque index and/or gingival index [,,,,,,,,,,,,], and the other 9 considered oral hygiene [,,,,]. Saliva assessment was addressed by seven studies [,,,,,,], and two investigated craniofacial development [,].
Some research highlights themes in a unique way, such as the regularity of dental attendance and type of dentist visited [], number of erupted teeth relative to age [], oral mucositis and ulceration, candidiasis, herpes and herpetic gingivo-stomatitis, oral petechiae, facial pain [], already [] addressed facial asymmetry and jaw hypoplasia, as well as trismus. Hutton 2010 mentioned traumatized teeth and [] calculated the root surface areas of mandibular teeth.
Furthermore, studies have been conducted in 16 countries worldwide. Notably, no studies have been performed in Oceania or Africa.
The most prevalent types of cancer studied were acute lymphoblastic leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, Rhabdomyosarcoma, Wilms tumor, and neuroblastoma. Other malignant conditions included Retinoblastoma, Fibroma, Medulloblastoma, Nasopharyngeal carcinoma, Langerhans cell histiocytoma, malignant teratoma, optical glioma, germinoma, leiomyosarcoma, and hepatoblastoma. Treatment modalities included chemotherapy and radiotherapy, with or without bone marrow transplantation.

Table 1.
Characteristics of the included studies.
Table 1.
Characteristics of the included studies.
| Study | Design | Country | Sample | Oral Health Conditions Case Definition Setting | Cancer Type | Treatment Modality | Study Funding |
|---|---|---|---|---|---|---|---|
| Halperson et al. (2022) [] | Cross-sectional | Israel | 121 | Dental caries; Dental developmental anomalies (DDA—includes five major groups: no disturbance identified, hypomineralization or hypoplasia, microdontia, root changes, and an absent tooth bud categorized as hypodontia); DMFT index | leukemia\lymphoma in 53 (45%) patients, solid tumors in 35 (29%) and other hematological conditions leading to BMT in 31 (26%) | Most patients (83, 69%) had received ChemoT without radiotherapy. Thirty-eight (31%) had received radiation therapy only or in combination with ChemoT. Fourteen (12%) of the cohort had received total body irradiation (TBI) 12 Gray and 15 (13%) radiation to the head and/or neck area (range of 27–70 Gray). The remaining nine patients had received radiotherapy to other areas (range of 30–70 Gray). Thirty percent of the cohort had undergone BMT | NR |
| Shayani et al. (2022) [] | Retrospective | Spain | 109 | DDE, microdontia, taurodontism, agenesis, root shortening (RS) | leukemias and lymphomas (41.3%) followed by solid non-CNS tumors (38.5%) and, finally, solid CNS tumors (20.2%) | ChemoT (CT); CT combined with radiotherapy (CT + RT); and CT + RT combined with hematopoietic stem cell transplantation (HSCT) | NI |
| Rabassa-Blanco et al. (2022) [] | Retrospective | Chile | 23 | missing or filled teeth index and the presence of gingivitis | ALL | ChemoT | NI |
| Stolze et al. (2022) [] | Cross-sectional | The Netherlands | 291 | unstimulated (UWS) and stimulated whole salivary flow rates (SWS) were measured according to internationally standardized procedures—categorized into ‘hyposalivation’ (<0.2 mL/min and <0.7 mL/min, respectively) and ‘severe hyposalivation’ (<0.1 mL/min and <0.5 mL/min, respectively); partic- ipants were asked to fill out the Dutch translation of the Xerostomia Inventory (XI) | Hematological malignancy (n = 216); brain tumor (n = 19); solid tumor (n = 57) | head and neck radiotherapy (H&N RT) or total body irradiation (TBI) without chronic graft versus host disease (cGVHD), a group of CCS with (a history of) cGVHD after HSCT, and a group of CCS treated with ChemoT and no H&N RT or TBI | NI |
| Tanem et al. (2022) [] | Cross-sectional | Norway | 46 | decayed-missing-filled index (DMFT), oral dryness, maximum mouth opening (MMO), fungal infection, and registration of dental developmental disturbances (DDD) in the form of hypodontia, microdontia, and enamel hypoplasia | brain tumors medulloblastoma (MB) and central nervous system supratentorial primitive neuroectodermal tumor (CNS-PNET). | ChemoT + craniospinal irradiation | Research Grant |
| Guagnano et al. (2022) [] | Cross-sectional | Italy | 52 | Decayed-missing-filled teeth (dmft/DMFT) index; Disturbances of enamel mineralisation using Aine rating scale; dental age estimation using panoramic radiographs; dental abnormalities using the Höltta Defect Index on panoramic radiographs—Valores médios para cada sexo, tipo de terapêutica e idade no diagnóstico (<5 anos ou ≥5 anos), os valores apresentados à frente são média da populção toda | ALL Acute Myeloblastic Leukemia Medulloblastoma Familiar Hemophagocitic Lymphohistiocitosis Lymphoma Juvenile Myelomonocytic Leukemia Wilms tumour Epatoblastoma Rhabdomyosarcoma Ewing-PNET Sarcoma Severe Aplastic Anaemia Xantoastocitoma Wide Cells Anaplastic Lymphoma Histiocytosis | CT and/or RT, Hematopoietic Stem Cell Transplantation (HSCT) or Bone Marrow Transplantation (BMT) | NI |
| Seremidi et al. (2021) [] | Retrospective | Greece | 70 | Microdontia, Malformed teeth, Oligodontia, Hypodontia, Enamel defects, Dental caries | central nervous system tumor, Solid Tumors and Lymphomas | ChemoT, or hemopoietic stem cell transplantatio | None |
| Proc et al. (2019) [] | Cross-sectional | Poland | 75 | dmft; DMFT; plaque index by silness and loe | ALL; Wilms tumor; Neuroblastoma; Rhabdosarcoma (RMS); Brain tumor; Hepatoblastoma; Acute non-lymphoblastic leukemia (ANLL) Non-Hodgkin’s lymphoma (B-NHL) Hodgkin’s lymphoma (HL); Primitive neuroectodermal tumor (PNET) Germinal tumour; Tumor ovari | RadioT & ChemoT | NI |
| Alnuaimi et al. (2018) [] | Retrospective | United Arab Emirates | 120 | Oral health problem: oral mucositis & ulceration, candidiasis, herpes and herpetic gingivo-stomatitis, gigival bleeding, gigivites, oral petechiae, dental caries, poor oral hygiene, facial pain/palsy, other | Leukaemic | ChemoT | NI |
| Çetiner et al. (2019) [] | Retrospective | Turkey | 53 | Gingival Index, Plaque Index, dmft/DMFT, dmfs/DMFS, craniofacial development | Hodgkin lymphoma, Non-Hodgkin lymphoma, Neuroblastoma, Wilms tumor, Retinoblastoma, Rhabdomyosarcoma, Nasopharynx carcinoma | ChemoT | NI |
| Olczak-Kowalczyk et al. (2018) [] | Case–control | Poland | 60 | DMFT; dmft; DMFS; dmfs: teeth/surfaces with white spot lesions–WSL (D1 + 2/d1 + 2), following the ICDAS-II criteria | neoplasm; medulloblastoma (12.5%), nephroblastoma (Wilms’tumour,10.8%), Burkitt’s lymphoma (10.8%), neuroblastoma (8.3%), rhab- domyosarcoma (RMS, 6.6%), Ewing’s sarcoma (5.8%), and less frequently: chondrosarcoma, hepatoblastoma, glioblas- toma, ependimoma, and osteosarcoma. | Multidrug therapy, adapted to each neoplasm type and including vincristine, cyclophosphamide, adriamycin, etopo- side, cisplatin, ifosfamide, actomycin, and methotrexate; ChemoT for the others | NI |
| Bica et al. (2017) [] | Retrospective | Romania | 36 | hypoplasia (hypomineralisation) of the enamel, microdontia and atypical eruption. | limphoblastic leukemia | ChemoT | NI |
| Krasuska-Sławińska et al. (2016) [] | Case–control | Poland | 60 | oral hygiene, gingiva (PI), dentition, and potential visible decrease in salivary secretion. | Different neoplasms | PCH—60 patients after at least 1 year ChemoT CG—60 generally healthy patients. | NR |
| Owosho et al. (2016) [] | Retrospective | United States of America | 13 | Facial asymmetry and jaw hypoplasia; Effects on the dental tissue causing tooth agenesis/hypodontia, root agenesis/stunting/malformation, and/or enamel hypoplasia; trismus, hyposalivation/xerostomia. | head and neck rhabdomyosarcoma (HNRMS)—Tumor sites were orbit in 1 patient and parameningeal in 12 (infratemporal fossa in 5, nasopharynx in 5, parapharyngeal in 1, and middle ear in 1) | multiagent ChemoT and IMRT—median radiation dose to the primary tumor was 50.4 Gy (range: 45–50.4 Gy), and the ChemoT agents were vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide | NI |
| Nemeth et al. (2014) [] | Case–control | Hungary | 38 | DMFT; unstimulated saliva flow rate—spitting method (USF); stimulated saliva flow rate—spitting method (SSF); palatal saliva flow rate using a Periotron meter (Oraflow Inc., Plainview, NY, USA) (PS); salivary buffer capacity using CRT buffer (Ivoclar Vivadent AG, Schaan, Lichtenstein) | NI | 18 patients BFM-95 = protocol for acute lymphoblastic lymphoma, Berlin-Frankfurt-Munster; 5 patients NBL-2 = protocol for neuroblastoma; 4 patients CWS 96 = protocol of Cooperative Soft Tissue Sarcoma Study Group; 4 patients SIOP 93 = international protocol of the Interna- tional Society of Paediatric Oncology; 3 patients BFM-98 = protocol for acute lymphoblastic lymphoma, Berlin-Frankfurt-Munster; 2 patients COSS-96 = protocol of Cooperative Os- teosarcoma Study Group; 2 patients DAL-HD 90 = protocol for Hodgkins disease, No patients had radiotherapy treatment, nor bone marrow transplantation, nor stem cell transplantation | NI |
| Nemeth et al. (2013) [] | Case–control | Hungary | 38 | DMFT; CPI; radiographic dental examination was used to analyze dental malforma- tions: agenesis, without third molars, microdontia, macrodontia, unerrupted teeth; root malformation | NI | 18 patients BFM-95 = protocol for acute lymphoblastic lymphoma, Berlin-Frankfurt-Munster; 5 patients NBL-2 = protocol for neuroblastoma; 4 patients CWS 96 = protocol of Cooperative Soft Tissue Sarcoma Study Group; 4 patients SIOP 93 = international protocol of the Interna- tional Society of Paediatric Oncology; 3 patients BFM-98 = protocol for acute lymphoblastic lymphoma, Berlin-Frankfurt-Munster; 2 patients COSS-96 = protocol of Cooperative Os- teosarcoma Study Group; 2 patients DAL-HD 90 = protocol for Hodgkins disease, No patients had radiotherapy treatment, nor bone marrow transplantation, nor stem cell transplantation | NI |
| Lauritano et al. (2012) [] | Prospective | Italy | 52 | DMFT, microdontia, enamel hypoplasia, dental agenesis, v-shaped roots | Thirty- nine patients were affected by lymphoblastic leukaemia (ALL), the remaining ones were affected by acute myeloblastic leukaemia (AML) | Patients were treated according to Italian Association of Paediatric Hematoncology (AIEOP)—Methotrexate + Vincristine + Daunoblastine + Prednisone + Desamethasone. Seven patients with ALL received cranial irradiation (18 Gy) in addition to ChemoT and cytotoxic treatment | NR |
| Hutton et al. (2010) [] | Retrospective | United Kingdom | 120 | DMFT index; dmft index; enamel opacities, fissure sealed, microdont; traumatized teeth; basic periodontal examination and gingival bleeding score in patients with fully erupted permanent incisors and first molars | Wilm’s tumour—29 patients (24.2%), rhabdomyosarcoma—10 patients (8.3%), Hodgkin’s lymphoma—14 patients (11.7%), non-Hodgkin’s lymphoma—10 patients (8.3%), neuroblastoma—21 patients (17.5%), and other solid tumour types—36 patients (30.0%) | ChemoT—four principal groups of chemo- therapeutic agent used: high-dose chemo- therapy with stem-cell rescue (HDCSCR); anthracycline drugs; alkylating agents; platinum drugs; and overlapping regimes | NR |
| Maciel et. al. (2009) [] | Case–control | Brazil | 56 | agenesis, microdontia, macrodontia, short roots, tapering roots, enlarged pulp chambers, supernumerary teeth, taurodontism, DMFT score, visible plaque index (VPI), gingival bleeding index (GBI), saliva flow | ALL | ChemoT, Chemo/radiotherapy, Chemo/radio/BMT | Research Grant |
| Çubukçu et al. (2008) [] | Case–control | Turkey | 62 | DMF/T, dmf/t | Non-Hodgkin lymphoma, Retinablastoma, Hodgkin lymphoma, Fibroma, Medulloblastoma, Wilms tumor, Nasopharyngeal carcinoma, Langerhans cell histiocytoma, Neuroblastoma, Malignant teratoma, Optical glioma, Rhabdomyosarcoma, Disgerminoma, Leiomyosarcoma, Hepatoblastoma | ChemoT | NI |
| Avşar et al. (2007) [] | Retrospective | Turkey | 96 | DMFT, The Silness-Loe Plaque Index (PI) and Gingival Index (GI), Saliva assessment included salivary flow rate, salivary buffer capacity, mutans streptococci, and lactobacilli counting, disturbances of enamel mineralization, disturbances in dental development | Hodgkin’s or non-Hodgkin’s lymphoma | ChemoT | NI |
| Marec-Berard et al. (2005) [] | Case–control | France | 27 | microdontia, excessive caries, root stunting, hypodontia, and enamel hypoplasia | nephroblastoma | Institutional protocol (SIOP 93 protocol) consisting of poly ChemoT with vincristine, actinomycin ± doxorubicin without any head and/or neck ir- radiation or high-dose ChemoT | NR |
| Oguz et al. (2004) [] | Case–control | Turkey | 36 | DMFT; DMFS; Loe–Silness GI; Sillnes–Loe PI; enamel defects and discolorations; root malformations; eruption status; agenesis; premature apexifications and microdontia | non- Hodgkin’s lymphomas (NHL) | Twenty-seven patients were treated according to BFM-90 B cell protocol; while the LSA2 L2 protocol was used in 4 patients, and the LMT-89 protocol was administered in five patients | NI |
| Duggal et al. (2003) [] | Case–control | United Kingdom | 69 | Calculation of root surface areas of mandibular teeth | Acute lymphoblastic leukaemia (43.3%); Wilms tumor (14.4%), Hodgkin’s disease (9.3%); CNS tumors (8.2%) Non Hodgkins lymphoma, acute myeloid leukaemia and other diagnoses | ChemoT, radiotherapy, and both chemo-and prophylactic cranial irradiation of between 16 and 22GY, or had received fractionated total body irradiation and a bone marrow transplant | NR |
| Pajari et al. (2001) [] | Retrospective | Finland | 36 | DMFT | 18 suffering from leukemia and 18 from solid tumors | combination ChemoT and 4 patients also received cranial irradiation | NI |
| Alpaslan et al. (1999) [] | Retrospective | NI | 32 | discoloration, enamel hypoplasia, crown/root malformation, unerupted teeth, premature apexification, microdontia, agenesis, gingival and plaque indexes, denatal caries, craniofacial growth | Hodgkin’s or non-Hodgkin’s lymphoma | ChemoT | NI |
| Kaste et al. (1998) [] | Retrospective | United States of America | 52 | dental abnormalities | Neuroblastoma | 8 received head and/or neck irradiation, either as part of a preparative regimen for bone marrow transplantation (n= 2) or as local therapy of a metastasis (n = 6) | NR |
| Duggal et al. (1997) [] | Case–control | United Kingdom | 46 | Enamel defects—modified developmental defects of enamel index (DDE index); DMFTS index; avaliação gengival | 22 acute lymphoblastic leukaemia; 6 Hodgkins disease; 4 Non- Hodgkins lymphoma; 6 brain tumours, 4 Wilm’s tumour; 4 other childhood malignancies. | Multi-drug ChemoT with or without cranial irradiation | NR |
| Kaste et al. (1997) [] | Retrospective | United States of America | 423 | Dental abnormalities: root stunting (abnormally shortened roots), microdontia (abnormallly small teeth), or hypodontia (absent teeth) | ALL | Multiagent ChemoT; In addition, cranial irradiation (1800 or 2400 cGy) was given to 243 of the 423 children (55.6%). | NR |
| Kaste et al. (1995) [] | Retrospective | United States of America | 22 | Dental abnormalities: root stunting, microdontia and hypodontia; multiple abnormalities. | Head and neck rhabdomyosarcoma | Multiagent ChemoT (including cyclophosphamide, Adriamy- cin, vincristine, and dactinomycin) and radiotherapy on four successive treatment regimens | NR |
| Sonis et al. (1995) [] | Case–control | Belgium | 52 | DMFT; dmft: Gengival index; Plaque index | 27 acute lymphoblastic leukaemia; 7 non-Hodgkin’s lymphoma; 7 Wilms’ tumour; 5 rhabdomyosarcoma; 6 different childhood cancers | ChemoT. Patients had not received any radiotherapy to the oral or the salivary gland region | NI |
| Dens et al. (1995) [] | Retrospective | NI | 64 | dmft; DMFT; OHI-S; modified loe and silness gingival index score | ALL | Varied combinations of chemotherapeutic agents: ChemoT alone (group 1); 1800 cGy (group 2); 2400 cGy (group 3) | NI |
| Näsman et al. (1994) [] | Case–control | Sweden | 76 | Dental caries, salivary flow, salivary microbial counts, enamel disturbances, and disturbances in dental development | BMT group: 15 children were treated for acute leukemia, 1 for a B-cell lymphoma,3 for Gaucher’s disease, 1 for a severe combined immunodeficiency. ChT group: 21 were treated for acute leukemia, 9 for lymphoma,6 for Wilm’s tumor, 6 for rhabdomyosarcoma,3 for histiocytosis-X, 3 for neuroblastoma, 3 for optic glioma, 3 for other CNS-tumors, and 3 for other tumors | Bone marrow transplantation (BMT group); ChemoT | NR |
| Fleming et al. (1993) [] | Case–control | Northern Ireland | 54 | Regularity of dental attendance; type of dentist visited; toothbrushing frequency; plaque presence on buccal and lingual surfaces; gengivitis (através do sangramento gengival ao passar com a sonda); DMFT index; dmft index | ALL | ChemoT | NR |
| Purdell-Lewis et al. (1988) [] | Cohort | United Kingdom | 45 | oral hygiene index; papilllary bleeding index; number of erupted teeth relative to age; number of carious or filled primary and permanent teeth; percentage of primary teeth with initial lesions; percentage of erupted incisors, canines or permanent first molars with opacities (1), rough surfaces (2), vertical grooves (3), hypoplastic horizontal grooves and pits scored using DDE-index | acute lymphatic leukaemia, neuroblastoma, wilm’s tumor, rhabdomyosarcoma, Histiocytosis X, acute non-lymphatic leukemia | poly ChemoT | NI |
NI—No information; NR—Not reported; RadioT—Radiotherapy; ChemoT—Chemotherapy; ALL—Acute limphoblastic leukaemia; DDE—Developmental defects of enamel (DDE).
3.3. Methodological Quality of the Included Studies
Most studies were categorized with high methodological quality (n = 21, 60%), while six had unclear methodological quality, and eight were of low methodological quality (Table 2).

Table 2.
Results from the methodological appraisal using JBI Critical Appraisal Checklist.
Studies mostly failed on stating strategies to deal with confounding factors (60.0%, n = 21) (item 6), clearly defining criteria for sample inclusion (54.3%, n = 19) (item 1), identifying confounding factors (31.4%, n = 11) (item 5), and describing, in detail, study subjects and the setting (22.9%, n = 8) (item 2). The remaining items had a performance of over 95%.
3.4. Data Synthesis
3.4.1. Dental Anomalies Prevalence
We were able to estimate specific prevalence rates for eight dental anomalies (Table 3) (forest plots are available in Supplementary Files S3–S11). Discoloration was the most prevalent dental anomaly, with a mean prevalence of 53% (0.53, 95% CI: 0.42; 0.65), but was less described (only in four studies) [,,,]. The second dental anomalies most prevalent, with 36% prevalence, were crown-root malformations (0.36, 95% CI: 0.28; 0.44) and agenesis (0.36, 95% CI: 0.27; 0.45), both described in 10 studies [,,,,,,,,,], followed by 32% prevalence of enamel hypoplasia (0.32, 95% CI: 0.21; 0.45), described in 13 studies [,,,,,,,,,,,,]. With 29% (0.29, 95% CI: 0.16; 0.43) prevalence, root development alterations were described in 10 studies [,,,,,,,,,]. Unerupted teeth had a mean prevalence of 24% (0.24, 95% CI: 0.15; 0.34), and this condition was described in four studies [,,,], with microdontia at 16% (0.16, 95% CI: 0.09; 0.24), but this was the most commonly described dental anomaly in 14 studies [,,,,,,,,,,,,,]. Lastly, hypodontia, reported in six studies [,,,,], had a prevalence of 13% (0.13, 95% CI: 0.05; 0.23), and macrodontia was the least prevalent dental anomaly, with 7% (0.07, 95% CI: 0.04; 0.12), being described in only 5 five studies [,,].

Table 3.
Prevalence data on dental anomalies in pediatric cancer patient survivors.
Furthermore, studies have been conducted in 16 countries worldwide. Notably, no studies have been performed in Oceania or Africa.
3.4.2. Dental Anomalies Risk in Pediatric Cancer Patient Survivors Compared to Controls
When comparing the prevalence of dental anomalies between cancer survivors and controls, five out of eight were significantly more prevalent among survivors (Table 4) (forest plots are available in Supplementary Files S12–S19). Root development alterations were 591% (OR = 6.91, 95% CI: 3.89; 12.29) more commonly found in survivors than in controls; microdontia was 518% (OR = 6.18, 95% CI: 2.45; 15.56), discoloration was 468% (OR = 5.68, 95% CI: 3.02; 10.7), agenesis was 350% (OR = 3.50, 95% CI: 1.98; 6.16), and enamel hypoplasia was 95% (OR = 1.95, 95% CI: 1.32, 2.88). The prevalence of crown-root malformation, unerupted teeth, and macrodontia was not significantly different between cancer survivors and controls.

Table 4.
Risk ratio on dental anomalies in cancer survivor pediatric patients.
Other oral manifestations, extending from dental development anomalies to soft tissue and saliva alterations, were reported in the studies but were not included in the meta-analysis due to a lack of data amenable for it; nevertheless, alternative synthesis methods were used.
No statistically significant differences were found on craniofacial growth among the controls and cancer survivors [,].
Hyposalivation in childhood cancer survivors is relatively high [,,], with more significant alterations found in stimulated salivary flow [,]. Ref. [] was the only study reporting no alterations in saliva flow rates. Studies did not find alterations in salivary buffer capacity [,], but a salivary microbial flora shift in patients who received radiation therapy was found, with an increased number of mutans streptococci and lactobacilli in saliva [,].
Caries experience was assessed through the calculation of decayed–missing–filled teeth and surfaces for primary (dmft, dmfs) and permanent (DMFT, DMFS) dentition. Permanent dentition scores (DMFT and DMFS) were, for the majority of the studies, higher in cancer survivors when compared with controls [,,,,,,]). Only one study reported a higher caries level in primary dentition [], and the others reported no differences between groups [,,].
3.5. Additional Analysis
We further assessed, through sensitivity analyses, whether the risk of bias (Table 5) and the female–male ratio (Table 6) could interfere with the estimates. Risk of bias only proved to be significant in the root development alteration (p < 0.0001).

Table 5.
Sensitivity analysis of risk of bias on prevalence using meta-regressions.

Table 6.
Sensitivity analysis of female and male ratio on prevalence data on dental anomalies in cancer survivor pediatric patients.
Female–male ratio showed a significant effect in the estimates concerning root development alteration (p < 0.0001), enamel hypoplasia (p = 0.0001), discoloration (p = 0.047), and microdontia (p = 0.0204), unveiling a possible sex-based difference.
4. Discussion
4.1. Summary of Main Findings
The results of the present systematic review estimated the pooled prevalence of the following dental anomalies as long-term dental sequelae in patients who had undergone cancer therapy during early childhood: discoloration, 53%; crown-root malformations and agenesis, 36%; enamel hypoplasia, 32%; root development alterations, 29%; unerupted teeth, 24%; microdontia, 16%; hypodontia, 13%; and macrodontia, 7%. Compared with controls, these dental anomalies were significantly more prevalent in cancer survivors and pediatric patients. Root development alterations were 591%, microdontia was 518%, discoloration was 468%, agenesis was 349%, and enamel hypoplasia was 95% more likely to be found in cancer survivors than in controls.
4.2. Implications for Practice and Research
As previously mentioned, the late side effects of chemotherapy and radiotherapy on the stomatognathic system in pediatric cancer survivors are numerous, which challenges clinical care and management in the dental setting. Regarding cancer types, it is perceived that the most prevalent cancers in children worldwide are leukemias, with the highest rate, followed by tumors of the central nervous system, then lymphomas, and others. [,,]. Thus, most patients receive chemotherapy without radiotherapy, but they may receive radiotherapy alone or in combination with chemotherapy. Radiation therapy to the head and/or neck area can range from 27 to 70 Gray []. And we also know that dental development or odontogenesis is a complex process that occurs over a long period of time, starting in intrauterine life and ending at 14–15 years of age [,]. Thus, each tooth goes through different stages of development, which when subjected to extrinsic or intrinsic factors, can result in the appearance of dental development defects. Depending on the stage of odontogenesis that is affected, different changes may occur; that is, if any changes occur during histodifferentiation, the structure of enamel and dentin may be altered. In turn, if they occur during morphodifferentiation, they may cause shape and size abnormalities of the teeth, and if the disturbances persist, they can damage root formation, resulting in a shortened or tapered root, which, in turn, can impair tooth eruption and occlusion. The first signs are expected after one to two years of anticancer treatment []. Some antineoplastics inhibit odontogenesis and eruption and can induce qualitative and quantitative changes in dental tissues. Regarding radiotherapy treatments, exposure to radiotherapy doses greater than 20 Gy has been shown to contribute to a greater risk of developing dental anomalies [].
Therefore, alterations in root development, microdontia, discoloration, agenesis, and enamel hypoplasia, which were the most common alterations recorded, had an impact on the quality of life.
With all this in mind, it is our understanding that, given the possibility of the presence of dental abnormality and increased caries risk as a consequence of cancer treatment, the most acceptable course of action should be to assume that the quality of life and oral implications are real, so the normal dental therapy scenario may increase the level of clinical priority for preventive screening and early screening.
Despite all the included oral manifestations, crown malformation, prevalence of unerupted teeth, and macrodontia, craniofacial growth was not statistically significant between controls and cancer survivors. However, a higher level of caries in the primary dentition has only been reported once [], as well as alterations in saliva flow rates [].
4.3. Strengths and Limitations
This study was conducted following PRISMA, a strict and widely advised guideline that has increased robustness and decreased reporting errors. Furthermore, a comprehensive literature search was conducted using a meticulous predefined protocol. Nevertheless, there are some limitations that need to be discussed. It is possible to see that there are several studies that address late health effects; however, in a non-systematic way and on multiple distinct points, this leads to a low sample size of children with cancer, where it is essential to obtain consistent results.
Dental abnormalities have been addressed, but studies on saliva alterations are scarce and have different objectives, making them inconclusive, [,,,,], as well as and despite reports of a higher prevalence of caries in the permanent dentition [,,,,,,]. In the deciduous dentition, the results showed that there were no differences between the groups [,,], with only one reporting the opposite []. One point that was not mentioned was malocclusion and occlusal disharmonies, which would be interesting given the high prevalence of changes in number and tooth development.
Thus, future studies should focus on data representativeness and method standardization to ensure more homogeneous evidence-based results. This information is extremely relevant to pediatric oncologists and to raise awareness among oral health professionals regarding the possible and predictable problems they are facing.
5. Conclusions
Childhood cancer survivors presented a higher risk of having dental alterations than control counterparts. Also, this group of people also presents considerable prevalence of such conditions. Additional analyses reveal possible sex-based differences that should be explored in future studies, as well as more longitudinal studies, as this is the only way to assess and understand the oral consequences of antineoplastic agents. These results collectively highlight the importance of oral healthcare and the prevention of disease in childhood cancer survivors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16010110/s1, Table S1, PRISMA 2020 checklist, Table S2, List of excluded studies with reasons. File S3. Forest plot of prevalence of root development alteration in survival pediatric cancer in comparison to those without root alterations, with mean effect size estimates and 95% confidence intervals. The size of squares reflects sample size, while continuous horizontal lines and the width of diamonds indicate the 95% confidence interval. The diamond and vertical dotted line represent the overall pooled estimate of root development alteration prevalence. File S4. Forest plots of prevalence of crown-root malformation. File S5. Forest plots of prevalence of unerupted teeth. File S6. Forest plots of prevalence of enamel hypoplasia. File S7. Forest plots of prevalence of hypodontia. File S8. Forest plots of prevalence of discoloration. File S9. Forest plots of prevalence of agenesis. File S10. Forest plots of prevalence of microdontia. File 11. Forest plots of prevalence of macrodontia. File 12. Forest plots of odds ratio of root development alteration. File S13. Forest plots of odds ratio of crown. File S14. Forest plots of odds ratio of unerupted teeth. File S15. Forest plots of odds ratio of enamel hypoplasia. File S16. Forest plots of odds ratio of discoloration. File S17. Forest plots of odds ratio of agenesis. File S18. Forest plots of odds ratio of microdontia. File 19. Forest plots of odds ratio of macrodontia. Refs [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.B. and L.B.L.; methodology, J.B. and L.B.L.; software, V.M.; validation, J.B., V.M. and L.B.L.; formal analysis, V.M.; data curation, J.P.L., J.B., V.M. and L.B.L.; writing—original draft preparation, J.B., V.M. and L.B.L.; writing—review and editing, J.P.L., I.R., J.B., V.M. and L.B.L.; supervision, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data were extracted from the original studies included in this systematic review. All data used for statistical analyses are presented in the manuscript and its Supplementary Files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International Incidence of Childhood Cancer, 2001–2010: A Population-Based Registry Study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002527-1. [Google Scholar]
- Proc, P.; Szczepańska, J.; Herud, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental Caries among Childhood Cancer Survivors. Medicine 2019, 98, e14279. [Google Scholar] [CrossRef] [PubMed]
- Halperson, E.; Matalon, V.; Goldstein, G.; Saieg Spilberg, S.; Herzog, K.; Fux-Noy, A.; Shmueli, A.; Ram, D.; Moskovitz, M. The Prevalence of Dental Developmental Anomalies among Childhood Cancer Survivors According to Types of Anticancer Treatment. Sci. Rep. 2022, 12, 4485. [Google Scholar] [CrossRef] [PubMed]
- Carl, W.; Sako, K.; Schaaf, N.G. Dental Complications in the Treatment of Rhabdomyosarcoma of the Oral Cavity in Children. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Themudo, R.; Botelho, J.; Machado, V. Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report. Dent. J. 2020, 8, 59. [Google Scholar] [CrossRef]
- Dury, D.C.; Roberts, M.W.; Miser, J.S.; Folio, J. Dental Root Agenesis Secondary to Irradiation Therapy in a Case of Rhabdomyosarcoma of the Middle Ear. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 595–599. [Google Scholar] [CrossRef]
- Jawad, H.; Hodson, N.A.; Nixon, P.J. A Review of Dental Treatment of Head and Neck Cancer Patients, before, during and after Radiotherapy: Part 2. Br. Dent. J. 2015, 218, 69–74. [Google Scholar] [CrossRef]
- Mod, D.; Mod, H.; Jha, A.K. Oral and Dental Complications of Head and Neck Radiotherapy and Their Management. J. Nepal. Health Res. Counc. 2013, 11, 300–304. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Alnuaimi, E.; Al Halabi, M.; Khamis, A.; Kowash, M. Oral Health Problems in Leukaemic Paediatric Patients in the United Arab Emirates: A Retrospective Study. Eur. J. Paediatr. Dent. 2018, 19, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bica, C.; Chincesan, M.; Esian, D.; Martha, K.; Ion, V.; Marinescu, L.A.; Earar, K.; Matei, M.N. Dental Development in Children After Chemotherapy. Rev. Chim. 2017, 68, 1397–1400. [Google Scholar] [CrossRef]
- Hutton, A.; Bradwell, M.; English, M.; Chapple, I. The Oral Health Needs of Children after Treatment for a Solid Tumour or Lymphoma: Oral Health Needs of Children. Int. J. Paediatr. Dent. 2010, 20, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.; Hopkins, K.; Jones, D.; Crom, D.; Greenwald, C.; Santana, V. Dental Abnormalities in Children Treated for Acute Lymphoblastic Leukemia. Leukemia 1997, 11, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Hopkins, K.P.; Bowman, L.C. Dental Abnormalities in Long-Term Survivors of Head and Neck Rhabdomyosarcoma. Med. Pediatr. Oncol. 1995, 25, 96–101. [Google Scholar] [CrossRef]
- Kaste, S.C.; Hopkins, K.P.; Bowman, L.C.; Santana, V.M. Dental Abnormalities in Children Treated for Neuroblastoma. Med. Pediatr. Oncol. 1998, 30, 22–27. [Google Scholar] [CrossRef]
- Owosho, A.A.; Brady, P.; Wolden, S.L.; Wexler, L.H.; Antonescu, C.R.; Huryn, J.M.; Estilo, C.L. Long-Term Effect of Chemotherapy–Intensity-Modulated Radiation Therapy (Chemo-IMRT) on Dentofacial Development in Head and Neck Rhabdomyosarcoma Patients. Pediatr. Hematol. Oncol. 2016, 33, 383–392. [Google Scholar] [CrossRef]
- Pajari, U.; Yliniemi, R.; Möttönen, M. The Risk of Dental Caries in Childhood Cancer Is Not High If the Teeth Are Caries-Free at Diagnosis. Pediatr. Hematol. Oncol. 2001, 18, 181–185. [Google Scholar] [CrossRef]
- Rabassa-Blanco, J.; Brunet-Llobet, L.; Marcote-Sinclair, P.; Balsells-Mejía, S.; Correa-Llano, M.G.; Miranda-Rius, J. Prevalence of, and Risk Factors for, Dental Sequelae in Adolescents Who Underwent Cancer Therapy during Childhood. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sonis, A.L.; Waber, D.P.; Sallan, S.; Tarbell, N.J. The Oral Health of Long-Term Survivors of Acute Lymphoblastic Leukaemia: A Comparison of Three Treatment Modalities. Eur. J. Cancer Part B Oral Oncol. 1995, 31, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Stolze, J.; Teepen, J.C.; Raber-Durlacher, J.E.; Loonen, J.J.; Kok, J.L.; Tissing, W.J.E.; de Vries, A.C.H.; Neggers, S.J.C.M.M.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; et al. Prevalence and Risk Factors for Hyposalivation and Xerostomia in Childhood Cancer Survivors Following Different Treatment Modalities—A Dutch Childhood Cancer Survivor Study Late Effects 2 Clinical Study (DCCSS LATER 2). Cancers 2022, 14, 3379. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan, G.; Alpaslan, C.; Gögen, H.; Aynur, O.; Çetiner, S.; Karadeniz, C. Disturbances in Oral and Dental Structures in Patients with Pediatric Lymphoma after chemotherapyA Preliminary Report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Avşar, A.; Elli, M.; Darka, Ö.; Pinarli, G. Long-Term Effects of Chemotherapy on Caries Formation, Dental Development, and Salivary Factors in Childhood Cancer Survivors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Çetiner, D.; Çetiner, S.; Uraz, A.; Alpaslan, G.H.; Alpaslan, C.; Toygar Memikoğlu, T.U.; Karadeniz, C. Oral and Dental Alterations and Growth Disruption Following Chemotherapy in Long-Term Survivors of Childhood Malignancies. Support. Care Cancer 2019, 27, 1891–1899. [Google Scholar] [CrossRef]
- Lauritano, D.; Petruzzi, M. Decayed, Missing and Filled Teeth Index and Dental Anomalies in Long-Term Survivors Leukaemic Children: A Prospective Controlled Study. Med. Oral 2012, 17, e977–e980. [Google Scholar] [CrossRef]
- Purdell-Lewis, D.J.; Stalman, M.S.; Leeuw, J.A.; Humphrey, G.B.; Kalsbeek, H. Long Term Results of Chemotherapy on the Developing Dentition: Caries Risk and Developmental Aspects. Commun. Dent. Oral Epidemiol. 1988, 16, 68–71. [Google Scholar] [CrossRef]
- Shayani, A.; Aravena, P.C.; Rodríguez-Salinas, C.; Escobar-Silva, P.; Diocares-Monsálvez, Y.; Angulo-Gutiérrez, C.; Rivera, C. Chemotherapy as a Risk Factor for Caries and Gingivitis in Children with Acute Lymphoblastic Leukemia: A Retrospective Cohort Study. Int. J. Paediatr. Dent. 2022, 32, 538–545. [Google Scholar] [CrossRef]
- Seremidi, K.; Kavvadia, K.; Kattamis, A.; Polychronopoulou, A. Dental Late Effects of Antineoplastic Treatment on Childhood Cancer Survivors: Radiographic Findings. Int. J. Paediatr. Dent. 2021, 31, 742–751. [Google Scholar] [CrossRef]
- Çubukçu, Ç.E.; Sevinir, B. Dental Health Indices of Long-Term Childhood Cancer Survivors Who Had Oral Supervision during Treatment: A Case–Control Study. Pediatr. Hematol. Oncol. 2008, 25, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Dens, F.; Boute, P.; Otten, J.; Vinckier, F.; Declerck, D. Dental Caries, Gingival Health, and Oral Hygiene of Long Term Survivors of Paediatric Malignant Diseases. Arch. Dis. Child. 1995, 72, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Duggal, M.S. Root Surface Areas in Long-Term Survivors of Childhood Cancer. Oral Oncol. 2003, 39, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Duggal, M.S.; Curzon, M.E.J.; Bailey, C.C. Dental Parameters in the Long Term Survivors of Childhood Cancer Compared with Siblings. Oral Oncol. 1997, 33, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.; Kinirons, M.J. Study of the Dental Health of Children in Remission from Acute Lymphoblastic Leukaemia in Northern Ireland. Commun. Dent. Oral Epidemiol. 1993, 21, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Krasuska-Sławińska, E.; Brożyna, A.; Dembowska-Bagińska, B.; Olczak-Kowalczyk, D. Factors Influencing Caries Incidence in Permanent Teeth in Children/Adolescents under and after Anti-Neoplastic Treatment. Contemp. Oncol. Współczesna Onkol. 2016, 1, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.C.C.; de Castro, C.G.; Brunetto, A.L.; Di Leone, L.P.; da Silveira, H.E.D. Oral Health and Dental Anomalies in Patients Treated for Leukemia in Childhood and Adolescence: Dental Anomalies in Leukemia Patients. Pediatr. Blood Cancer 2009, 53, 361–365. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Azzi, D.; Chaux-Bodard, A.G.; Lagrange, H.; Gourmet, R.; Bergeron, C. Long-Term Effects on Chemotherapy on Dental Status in Children Treated for Nephroblastoma. Pediatr. Hematol. Oncol. 2005, 22, 581–588. [Google Scholar] [CrossRef]
- Näsman, M.; Björk, O.; Söderhäll, S.; Ringdén, O.; Dahllöf, G. Disturbances in the Oral Cavity in Pediatric Long-Term Survivors after Different Forms of Antineoplastic Therapy. Pediatr. Dent. 1994, 16, 217–223. [Google Scholar]
- Nemeth, O.; Hermann, P.; Kivovics, P.; Garami, M. Long-Term Effects of Chemotherapy on Dental Status of Children Cancer Survivors. Pediatr. Hematol. Oncol. 2013, 30, 208–215. [Google Scholar] [CrossRef]
- Nemeth, O.; Kivovics, M.; Pinke, I.; Marton, K.; Kivovics, P.; Garami, M. Late Effects of Multiagent Chemotherapy on Salivary Secretion in Children Cancer Survivors. J. Am. Coll. Nutr. 2014, 33, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Cetiner, S.; Karadeniz, C.; Alpaslan, G.; Alpaslan, C.; Pinarli, G. Long-Term Effects of Chemotherapy on Orodental Structures in Children with Non-Hodgkin’s Lymphoma. Eur. J. Oral Sci. 2004, 112, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Krasuska-Sławińska, E.; Brożyna, A.; Turska-Szybka, A.; Dembowska-Bagińska, B. Dental Caries in Children and Adolescents During and After Antineoplastic Chemotherapy. J. Clin. Pediatr. Dent. 2018, 42, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Guagnano, R.; Romano, F.; Berger, M.; Fagioli, F.; Vallone, V.; Bello, L.; Vitale, M.C.; Defabianis, P. Long-Term Effect of Anticancer Therapy on Dentition of Italian Children in Remission from Malignant Disease: A Cross-Sectional Study. Eur. J. Paediatr. Dent. 2022, 23, 131–136. [Google Scholar] [CrossRef]
- Tanem, K.E.; Stensvold, E.; Wilberg, P.; Skaare, A.B.; Brandal, P.; Herlofson, B.B. Oral and Dental Late Effects in Long-Term Survivors of Childhood Embryonal Brain Tumors. Support. Care Cancer 2022, 30, 10233–10241. [Google Scholar] [CrossRef]
- Seremidi, K.; Kloukos, D.; Polychronopoulou, A.; Kattamis, A.; Kavvadia, K. Late Effects of Chemo and Radiation Treatment on Dental Structures of Childhood Cancer Survivors. A Systematic Review and Meta-analysis. Head Neck 2019, 41, 3422–3433. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Aggarwal, A.; Pai, K.M. Orofacial Mani-festations of Leukemic Children on Treatment: A Descriptive Study. Int J Clin Pediatr Dent 2018, 11, 193–198. [Google Scholar]
- Alberth, M.; Kovalecz, G.; Nemes, J.; Math, J.; Kiss, C.; Marton, I.J. Oral Health of Long-Term Childhood Cancer Survivors. Pediatr Blood Cancer 2004, 43, 88–90. [Google Scholar] [CrossRef]
- Ali, M.; Nurelhuda, N. Oral health status and its determinants in children with leukaemia at the Radiation and Isotope Center Khartoum, Khartoum State, Sudan. Sudan. J. Paediatr. 2019, 19, 93–100. [Google Scholar] [CrossRef]
- Azher, U.; Shiggaon, N. Oral health status of children with acute lymphoblastic leukemia undergoing chemotherapy. Indian J. Dent. Res. 2013, 24, 523. [Google Scholar] [CrossRef] [PubMed]
- Velten, D.B.; Zandonade, E.; Miotto, M.H.M.d.B. Prevalence of oral manifestations in children and adolescents with cancer submitted to chemotherapy. BMC Oral Heal. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Childers, N.K.; Stinnett, E.A.; Wheeler, P.; Wright, J.; Castleberry, R.P.; Dasanayake, A.P. Oral complications in children with cancer. Oral Surgery, Oral Med. Oral Pathol. 1993, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Bezerra, P.M.M.; Damascena, L.C.L.; Ribeiro, I.L.A.; Bonan, P.R.F.; de Sousa, S.A.; Almeida, L.d.F.D.; Valença, A.M.G. Impact of Saliva and Cariogenic Microbiota on the Chemotherapy-Induced Oral Mucositis in Oncopediatric Patients: A Preliminary Longitudinal Study. Int. J. Dent. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.B. Childhood Leukemias: Initial Oral Manifestations. J. Am. Dent. Assoc. 1971, 83, 159–164. [Google Scholar] [CrossRef]
- El-Housseiny, A.A.; Saleh, S.M.; El-Masry, A.A.; Allam, A.A. Assessment of Oral Complications in Children Receiving Chemotherapy. J. Clin. Pediatr. Dent. 2007, 31, 267–273. [Google Scholar] [CrossRef]
- Fayle, S.A.; Curzon, M.E. Oral complications in pediatric oncology patients. Pediatr Dent. 1991, 13, 289–295. [Google Scholar]
- Gandhi, K.; Datta, G.; Ahuja, S.; Saxena, T.; Datta, A.G. Prevalence of Oral Complications occurring in a Popu-lation of Pediatric Cancer Patients receiving Chemotherapy. Int Int. J. Clin. Pediatr. Dent. 2017, 10, 166–171. [Google Scholar]
- Gravina, H.G.; De Morán, E.G.; Zambrano, O.; Chourio, M.L.; De Valero, S.R.; Robertis, S.; Mesa, L. Oral Candidiasis in children and adolescents with cancer. Med. Oral. Patol. Oral. Cir. Bucal. 2007, 12, e419-423. [Google Scholar]
- Gupta, A.; Marwaha, M.; Bansal, K.; Sachdeva, A.; Gupta, A. Dental Awareness among Parents and Oral Health of Paediatric Cancer Patients Receiving Chemotherapy. J Clin Diagn Res. 2016, 10, 92–95. [Google Scholar] [CrossRef]
- Hegde, A.; Joshi, S.; Rai, K.; Shetty, S. Evaluation of Oral Hygiene Status, Salivary Characteristics and Dental Caries Experience in Acute Lymphoblastic Leukemic (ALL) Children. J. Clin. Pediatr. Dent. 2011, 35, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Hovi, L.; Saarinen, U.M.; Donner, U.; Lindqvist, C. Opportunistic Osteomyelitis in the Jaws of Children on Immunosuppressive Chemotherapy. J. Pediatr. Hematol. 1996, 18, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Hopkins, K.P.; Jenkins, J.J. Abnormal odontogenesis in children treated with radiation and chemotherapy: Imaging findings. Am. J. Roentgenol. 1994, 162, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Kowlessar, A.; Naidu, R.; Ramroop, V.; Nurse, J.; Dookie, K.; Bodkyn, C.; Lalchandani, S. Oral health among children attending an oncology clinic in Trinidad. Clin. Exp. Dent. Res. 2019, 5, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Kung, A.Y.; Zhang, S.; Zheng, L.W.; Wong, G.H.; Chu, C.H. Oral Health Status of Chinese Paediatric and Adolescent Oncology Patients with Chemotherapy in Hong Kong: A Pilot Study. Open Dent. J. 2015, 9, 21–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levy-Polack, M.P.; Sebelli, P.; Polack, N.L. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spéc. Care Dent. 1998, 18, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Lula, E.C.O.; Lula, C.E.O.; Alves, C.M.C.; Lopes, F.F.; Pereira, A.L.A. Chemotherapy-induced oral complications in leukemic patients. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1681–1685. [Google Scholar] [CrossRef]
- Marangoni-Lopes, L.; Rodrigues, L.; Mendonça, R.; Santos, M.N.-D. Radiotherapy changes salivary properties and impacts quality of life of children with Hodgkin disease. Arch. Oral Biol. 2016, 72, 99–105. [Google Scholar] [CrossRef]
- Mathur, V.P.; Kalra, G.; Dhillon, J.K. Oral health in children with leukemia. Indian J. Palliat. Care 2012, 18, 12–18. [Google Scholar] [CrossRef]
- Morais, E.F.; Lira, J.A.S.; Macedo, R.A.P.; Santos, K.S.; Elias, C.T.V.; Arruda-Morais, M.L.S. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz. J. Otorhinolaryngol. 2014, 80, 78–85. [Google Scholar]
- Mougeot, J.-L.C.; Stevens, C.B.; Almon, K.G.; Paster, B.J.; Lalla, R.V.; Brennan, M.T.; Mougeot, F.B. Caries-associated oral microbiome in head and neck cancer radiation patients: A longitudinal study. J. Oral Microbiol. 2019, 11, 1586421. [Google Scholar] [CrossRef] [PubMed]
- Nasim, V.S.; Shetty, Y.R.; Hegde, A.M. Dental Health Status in Children with Acute Lymphoblastic Leukemia. J. Clin. Pediatr. Dent. 2007, 31, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.E.M.; Westphalen, F.H. Analysis of oral complications related to cancer therapy. Arch. Oral Res. 2013, 9, 159. [Google Scholar] [CrossRef]
- Nikoui, M.; Lalonde, B. Manifestations bucco-dentaires de la leucémie chez l'enfant [Oro-dental manifestations of leukemia in children]. J. Can Dent Assoc. 1996, 62, 443–450. [Google Scholar] [PubMed]
- O'Sullivan, E.A.; Duggal, M.S.; Bailey, C.C. Changes in the oral health of children during treatment for acute lymphoblastic leukaemia. Int. J. Paediatr. Dent. 2009, 4, 31–34. [Google Scholar] [CrossRef]
- Olszewska, K.; Mielnik-Błaszczak, M. An Assessment of the Number of Cariogenic Bacteria in the Saliva of Children with Chemotherapy-Induced Neutropenia. Adv. Clin. Exp. Med. 2016, 25, 11–19. [Google Scholar] [CrossRef]
- Orback, R.; Orbak, Z. Oral condition of patients with leukemia and lymphoma J. Nihon. Univ. Sch. Dent. 1997, 39, 67–70. [Google Scholar] [CrossRef]
- Ou-Yang, L.-W.; Chang, P.-C.; Tsai, A.; Jaing, T.-H.; Lin, S.-Y. Salivary microbial counts and buffer capacity in children with acute lymphoblastic leukemia. Pediatr. Dent. 2010, 32. [Google Scholar]
- Parra, J.J.; Alvarado, M.C.; Monsalve, P.; Costa, A.L.F.; Montesinos, G.A.; Parra, P.A. Oral health in children with acute lymphoblastic leukaemia: Before and after chemotherapy treatment. Eur. Arch. Paediatr. Dent. 2019, 21, 129–136. [Google Scholar] [CrossRef]
- Ponce-Torres, E.; Ruíz-Rodríguez, M.d.S.; Alejo-González, F.; Hernández-Sierra, J.F.; de Pozos-Guillén, A. Oral Manifestations in Pediatric Patients Receiving Chemotherapy for Acute Lymphoblastic Leukemia. J. Clin. Pediatr. Dent. 2010, 34, 275–279. [Google Scholar] [CrossRef]
- Ribeiro, I.L.A.; Silva, S.M.; Limeira, R.R.T.; Bonan, P.R.F.; Valença, A.M.G.; Neto, E.A.d.L.; de Castro, R.D. Differences between the oral changes presented by patients with solid and hematologic tumors during the chemotherapeutic treatment. J. Appl. Oral Sci. 2020, 28, e20190020. [Google Scholar] [CrossRef] [PubMed]
- Ritwik, P. Dental Care for Patients With Childhood Cancers. Ochsner J. 2018, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E.; Hopkins, K.; Wilbur, R.B. Acute necrotizing Ulcerative Gengivitis in Children with Cancer. Am. J. Dis. Child. 1983, 137. [Google Scholar]
- Sahai, P.; Mohanti, B.K.; Sharma, A.; Thakar, A.; Bhasker, S.; Kakkar, A.; Sharma, M.C.; Upadhyay, A.D. Clinical outcome and morbidity in pediatric patients with nasopharyngeal cancer treated with chemoradiotherapy. Pediatr. Blood Cancer 2016, 64, 259–266. [Google Scholar] [CrossRef]
- Sepúlveda-Tebache, E.; Brethauer-Meier, U.; Jiménez-Moraga, M.; Mora- les-Figueroa, R.; Rojas-Castro, J.; Le Fort-Canales, P. Detección del virus herpes simple en lesiones de la mucosa oral en pacientes con terapia oncológica. Med. Oral. 2003, 8, 329–333. [Google Scholar] [PubMed]
- Tao, C.-J.; Liu, X.; Tang, L.-L.; Mao, Y.-P.; Chen, L.; Li, W.-F.; Yu, X.-L.; Liu, L.-Z.; Zhang, R.; Lin, A.-H.; et al. Long-term outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin. J. Cancer 2013, 32, 525–532. [Google Scholar] [CrossRef]
- Velten, D.B.; Zandonade, E.; Miotto, M.H.M.d.B. Prevalence of oral manifestations in children and adolescents with cancer submitted to chemotherapy. BMC Oral Heal. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Visnapuu, V.; Peltonen, S.; Alivuotila, L.; Happonen, R.-P.; Peltonen, J. Craniofacial and oral alterations in patients with Neurofibromatosis 1. Orphanet J. Rare Dis. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Yang, X.; Que, J.; Du, Q.; Zhang, Q.; Zou, J. Oral Health, Caries Risk Profiles, and Oral Microbiome of Pediatric Patients with Leukemia Submitted to Chemotherapy. BioMed Res. Int. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- White, G.E.; Cambridge, M. Oral manifestations of leukemia in children. Oral. Surg. 1970, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).