Attenuation of the BOLD fMRI Signal and Changes in Functional Connectivity Affecting the Whole Brain in Presence of Brain Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Image Acquisition
2.3. Paradigms
2.4. Data Analysis
2.5. Statistical Analysis
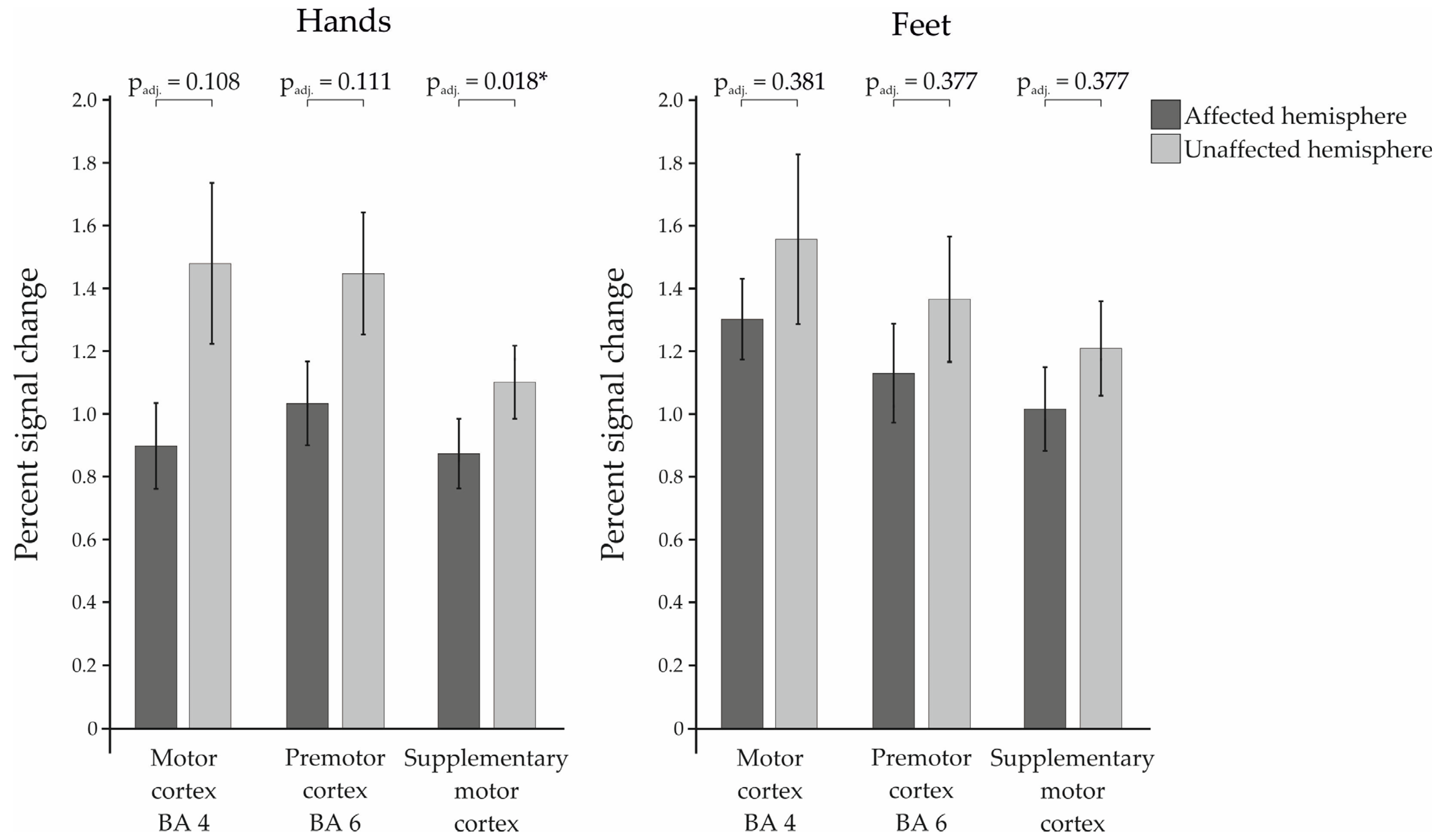
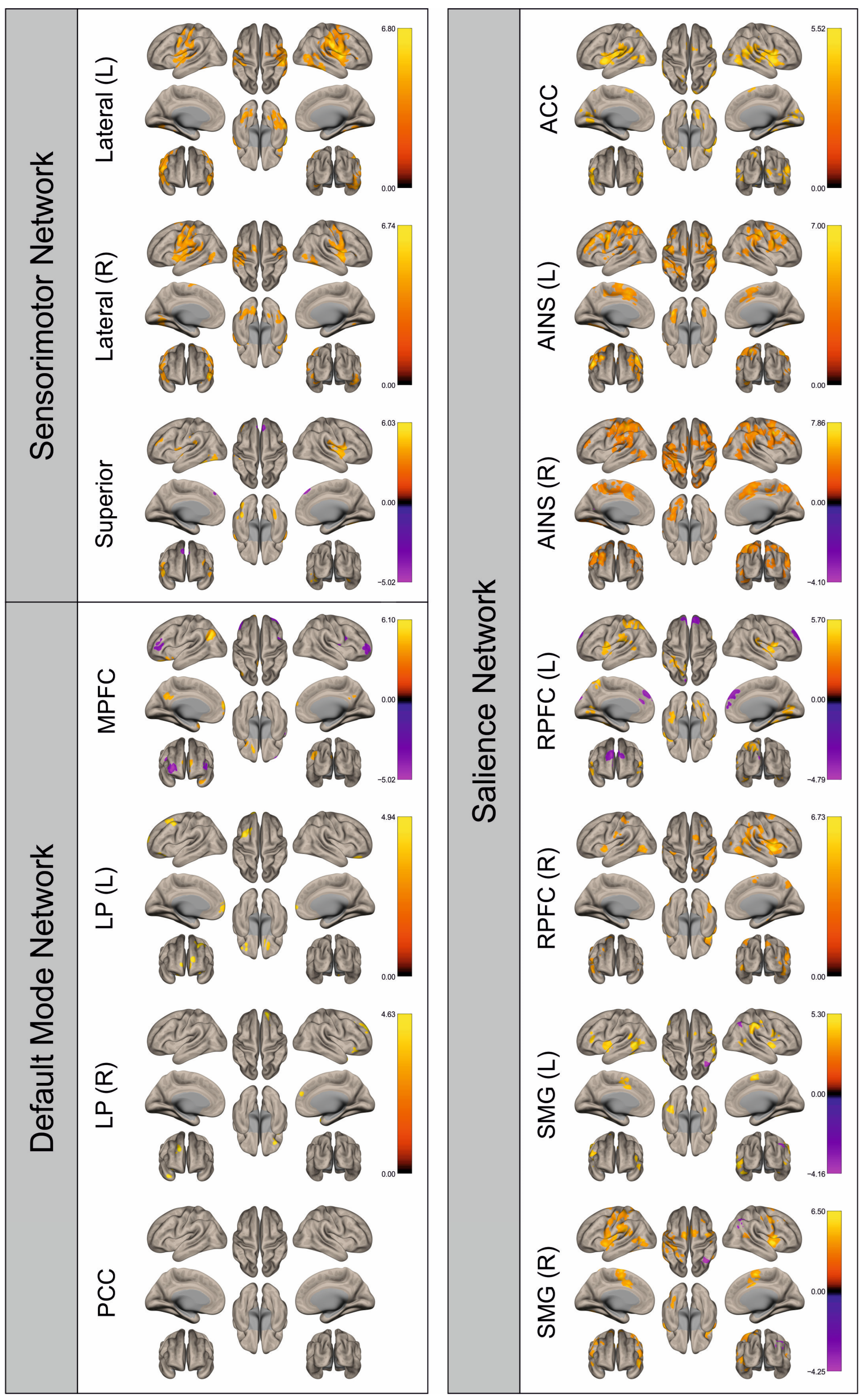
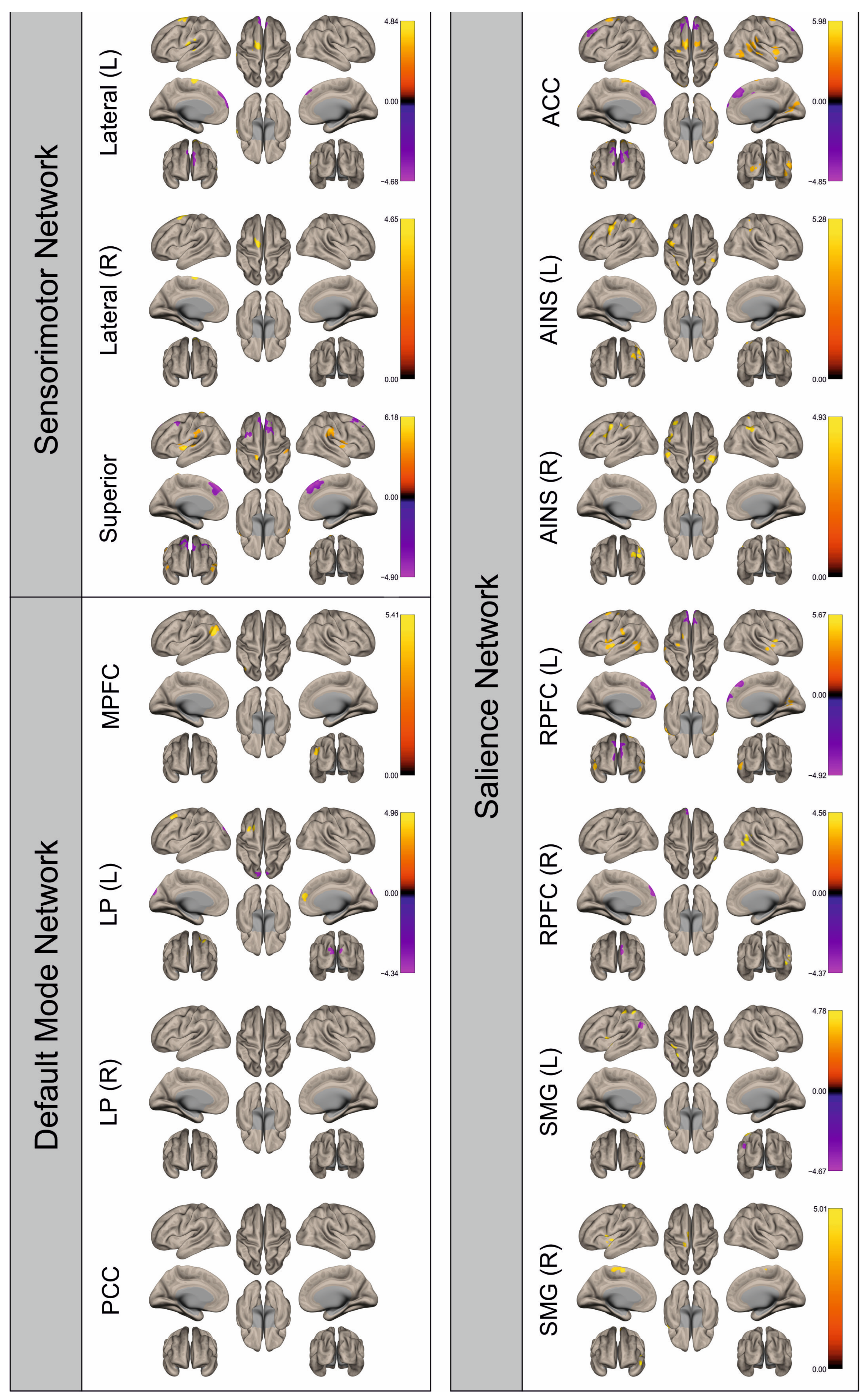
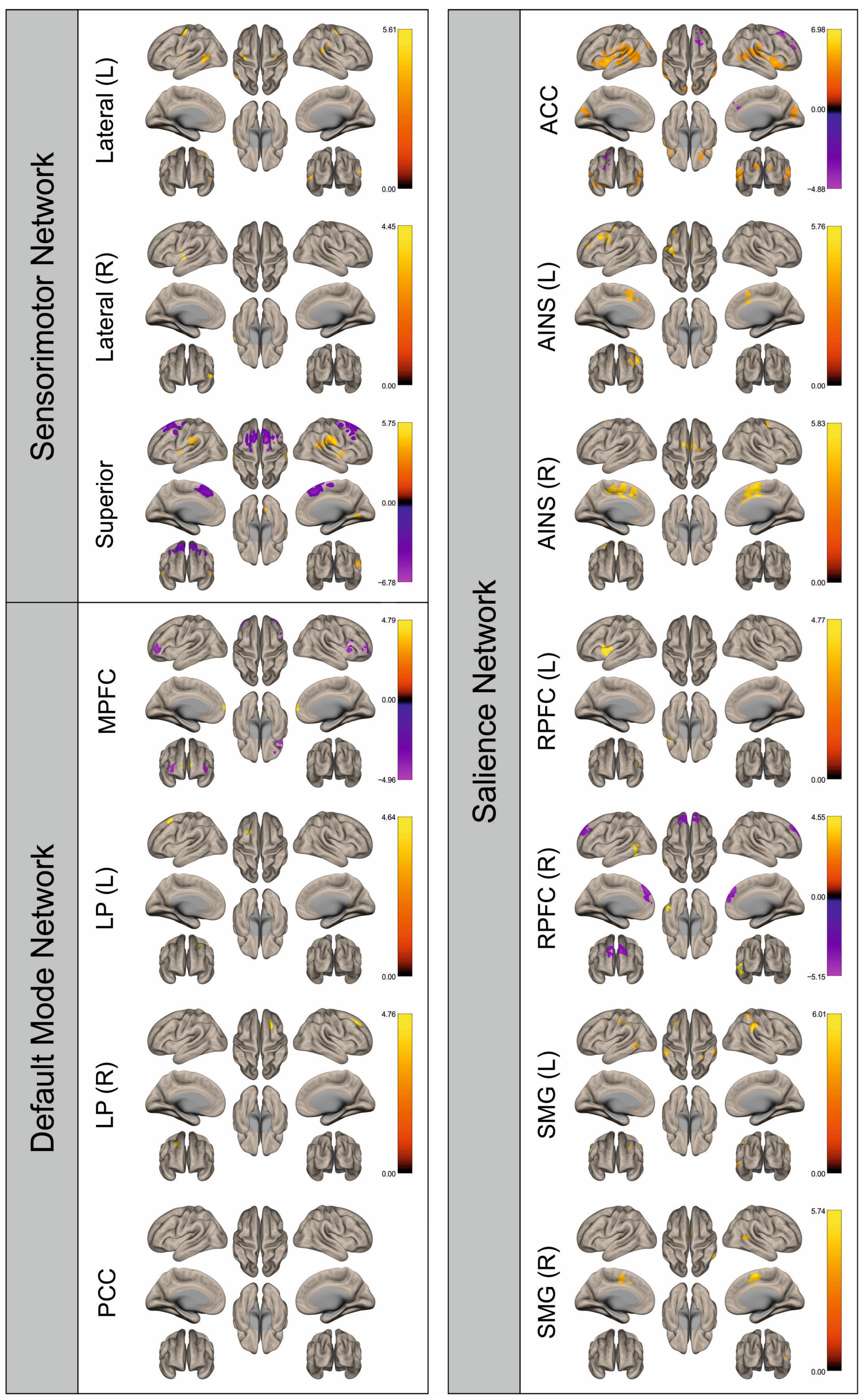
3. Results
3.1. Percent Signal Change
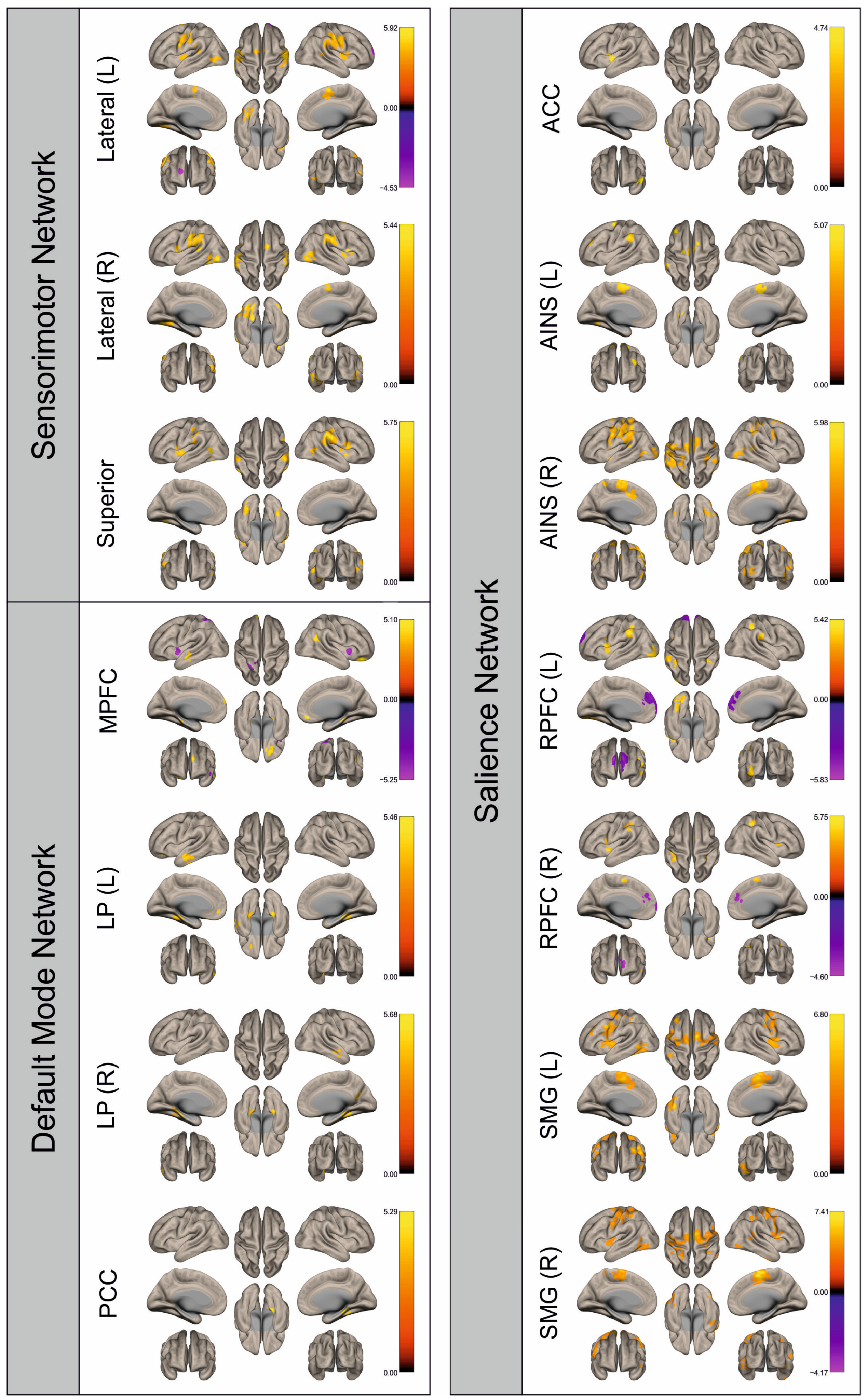
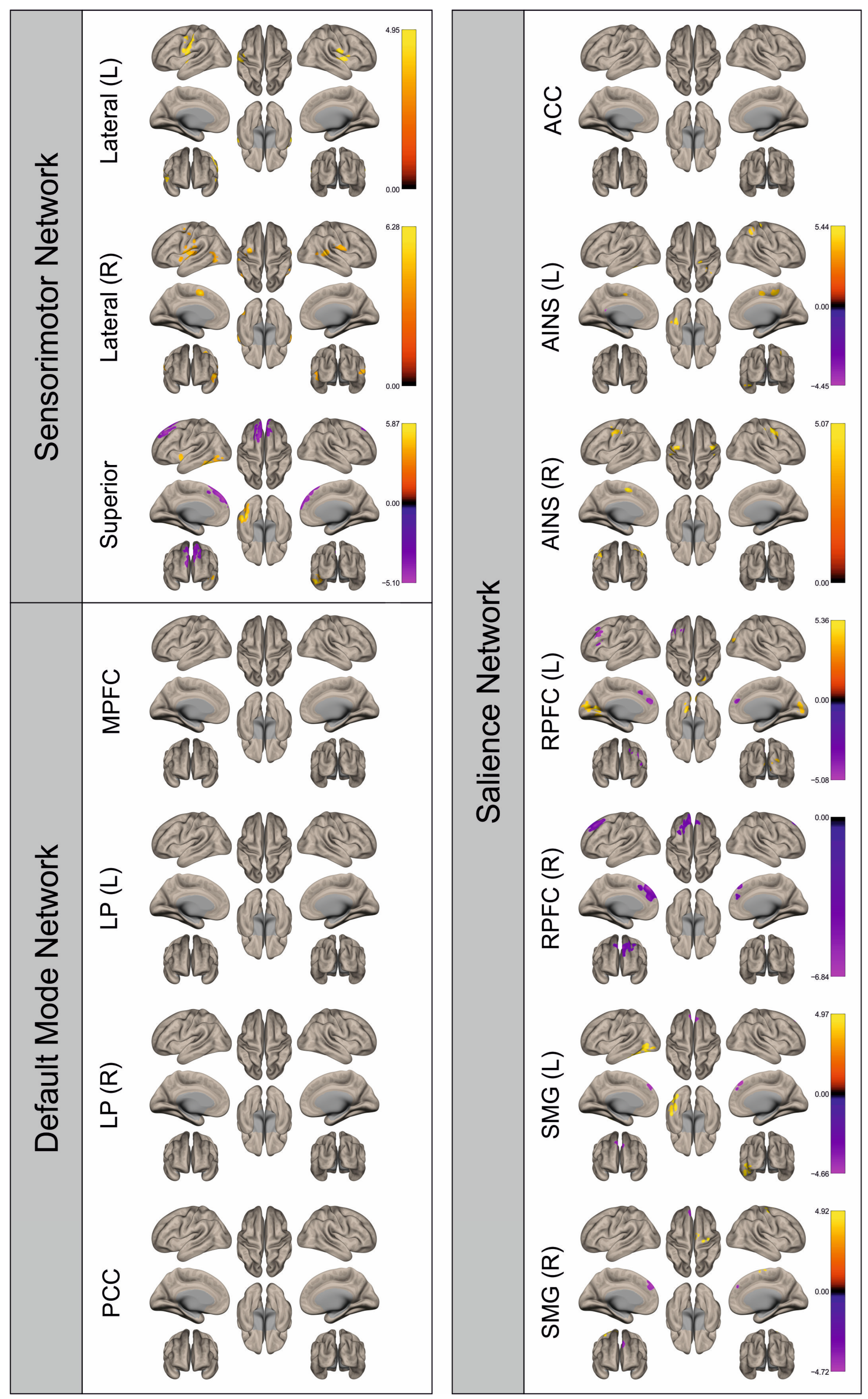
3.2. Functional Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gállego Pérez-Larraya, J.; Hildebrand, J. Brain metastases. Handb. Clin. Neurol. 2014, 121, 1143–1157. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Wright, C.H.; Barnholtz-Sloan, J.S. Brain metastases: Epidemiology. Handb. Clin. Neurol. 2018, 149, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.S.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampson, J.H.; et al. Current multidisciplinary management of brain metastases. Cancer 2020, 126, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.-M. The Management of Brain Metastases-Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef] [PubMed]
- Vysotski, S.; Madura, C.; Swan, B.; Holdsworth, R.; Lin, Y.; Del Munoz Rio, A.; Wood, J.; Kundu, B.; Penwarden, A.; Voss, J.; et al. Preoperative FMRI Associated with Decreased Mortality and Morbidity in Brain Tumor Patients. Interdiscip. Neurosurg. 2018, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Feldman, S.C.; Schulder, M.; Kalnin, A.J.; Holodny, A.I.; Zimmerman, A.; Sinensky, R.; Rao, S. The effect of tumour type and distance on activation in the motor cortex. Neuroradiology 2005, 47, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, D.; Olson, J.; Ali, S.; Fan, T.; Mao, H. Re-examine tumor-induced alterations in hemodynamic responses of BOLD fMRI: Implications in presurgical brain mapping. Acta Radiol. 2012, 53, 802–811. [Google Scholar] [CrossRef]
- Hense, K.; Plank, T.; Wendl, C.; Dodoo-Schittko, F.; Bumes, E.; Greenlee, M.W.; Schmidt, N.O.; Proescholdt, M.; Rosengarth, K. fMRI Retinotopic Mapping in Patients with Brain Tumors and Space-Occupying Brain Lesions in the Area of the Occipital Lobe. Cancers 2021, 13, 2439. [Google Scholar] [CrossRef]
- Hou, B.L.; Bradbury, M.; Peck, K.K.; Petrovich, N.M.; Gutin, P.H.; Holodny, A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage 2006, 32, 489–497. [Google Scholar] [CrossRef]
- Zacà, D.; Jovicich, J.; Nadar, S.R.; Voyvodic, J.T.; Pillai, J.J. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J. Magn. Reson. Imaging 2014, 40, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Hubbe, U.; Ziyeh, S.; Hennig, J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am. J. Neuroradiol. 2000, 21, 1055–1063. [Google Scholar]
- Lüdemann, L.; Förschler, A.; Grieger, W.; Zimmer, C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J. Magn. Reson. Imaging 2006, 23, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Sul, J.; Posner, J.B. Brain Metastases: Epidemiology and Pathophysiology. In Brain Metastases; Springer: Boston, MA, USA, 2007; pp. 1–21. [Google Scholar]
- Ghumman, S.; Fortin, D.; Noel-Lamy, M.; Cunnane, S.C.; Whittingstall, K. Exploratory study of the effect of brain tumors on the default mode network. J. Neurooncol 2016, 128, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Ding, X.; Xiong, M.; Zhang, F.; Luo, Y.; Ding, J.; Ding, Z. Alterations of functional and structural connectivity in patients with brain metastases. PLoS ONE 2020, 15, e0233833. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, F.; Bosma, I.; Klein, M.; Baayen, J.C.; Reijneveld, J.C.; Postma, T.J.; Heimans, J.J.; van Dijk, B.W.; de Munck, J.C.; de Jongh, A.; et al. How do brain tumors alter functional connectivity? A magnetoencephalography study. Ann. Neurol. 2006, 59, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Maesawa, S.; Bagarinao, E.; Fujii, M.; Futamura, M.; Motomura, K.; Watanabe, H.; Mori, D.; Sobue, G.; Wakabayashi, T. Evaluation of resting state networks in patients with gliomas: Connectivity changes in the unaffected side and its relation to cognitive function. PLoS ONE 2015, 10, e0118072. [Google Scholar] [CrossRef] [PubMed]
- Mallela, A.N.; Peck, K.K.; Petrovich-Brennan, N.M.; Zhang, Z.; Lou, W.; Holodny, A.I. Altered Resting-State Functional Connectivity in the Hand Motor Network in Glioma Patients. Brain Connect. 2016, 6, 587–595. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Stoecklein, S.; Galiè, F.; Ren, J.; Schmutzer, M.; Unterrainer, M.; Albert, N.L.; Kreth, F.-W.; Thon, N.; Liebig, T.; et al. Resting-state fMRI detects alterations in whole brain connectivity related to tumor biology in glioma patients. Neuro-oncology 2020, 22, 1388–1398. [Google Scholar] [CrossRef]
- Tordjman, M.; Madelin, G.; Gupta, P.K.; Cordova, C.; Kurz, S.C.; Orringer, D.; Golfinos, J.; Kondziolka, D.; Ge, Y.; Wang, R.L.; et al. Functional connectivity of the default mode, dorsal attention and fronto-parietal executive control networks in glial tumor patients. J. Neuro-Oncol. 2021, 152, 347–355. [Google Scholar] [CrossRef]
- van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A data acquisition perspective. NeuroImage 2012, 62, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M.; Burgess, G.C.; Harms, M.P.; Petersen, S.E.; Schlaggar, B.L.; Corbetta, M.; Glasser, M.F.; Curtiss, S.; Dixit, S.; Feldt, C.; et al. Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage 2013, 80, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.S.; Harms, M.P.; Snyder, A.Z.; Jenkinson, M.; Wilson, J.A.; Glasser, M.F.; Barch, D.M.; Archie, K.A.; Burgess, G.C.; Ramaratnam, M.; et al. Human Connectome Project informatics: Quality control, database services, and data visualization. NeuroImage 2013, 80, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 2013, 80, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Krienen, F.M.; Castellanos, A.; Diaz, J.C.; Yeo, B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.-P.; Frith, C.D.; Frackowiak, R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.L.; Valabregue, R.; Poline, J. Region of interest analysis using an SPM toolbox. NeuroImage 2002, 16, 497. [Google Scholar]
- Eickhoff, S.B.; Stephan, K.E.; Mohlberg, H.; Grefkes, C.; Fink, G.R.; Amunts, K.; Zilles, K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 2005, 25, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Paus, T.; Caspers, S.; Grosbras, M.-H.; Evans, A.C.; Zilles, K.; Amunts, K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 2007, 36, 511–521. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Heim, S.; Zilles, K.; Amunts, K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 2006, 32, 570–582. [Google Scholar] [CrossRef]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 2011, 54, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.R.; Collins, D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage 2009, 47, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Esposito, R.; Mattei, P.A.; Briganti, C.; Romani, G.L.; Tartaro, A.; Caulo, M. Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS ONE 2012, 7, e40231. [Google Scholar] [CrossRef]
- Carter, A.R.; Astafiev, S.V.; Lang, C.E.; Connor, L.T.; Rengachary, J.; Strube, M.J.; Pope, D.L.W.; Shulman, G.L.; Corbetta, M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 2010, 67, 365–375. [Google Scholar] [CrossRef]
- Warren, J.E.; Crinion, J.T.; Lambon Ralph, M.A.; Wise, R.J.S. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain 2009, 132, 3428–3442. [Google Scholar] [CrossRef]
- Kovács, Á.; Emri, M.; Opposits, G.; Pisák, T.; Vandulek, C.; Glavák, C.; Szalai, Z.; Biró, G.; Bajzik, G.; Repa, I. Changes in functional MRI signals after 3D based radiotherapy of glioblastoma multiforme. J. Neuro-Oncol. 2015, 125, 157–166. [Google Scholar] [CrossRef]
- Kesler, S.R.; Wefel, J.S.; Hosseini, S.M.H.; Cheung, M.; Watson, C.L.; Hoeft, F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc. Natl. Acad. Sci. USA 2013, 110, 11600–11605. [Google Scholar] [CrossRef]
- Mentzelopoulos, A.; Gkiatis, K.; Karanasiou, I.; Karavasilis, E.; Papathanasiou, M.; Efstathopoulos, E.; Kelekis, N.; Kouloulias, V.; Matsopoulos, G.K. Chemotherapy-Induced Brain Effects in Small-Cell Lung Cancer Patients: A Multimodal MRI Study. Brain Topogr. 2021, 34, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Simó, M.; Rifà-Ros, X.; Vaquero, L.; Ripollés, P.; Cayuela, N.; Jové, J.; Navarro, A.; Cardenal, F.; Bruna, J.; Rodríguez-Fornells, A. Brain functional connectivity in lung cancer population: An exploratory study. Brain Imaging Behav. 2018, 12, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wu, D.; Zeng, L.-L.; Shen, H.; Hu, D.; Qiu, S. Radiation-induced functional connectivity alterations in nasopharyngeal carcinoma patients with radiotherapy. Medicine 2016, 95, e4275. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Seitzman, B.A.; Ballard, N.; Petersen, S.E.; Shimony, J.S.; Leuthardt, E.C. Human Brain Functional Network Organization Is Disrupted after Whole-Brain Radiation Therapy. Brain Connect. 2020, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, R.A.; Jost, E.; Shaw, K.; Brennan, N.P.; Peck, K.K.; Holodny, A.I. Cortical Plasticity in the Setting of Brain Tumors. Top. Magn. Reson. Imaging TMRI 2016, 25, 25–30. [Google Scholar] [CrossRef]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Foerschler, A.; Meyer, B.; Ringel, F. Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS ONE 2013, 8, e75403. [Google Scholar] [CrossRef]
- Bryszewski, B.; Pfajfer, L.; Antosik-Biernacka, A.; Tybor, K.; Smigielski, J.; Zawirski, M.; Majos, A. Functional rearrangement of the primary and secondary motor cortex in patients with primary tumors of the central nervous system located in the region of the central sulcus depending on the histopathological type and the size of tumor: Examination by means of functional magnetic resonance imaging. Pol. J. Radiol. 2012, 77, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Majos, A.; Bryszewski, B.; Kośla, K.N.; Pfaifer, L.; Jaskólski, D.; Stefańczyk, L. Process of the Functional Reorganization of the Cortical Centers for Movement in GBM Patients: fMRI Study. Clin. Neuroradiol. 2017, 27, 71–79. [Google Scholar] [CrossRef]
- Peck, K.K.; Bradbury, M.S.; Hou, B.L.; Brennan, N.P.; Holodny, A.I. The role of the Supplementary Motor Area (SMA) in the execution of primary motor activities in brain tumor patients: Functional MRI detection of time-resolved differences in the hemodynamic response. Med. Sci. Monit. 2009, 15, MT55-62. [Google Scholar]



| Paradigm | Total | Left Hemisphere | Right Hemisphere | |
|---|---|---|---|---|
| Hand movement | N | 29 | 16 | 13 |
| Male | 13 | 8 | 5 | |
| Female | 16 | 8 | 8 | |
| Mean age in years (range) | 59.90 (31–85) | 58.50 (31–74) | 61.62 (36–85) | |
| Lung carcinoma | 11 | 7 | 4 | |
| Breast cancer | 5 | 2 | 3 | |
| Melanoma | 4 | 2 | 2 | |
| Other primary tumors | 7 | 3 | 4 | |
| Unknown primary tumor | 2 | 2 | ||
| Systemic therapy + radiotherapy | 4 | 3 | 1 | |
| Systemic therapy | 11 | 3 | 8 | |
| Radiotherapy | 1 | 1 | ||
| No previous therapy | 10 | 9 | 1 | |
| Unknown previous therapies | 3 | 1 | 2 | |
| Foot movement | N | 16 | 7 | 9 |
| Male | 7 | 3 | 4 | |
| Female | 9 | 4 | 5 | |
| Mean age in years (range) | 57.63 (36–67) | 57.57 (48–67) | 57.67 (36–67) | |
| Lung carcinoma | 8 | 4 | 4 | |
| Breast cancer | 3 | 1 | 2 | |
| Melanoma | 2 | 1 | 1 | |
| Other primary tumors | 2 | 2 | ||
| Unknown primary tumor | 1 | 1 | ||
| Systemic therapy + radiotherapy | 1 | 1 | ||
| Systemic therapy | 7 | 1 | 6 | |
| No previous therapy | 6 | 5 | 1 | |
| Unknown previous therapies | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angstwurm, P.; Hense, K.; Rosengarth, K.; Strotzer, Q.; Schmidt, N.O.; Bumes, E.; Hau, P.; Pukrop, T.; Wendl, C. Attenuation of the BOLD fMRI Signal and Changes in Functional Connectivity Affecting the Whole Brain in Presence of Brain Metastasis. Cancers 2024, 16, 2010. https://doi.org/10.3390/cancers16112010
Angstwurm P, Hense K, Rosengarth K, Strotzer Q, Schmidt NO, Bumes E, Hau P, Pukrop T, Wendl C. Attenuation of the BOLD fMRI Signal and Changes in Functional Connectivity Affecting the Whole Brain in Presence of Brain Metastasis. Cancers. 2024; 16(11):2010. https://doi.org/10.3390/cancers16112010
Chicago/Turabian StyleAngstwurm, Pia, Katharina Hense, Katharina Rosengarth, Quirin Strotzer, Nils Ole Schmidt, Elisabeth Bumes, Peter Hau, Tobias Pukrop, and Christina Wendl. 2024. "Attenuation of the BOLD fMRI Signal and Changes in Functional Connectivity Affecting the Whole Brain in Presence of Brain Metastasis" Cancers 16, no. 11: 2010. https://doi.org/10.3390/cancers16112010
APA StyleAngstwurm, P., Hense, K., Rosengarth, K., Strotzer, Q., Schmidt, N. O., Bumes, E., Hau, P., Pukrop, T., & Wendl, C. (2024). Attenuation of the BOLD fMRI Signal and Changes in Functional Connectivity Affecting the Whole Brain in Presence of Brain Metastasis. Cancers, 16(11), 2010. https://doi.org/10.3390/cancers16112010








