Bimodal Effect of NKG2A Blockade on Intratumoral and Systemic CD8 T Cell Response Induced by Cancer Vaccine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Ethic Approval
2.3. Tumor Cell Line
2.4. Generation of Vaccine Constructs
2.5. NKG2A Blocking Antibody
2.6. In Vivo Tumor Experiments
2.7. Ex Vivo Cell Preparation
2.7.1. Tumors
2.7.2. Spleen
2.7.3. Peripheral Blood
2.7.4. Bone Marrow
2.8. Ex Vivo T Cell Stimulation
2.9. Bone Marrow-Dendritic Cells (BM-DCs)
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
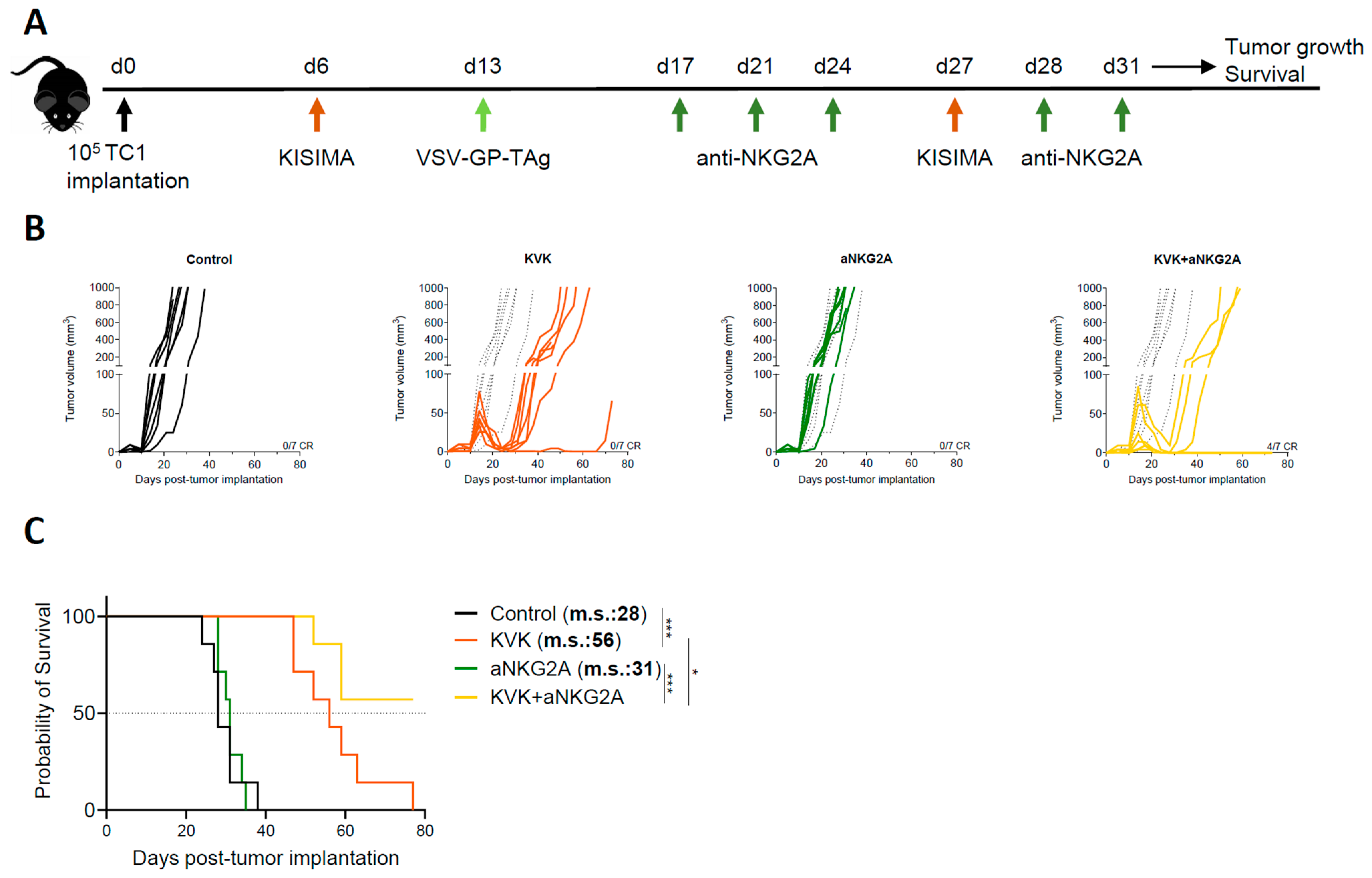
3.1. Combination Treatment with Anti-NKG2A Improves the Antitumoral Efficacy of KISIMA—VSV-GP-TAg Heterologous Prime-Boost Vaccination
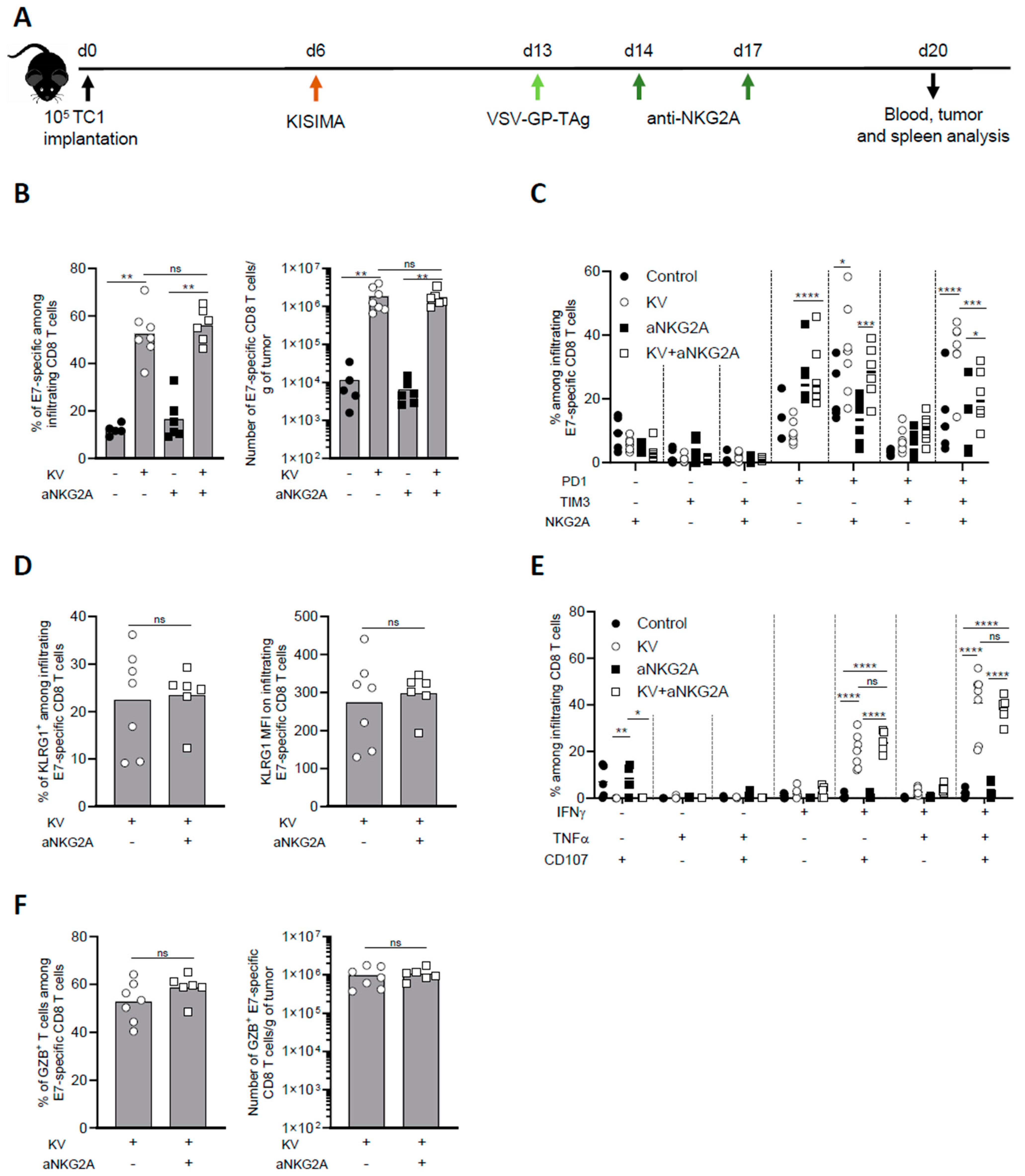
3.2. NKG2A Blockade Reduces Exhaustion of Tumor Infiltrating Antigen-Specific CD8 T Cells
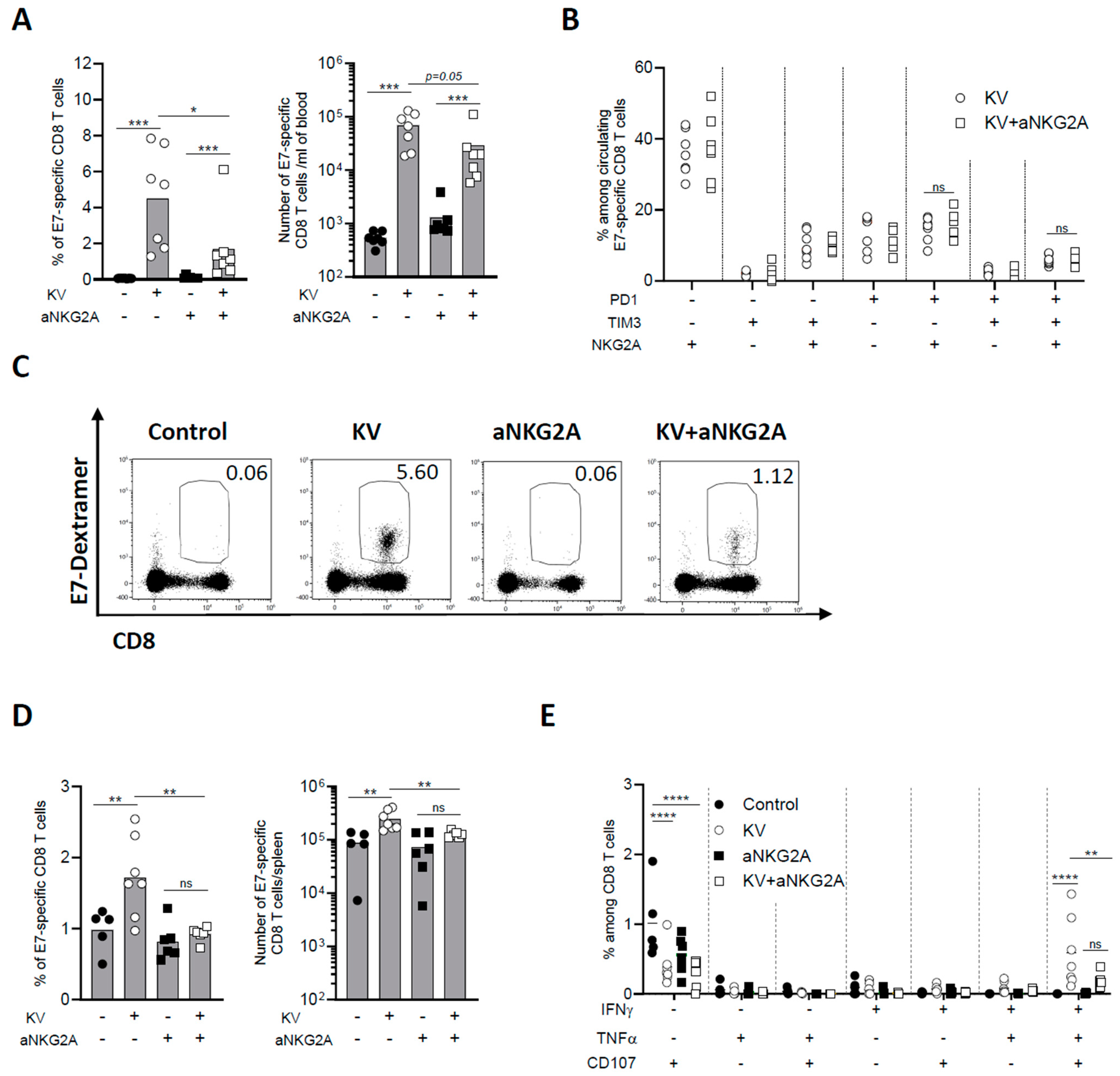
3.3. NKG2A Blockade Dampens Peripheral CD8 T Cell Response Induced by KISIMA—VSV-GP-TAg Vaccination
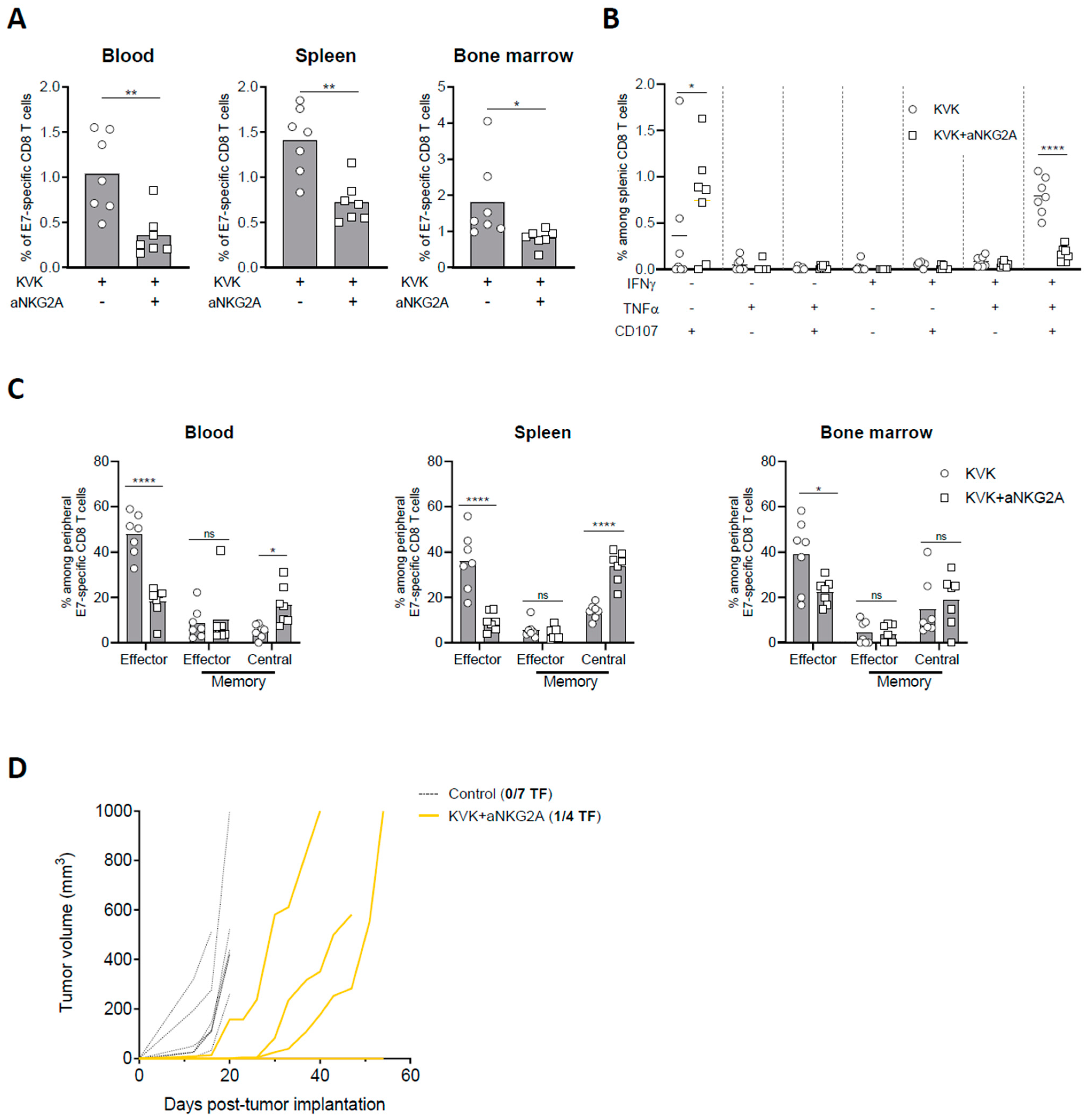
3.4. NKG2A Blockade Affects the Establishment of Long-Term Antigen-Specific CD8 T Cell Memory Response Induced by KISIMA—VSV-GP-TAg Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niemi, J.V.L.; Sokolov, A.V.; Schiöth, H.B. Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments. Cancers 2022, 14, 5163. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Berardino-Besson, W.D.; Gaertner, H.; Carboni, S.; Dunand-Sauthier, I.; Cerini, F.; Suso-Inderberg, E.-M.; Wälchli, S.; König, S.; Salazar, A.M.; et al. Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-Penetrating Peptide-Based Vaccines and Adjuvants. Mol. Ther. 2016, 24, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Mayol, J.-F.; Carboni, S.; Besson, W.D.B.; Dupuychaffray, E.; Nelde, A.; Stevanovic, S.; Santiago-Raber, M.-L.; Walker, P.R.; Derouazi, M. Targeting Self- and Neoepitopes with a Modular Self-Adjuvanting Cancer Vaccine. JCI Insight 2019, 127305. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Stubbert, L.J.; Jahedi, R.Z.; Geiβ, Y.; Kimpel, J.; Dold, C.; Tober, R.; Volk, A.; Klein, S.; Dietrich, U.; et al. Re-Engineering Vesicular Stomatitis Virus to Abrogate Neurotoxicity, Circumvent Humoral Immunity, and Enhance Oncolytic Potency. Cancer Res. 2014, 74, 3567–3578. [Google Scholar] [CrossRef] [PubMed]
- Tober, R.; Banki, Z.; Egerer, L.; Muik, A.; Behmüller, S.; Kreppel, F.; Greczmiel, U.; Oxenius, A.; von Laer, D.; Kimpel, J. VSV-GP: A Potent Viral Vaccine Vector That Boosts the Immune Response upon Repeated Applications. J. Virol. 2014, 88, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.-M.; Urbiola, C.; Das, K.; Spiesschaert, B.; Kimpel, J.; Heinemann, F.; Stierstorfer, B.; Müller, P.; Petersson, M.; Erlmann, P.; et al. The Lytic Activity of VSV-GP Treatment Dominates the Therapeutic Effects in a Syngeneic Model of Lung Cancer. Br. J. Cancer 2019, 121, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Belnoue, E.; Rossi, M.; Hofer, T.; Danklmaier, S.; Nolden, T.; Schreiber, L.-M.; Angerer, K.; Kimpel, J.; Hoegler, S.; et al. A Modular Self-Adjuvanting Cancer Vaccine Combined with an Oncolytic Vaccine Induces Potent Antitumor Immunity. Nat. Commun. 2021, 12, 5195. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Rossi, M.; Carboni, S.; Besson, W.D.B.; von Laer, D.; Wollmann, G.; Derouazi, M.; Santiago-Raber, M.-L. Heterologous Prime-Boost Vaccination with a Peptide-Based Vaccine and Viral Vector Reshapes Dendritic Cell, CD4+ and CD8+ T Cell Phenotypes to Improve the Antitumor Therapeutic Effect. Cancers 2021, 13, 6107. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Carlino, M.; Weber, J.S.; Meniawy, T.; Taylor, M.H.; Kim, K.; McKean, M.; Long, G.V.; Faries, M.; Cowey, C.L.; et al. Abstract CT224: MRNA-4157, a Personalized Cancer Vaccine, in Combination with Pembrolizumab, Demonstrates Trend for Improved Recurrence Free Survival Compared to Pembrolizumab Alone in Adjuvant Melanoma Patients across Tumor Mutational Burden Subgroups. Cancer Res. 2023, 83, CT224. [Google Scholar] [CrossRef]
- Khattak, A.; Carlino, M.; Meniawy, T.; Ansstas, G.; Medina, T.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Abstract CT001: A Personalized Cancer Vaccine, MRNA-4157, Combined with Pembrolizumab versus Pembrolizumab in Patients with Resected High-Risk Melanoma: Efficacy and Safety Results from the Randomized, Open-Label Phase 2 MRNA-4157-P201/Keynote-942 Trial. Cancer Res. 2023, 83, CT001. [Google Scholar] [CrossRef]
- Manser, A.R.; Uhrberg, M. Age-Related Changes in Natural Killer Cell Repertoires: Impact on NK Cell Function and Immune Surveillance. Cancer Immunol. Immunother. 2016, 65, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Borst, L.; van der Burg, S.H.; van Hall, T. The NKG2A–HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 5549–5556. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.-C.; Chiou, S.-H.; Lin, H.-H.; Chow, S.-N.; Huang, S.-C.; Ho, H.-N.; Hsu, S.-M. Up-Regulation of Inhibitory Natural Killer Receptors CD94/NKG2A with Suppressed Intracellular Perforin Expression of Tumor-Infiltrating CD8+ T Lymphocytes in Human Cervical Carcinoma. Cancer Res. 2005, 65, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, H.; Ning, Z. Implications of NKG2A in Immunity and Immune-Mediated Diseases. Front. Immunol. 2022, 13, 960852. [Google Scholar] [CrossRef] [PubMed]
- Salomé, B.; Sfakianos, J.P.; Ranti, D.; Daza, J.; Bieber, C.; Charap, A.; Hammer, C.; Banchereau, R.; Farkas, A.M.; Ruan, D.F.; et al. NKG2A and HLA-E Define an Alternative Immune Checkpoint Axis in Bladder Cancer. Cancer Cell 2022, 40, 1027–1043.e9. [Google Scholar] [CrossRef] [PubMed]
- Ducoin, K.; Oger, R.; Mutala, L.B.; Deleine, C.; Jouand, N.; Desfrançois, J.; Podevin, J.; Duchalais, E.; Cruard, J.; Benlalam, H.; et al. Targeting NKG2A to Boost Anti-Tumor CD8 T-Cell Responses in Human Colorectal Cancer. Oncoimmunology 2022, 11, 2046931. [Google Scholar] [CrossRef] [PubMed]
- van Montfoort, N.; Borst, L.; Korrer, M.J.; Sluijter, M.; Marijt, K.A.; Santegoets, S.J.; van Ham, V.J.; Ehsan, I.; Charoentong, P.; André, P.; et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 2018, 175, 1744–1755.e15. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef]
- Zander, R.; Cui, W. Exhausted CD8+ T Cells Face a Developmental Fork in the Road. Trends Immunol. 2023, 44, 276–286. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2021, 11, 622509. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Aldemir, H.; Breart, B.; Ferlin, W.G. Expression of CD94–NKG2A Inhibitory Receptor Is Restricted to a Subset of CD8+ T Cells. Trends Immunol. 2003, 24, 162–164. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.J.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E Is Expressed on Trophoblast and Interacts with CD94/NKG2 Receptors on Decidual NK Cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.T.; van Riet, S.; van Schadewijk, A.; Fiocco, M.; van Hall, T.; Taube, C.; Hiemstra, P.S.; van der Burg, S.H. The Positive Prognostic Effect of Stromal CD8+ Tumor-Infiltrating T Cells Is Restrained by the Expression of HLA-E in Non-Small Cell Lung Carcinoma. Oncotarget 2015, 7, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- de Kruijf, E.M.; Sajet, A.; van Nes, J.G.H.; Natanov, R.; Putter, H.; Smit, V.T.H.B.M.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.H.; Kuppen, P.J.K. HLA-E and HLA-G Expression in Classical HLA Class I-Negative Tumors Is of Prognostic Value for Clinical Outcome of Early Breast Cancer Patients. J. Immunol. 2010, 185, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Jasinski-Bergner, S.; Quandt, D.; Stoehr, C.; Bukur, J.; Wach, S.; Legal, W.; Taubert, H.; Wullich, B.; Hartmann, A. HLA-E Expression and Its Clinical Relevance in Human Renal Cell Carcinoma. Oncotarget 2016, 7, 67360–67372. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; Hall, T. van HLA-E Expression by Gynecological Cancers Restrains Tumor-Infiltrating CD8+ T Lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef]
- Borst, L.; Sluijter, M.; Sturm, G.; Charoentong, P.; Santegoets, S.J.; Gulijk, M.; Elsas, M.J.; Groeneveldt, C.; Montfoort, N.; Finotello, F.; et al. NKG2A Is a Late Immune Checkpoint on CD8 T Cells and Marks Repeated Stimulation and Cell Division. Int. J. Cancer 2022, 150, 688–704. [Google Scholar] [CrossRef]
- Tinker, A.V.; Hirte, H.W.; Provencher, D.; Butler, M.; Ritter, H.; Tu, D.; Azim, H.A.; Paralejas, P.; Grenier, N.; Hahn, S.-A.; et al. Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results from the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jenkins, R.W.; Sullivan, R.J. Mechanisms of Resistance to Immune Checkpoint Blockade. Am. J. Clin. Dermatol. 2019, 20, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non–Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic Resistance to Immune Checkpoint Blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, E.; Carboni, S.; di Berardino-Besson, W.; Moyat, M.; Belnoue, E.; Devy-Dimanche, L.; Rossi, M. Bimodal Effect of NKG2A Blockade on Intratumoral and Systemic CD8 T Cell Response Induced by Cancer Vaccine. Cancers 2024, 16, 2036. https://doi.org/10.3390/cancers16112036
Riva E, Carboni S, di Berardino-Besson W, Moyat M, Belnoue E, Devy-Dimanche L, Rossi M. Bimodal Effect of NKG2A Blockade on Intratumoral and Systemic CD8 T Cell Response Induced by Cancer Vaccine. Cancers. 2024; 16(11):2036. https://doi.org/10.3390/cancers16112036
Chicago/Turabian StyleRiva, Erika, Susanna Carboni, Wilma di Berardino-Besson, Mati Moyat, Elodie Belnoue, Laetitia Devy-Dimanche, and Matteo Rossi. 2024. "Bimodal Effect of NKG2A Blockade on Intratumoral and Systemic CD8 T Cell Response Induced by Cancer Vaccine" Cancers 16, no. 11: 2036. https://doi.org/10.3390/cancers16112036
APA StyleRiva, E., Carboni, S., di Berardino-Besson, W., Moyat, M., Belnoue, E., Devy-Dimanche, L., & Rossi, M. (2024). Bimodal Effect of NKG2A Blockade on Intratumoral and Systemic CD8 T Cell Response Induced by Cancer Vaccine. Cancers, 16(11), 2036. https://doi.org/10.3390/cancers16112036




