The Influence of Preoperative Anticoagulant and Antiplatelet Therapy on Rebleeding Rates in Patients Suffering from Spinal Metastatic Cancer: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akram, H.; Allibone, J. Spinal surgery for palliation in malignant spinal cord compression. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K.; Global Spine Tumor Study Group. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobin, N.P.; Foukakis, T.; De Petris, L.; Bergh, J. The importance of molecular markers for diagnosis and selection of targeted treatments in patients with cancer. J. Intern. Med. 2015, 278, 545–570. [Google Scholar] [CrossRef] [PubMed]
- Klimo, P., Jr.; Schmidt, M.H. Surgical management of spinal metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; Hassan, S.; Thumallapally, N.; Dai, Q. Rivaroxaban-Induced Nontraumatic Spinal Subdural Hematoma: An Uncommon Yet Life-Threatening Complication. Case Rep. Hematol. 2015, 2015, 275380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhanot, K.; Widdifield, J.; Huang, A.; Paterson, J.M.; Shultz, D.B.; Finkelstein, J. Survival after surgery for spinal metastases: A population-based study. Can. J. Surg. 2022, 65, E512–E518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loblaw, D.A.; Laperriere, N.J.; Mackillop, W.J. A population-based study of malignant spinal cord compression in Ontario. Clin. Oncol. (R. Coll. Radiol.) 2003, 15, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.T.; Gao, Y.; Duchman, K.R.; Pugely, A.J. The Impact of Current Smoking and Smoking Cessation on Short-Term Morbidity Risk after Lumbar Spine Surgery. Spine 2016, 41, 577–584. [Google Scholar] [CrossRef] [PubMed]
- El-Kadi, M.; Donovan, E.; Kerr, L.; Cunningham, C.; Osio, V.; Abdallah, S.; Kazan, J. Risk factors for postoperative spinal infection: A retrospective analysis of 5065 cases. Surg. Neurol. Int. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzman, J.Z.; Skovrlj, B.; Shin, J.; Hecht, A.C.; Qureshi, S.A.; Iatridis, J.C.; Cho, S.K. The impact of diabetes mellitus on patients undergoing degenerative cervical spine surgery. Spine 2014, 39, 1656–1665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzman, J.Z.; Iatridis, J.C.; Skovrlj, B.; Cutler, H.S.; Hecht, A.C.; Qureshi, S.A.; Cho, S.K. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine 2014, 39, 1596–1604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhuang, T.; Feng, A.Y.; Shapiro, L.M.; Hu, S.S.; Gardner, M.; Kamal, R.N. Is Uncontrolled Diabetes Mellitus Associated with Incidence of Complications after Posterior Instrumented Lumbar Fusion? A National Claims Database Analysis. Clin. Orthop. Relat. Res. 2021, 479, 2726–2733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mikhail, C.; Pennington, Z.; Arnold, P.M.; Brodke, D.S.; Chapman, J.R.; Chutkan, N.; Daubs, M.D.; DeVine, J.G.; Fehlings, M.G.; Gelb, D.E.; et al. Minimizing Blood Loss in Spine Surgery. Glob. Spine J. 2020, 10 (Suppl. S1), 71S–83S. [Google Scholar] [CrossRef] [PubMed]
- Minakata, K.; Bando, K.; Tanaka, S.; Takanashi, S.; Konishi, H.; Miyamoto, Y.; Ueshima, K.; Yasuno, S.; Ueda, Y.; Okita, Y.; et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ. J. 2014, 78, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Puvanesarajah, V.; Jain, A.; Hess, D.E.; Shimer, A.L.; Shen, F.H.; Hassanzadeh, H. Complications and Mortality after Lumbar Spinal Fusion in Elderly Patients with Late Stage Renal Disease. Spine 2016, 41, E1298–E1302. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; White, S.J.; Ye, I.B.; Mikhail, C.M.; Cheung, Z.B.; Cho, S.K. The Effects of Liver Disease on Surgical Outcomes Following Adult Spinal Deformity Surgery. World Neurosurg. 2019, 130, e498–e504. [Google Scholar] [CrossRef] [PubMed]
- Qato, D.M.; Alexander, G.C.; Conti, R.M.; Johnson, M.; Schumm, P.; Lindau, S.T. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008, 300, 2867–2878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porto, G.B.F.; Jeffrey Wessell, D.O.; Alvarado, A.; Arnold, P.M.; Buchholz, A.L. Anticoagulation and Spine Surgery. Glob. Spine J. 2020, 10 (Suppl. S1), 53S–64S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levi, M.M.; Vink, R.; de Jonge, E. Management of bleeding disorders by prohemostatic therapy. Int. J. Hematol. 2002, 76 (Suppl. S2), 139–144. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, J.M.; Petrizzo, A.; Vaswani, R.; Goldstein, J.A.; Bendo, J.A. Does aspirin administration increase perioperative morbidity in patients with cardiac stents undergoing spinal surgery? Spine 2015, 40, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Eikelboom, J.; Douketis, J.; Wallentin, L.; Oldgren, J.; Yang, S.; Themeles, E.; Heidbuchel, H.; Avezum, A.; Reilly, P.; et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: Results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012, 126, 343–348, Erratum in: Circulation 2012, 126, e160. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.M.; Dalolio, M.; Guzman, R.; Mariani, L.; Schaeren, S.; Kamenova, M.; Soleman, J. Direct Oral Anticoagulants in Patients Undergoing Spine Surgery. World Neurosurg. 2019, 125, e1034–e1041. [Google Scholar] [CrossRef] [PubMed]
- Goes, R.; Muskens, I.S.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D.; Moojen, W.A. Risk of aspirin continuation in spinal surgery: A systematic review and meta-analysis. Spine J. 2017, 17, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Czigléczki, G.; Mezei, T.; Pollner, P.; Horváth, A.; Banczerowski, P. Prognostic Factors of Surgical Complications and Overall Survival of Patients with Metastatic Spinal Tumor. World Neurosurg. 2018, 113, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.N.; Refaai, M.A.; Milling, T.J.; Lewis, B.; Goldberg-Alberts, R.; Hug, B.A.; Sarode, R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: A phase 3b, open-label, non-inferiority, randomised trial. Lancet 2015, 385, 2077–2087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palaiodimos, L.; Miles, J.; Kokkinidis, D.G.; Barkolias, C.; Jonnalagadda, A.K.; Papaconstantinou, D.; Frountzas, M.; Misiakos, E.P.; Schizas, D. Reversal of Novel Anticoagulants in Emergent Surgery and Trauma: A Comprehensive Review and Proposed Management Algorithm. Curr. Pharm. Des. 2018, 24, 4540–4553. [Google Scholar] [CrossRef] [PubMed]
- Steib, A.; Hadjiat, F.; Skibba, W.; Steib, J.P.; French Spine Surgery Society. Focus on perioperative management of anticoagulants and antiplatelet agents in spine surgery. Orthop. Traumatol. Surg. Res. 2011, 97 (Suppl. S6), S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Beynon, C.; Potzy, A.; Unterberg, A.W.; Sakowitz, O.W. Prothrombin complex concentrate facilitates emergency spinal surgery in anticoagulated patients. Acta Neurochir. 2014, 156, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.V.; Wang, K.; Sethi, I.; Zhang, B.; Margalit, A.; Puvanesarajah, V.; Jain, A. Spine Surgery and Preoperative Hemoglobin, Hematocrit, and Hemoglobin A1c: A Systematic Review. Glob. Spine J. 2022, 12, 155–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, J.H.; Chancer, Z.; Mazzeffi, M.A.; McNeil, J.S.; Sokolow, M.J.; Gaines, T.M.; Reif, M.M.; Trinh, A.T.; Wellington, I.J.; Camacho, J.E.; et al. Impact of Preoperative Platelet Count on Bleeding Risk and Allogeneic Transfusion in Multilevel Spine Surgery. Spine 2021, 46, E65–E72. [Google Scholar] [CrossRef] [PubMed]
- Strony, J.T.; Ahn, J.; Du, J.Y.; Ahn, U.M.; Haase, L.; Ahn, N.U. The Effect of High-Normal Preoperative International Normalized Ratios on Outcomes and Complications after Anterior Cervical Spine Surgery. Orthopedics 2024, 47, e26–e32. [Google Scholar] [CrossRef] [PubMed]
- Horlocker, T.T.; Nuttall, G.A.; Dekutoski, M.B.; Bryant, S.C. The accuracy of coagulation tests during spinal fusion and instrumentation. Anesth. Analg. 2001, 93, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zaw, A.S.; Reyes, M.R.; Malhotra, R.; Wu, P.H.; Makandura, M.C.; Thambiah, J.; Liu, G.K.; Wong, H.K. Versatility of percutaneous pedicular screw fixation in metastatic spine tumor surgery: A prospective analysis. Ann. Surg. Oncol. 2015, 22, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Shin, S.H.; Yoo, H.; Lee, S.H.; Kim, K.J.; Jahng, T.A.; Gwak, H.S. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: Prognostic factors for functional outcome and patients’ survival. Spine J. 2012, 12, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Ebata, S.; Haro, H. Influence of Postoperative Hypertension on the Development of Spinal Epidural Hematoma. Orthop. Surg. 2017, 9, 386–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, A.; Meng, S.; Wang, C.; Zhao, X.; Han, S.; Zhang, H.; Shen, Y.; Zhu, K.; Zhou, D.; Su, K.; et al. Severe Symptomatic Epidural Hematoma Following Percutaneous Endoscopic Unilateral Laminectomy for Bilateral Decompression (Endo-ULBD)-Series Report and Management Strategies. Orthop. Surg. 2023, 15, 2342–2353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sioutos, P.J.; Arbit, E.; Meshulam, C.F.; Galicich, J.H. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer 1995, 76, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Takahashi, M.; Inagaki, J.; Kobayashi, H.; Sugiura, H.; Yamamura, S.; Iwata, H. Clinical results of nonsurgical treatment for spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Maranzano, E.; Latini, P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Lener, S.; Wipplinger, C.; Hernandez, R.N.; Hussain, I.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.A.; Kim, E.; Härtl, R. Defining the MIS-TLIF: A Systematic Review of Techniques and Technologies Used by Surgeons Worldwide. Glob. Spine J. 2020, 10 (Suppl. S2), 151S–167S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
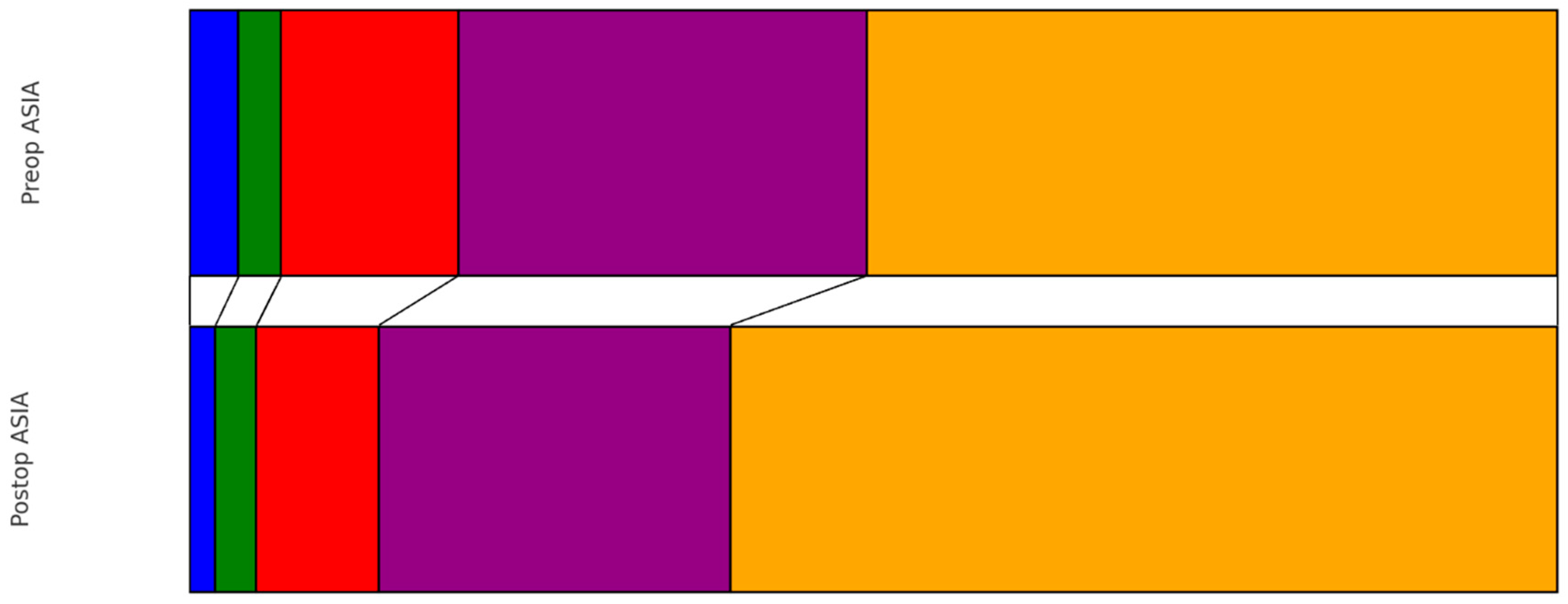
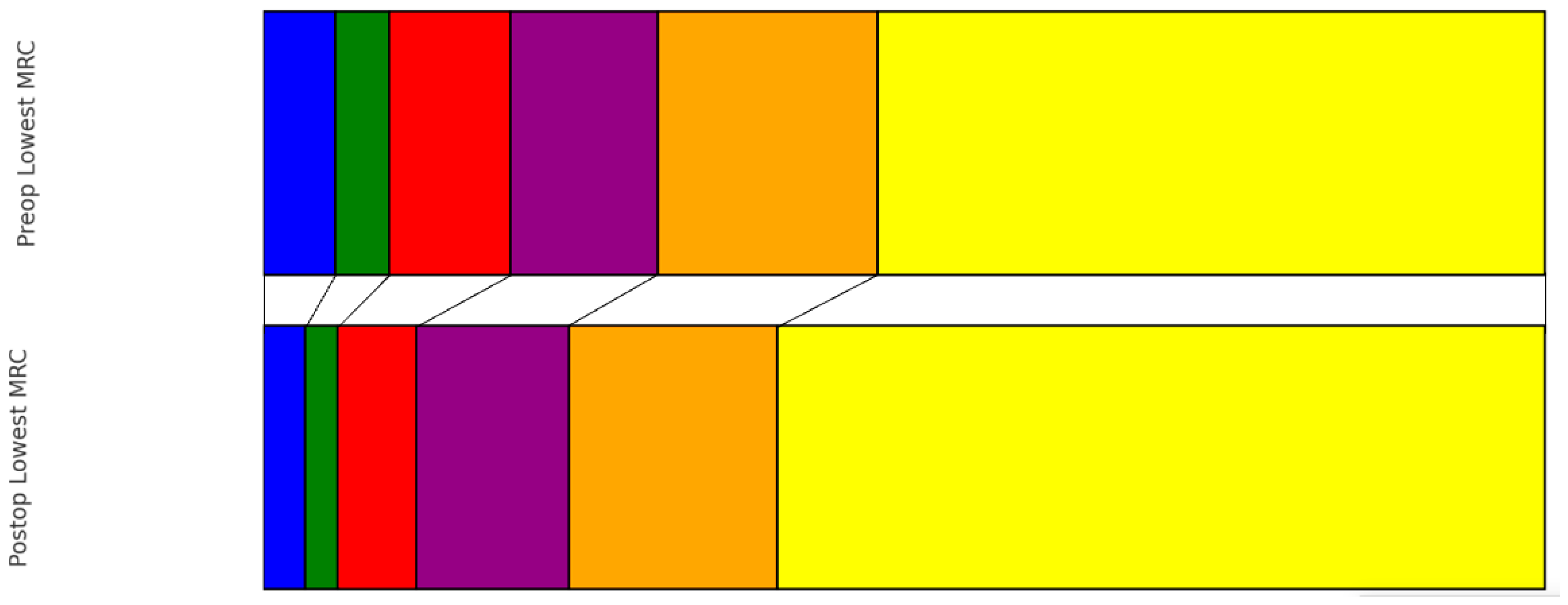
| Risk Factor | Number (%) |
|---|---|
| Smoking history | 57 (19.7) |
| Hypertension | 123 (42.4) |
| Diabetes mellitus | 31 (10.7) |
| Coronary heart disease | 41 (14.1) |
| Hepatopathy | 24 (8.3) |
| Nephropathy | 56 (19.3) |
| Coagulation disorder | 1 (0.3) |
| Anticoagulant Intake | Number (%) |
|---|---|
| Apixaban | 8 (2.8) |
| Heparin | 1 (0.3) |
| Edoxaban | 1 (0.3) |
| Clopidogrel | 2 (0.7) |
| Acenocumarol | 5 (1.7) |
| Acetylsalicylic acid | 45 (15.5) |
| Rivaroxaban | 3 (1) |
| Multiple | 1 (0.3) |
| Unknown | 4 (1.3) |
| Perioperative Complication | Number (%) |
|---|---|
| Incidental durotomy | 11 (3.8) |
| Cement leakage | 1 (0.3) |
| Wound healing disorder | 2 (0.7) |
| Surgery Level | Number (%) |
|---|---|
| Cervical | 39 (13.4) |
| Thoracic | 160 (55.2) |
| Lumbar | 76 (26.2) |
| Sacral | 2 (0.7) |
| Multiregional | 13 (4.5) |
| Rebleeding | No Rebleeding | p-Value | |
|---|---|---|---|
| Preop reversion of anticoagulants | 1 (11.1) | 3 (1.1) | 0.121 |
| Administration of coagulation factors | |||
| Preop | 0 | 9 (3.2) | 0.581 |
| Intraop | 4 (44.4) | 90 (32.5) | 0.492 |
| Postop | 1 (11.1) | 27 (9.7) | 0.913 |
| Intraoperative complications | 0 | 13 (4.7) | 0.503 |
| Use of drainage | 7 (77.8) | 169 (61.0) | 0.491 |
| Preoperative embolization | 0 | 9 (3.2) | 0.582 |
| Anticoagulant intake | 3 (33.3) | 67 (24.2) | 0.530 |
| Duration of surgery (min.; median; IQR) | 153 (82, 234) | 157 (104, 254) | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orban, C.; Abramovic, A.; Gmeiner, R.; Lener, S.; Demetz, M.; Thomé, C. The Influence of Preoperative Anticoagulant and Antiplatelet Therapy on Rebleeding Rates in Patients Suffering from Spinal Metastatic Cancer: A Retrospective Cohort Study. Cancers 2024, 16, 2052. https://doi.org/10.3390/cancers16112052
Orban C, Abramovic A, Gmeiner R, Lener S, Demetz M, Thomé C. The Influence of Preoperative Anticoagulant and Antiplatelet Therapy on Rebleeding Rates in Patients Suffering from Spinal Metastatic Cancer: A Retrospective Cohort Study. Cancers. 2024; 16(11):2052. https://doi.org/10.3390/cancers16112052
Chicago/Turabian StyleOrban, Christoph, Anto Abramovic, Raphael Gmeiner, Sara Lener, Matthias Demetz, and Claudius Thomé. 2024. "The Influence of Preoperative Anticoagulant and Antiplatelet Therapy on Rebleeding Rates in Patients Suffering from Spinal Metastatic Cancer: A Retrospective Cohort Study" Cancers 16, no. 11: 2052. https://doi.org/10.3390/cancers16112052
APA StyleOrban, C., Abramovic, A., Gmeiner, R., Lener, S., Demetz, M., & Thomé, C. (2024). The Influence of Preoperative Anticoagulant and Antiplatelet Therapy on Rebleeding Rates in Patients Suffering from Spinal Metastatic Cancer: A Retrospective Cohort Study. Cancers, 16(11), 2052. https://doi.org/10.3390/cancers16112052







