Hormone Receptor Expression and Activity for Different Tumour Locations in Patients with Advanced and Recurrent Endometrial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Patients
2.3. Tumour Locations
- Uterine biopsies from patients with advanced EC (cervix, uterus);
- Lymphogenic tumour location (inguinal lymph node, para-aortic lymph node, pelvic lymph node, supraclavicular lymph node);
- Hematogenous tumour location (liver, lung);
- Intra-abdominal tumour location (colon, peritoneum, omentum);
- Port-site tumour location (surgical scar, umbilical cord);
- Vaginal vault tumour location from recurrent EC patients.
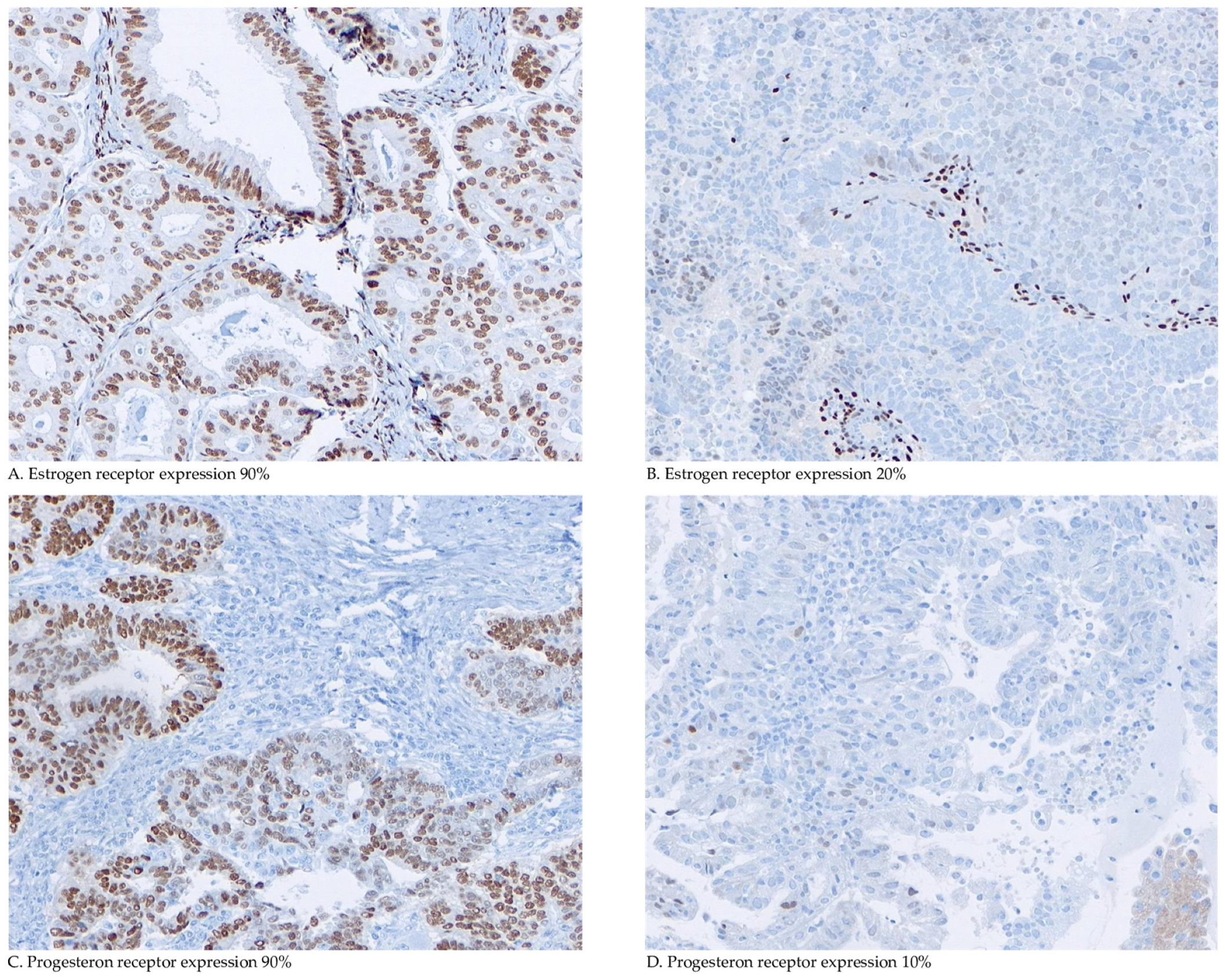
2.4. Immunohistochemical Staining and Scoring
2.4.1. RNA Isolation
2.4.2. ER Pathway Activity Test
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
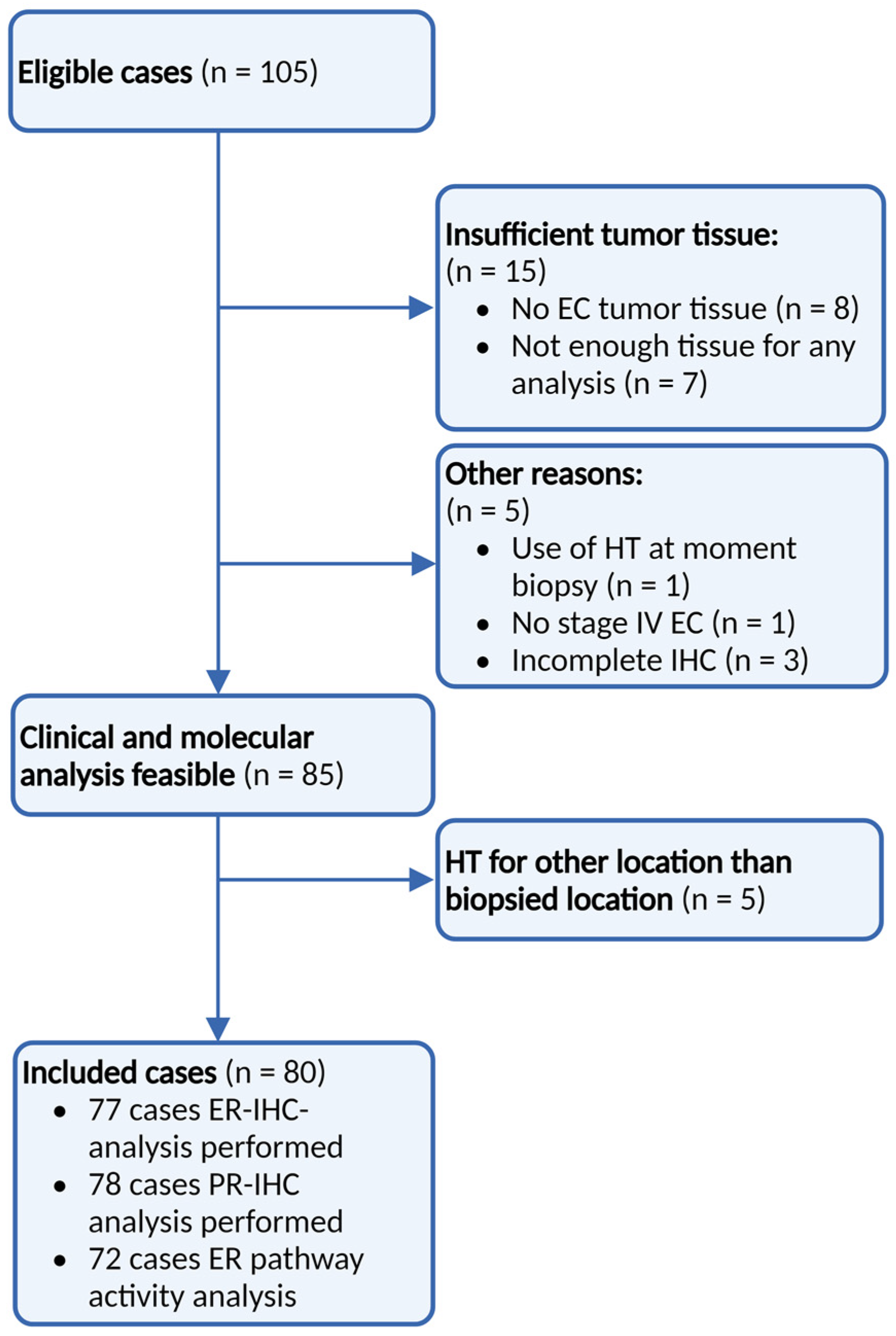
3.1. Patients
3.2. Samples
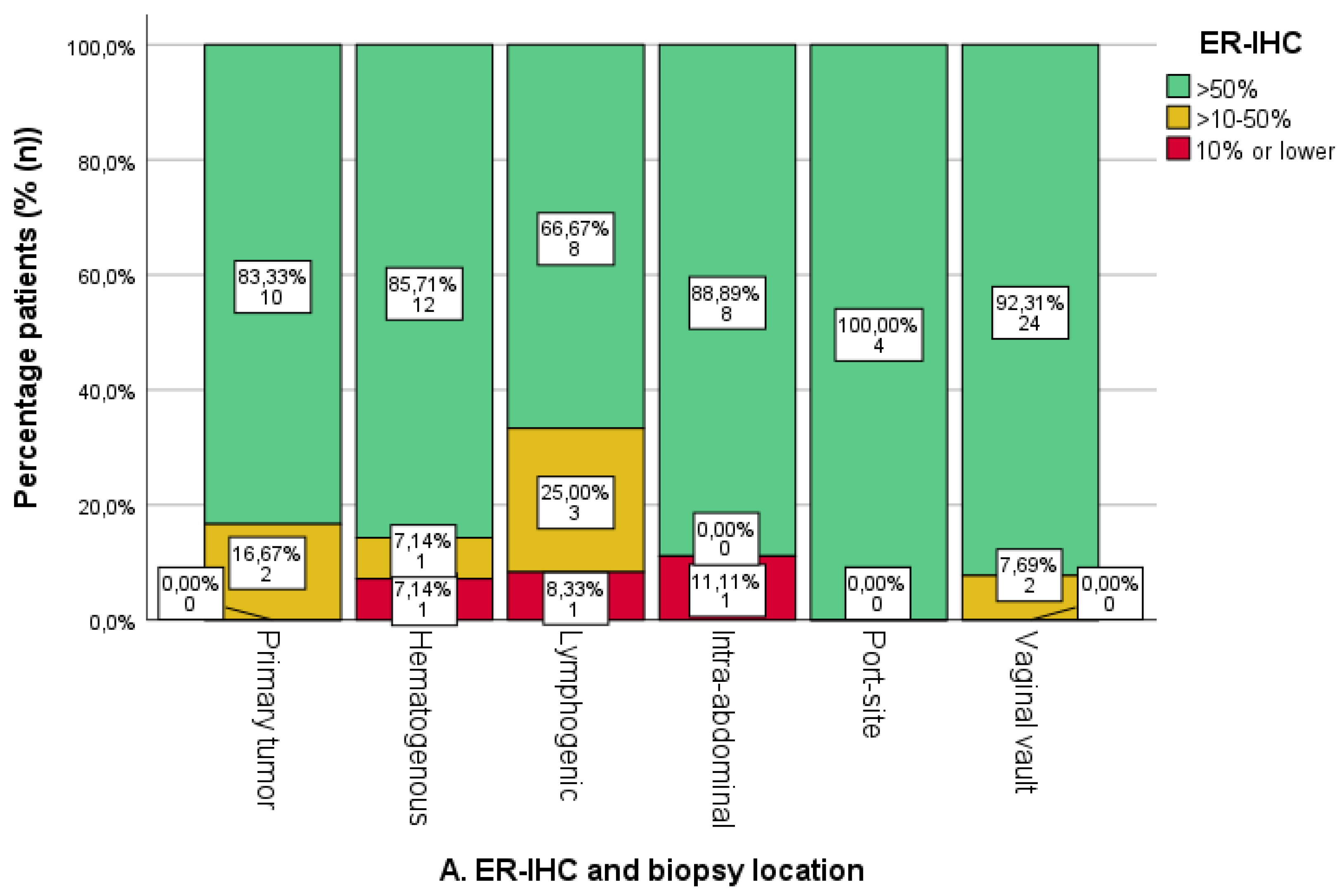
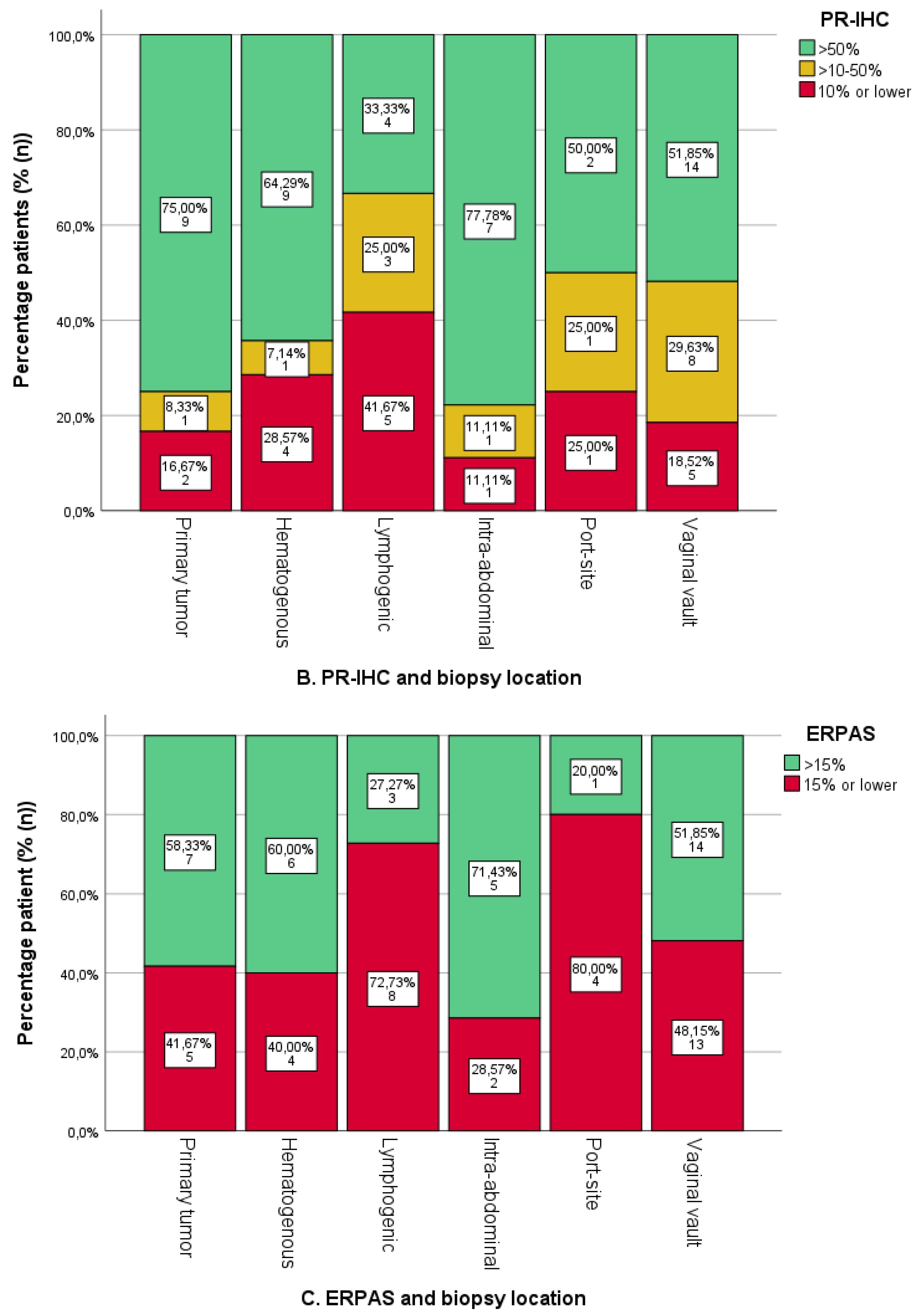
3.3. Expression of ER/PR-IHC and ER Pathway in Relation to Pattern of Spread
3.4. Expression of ER/PR-IHC and ER Pathway Activity in Relation to Tumour Grade
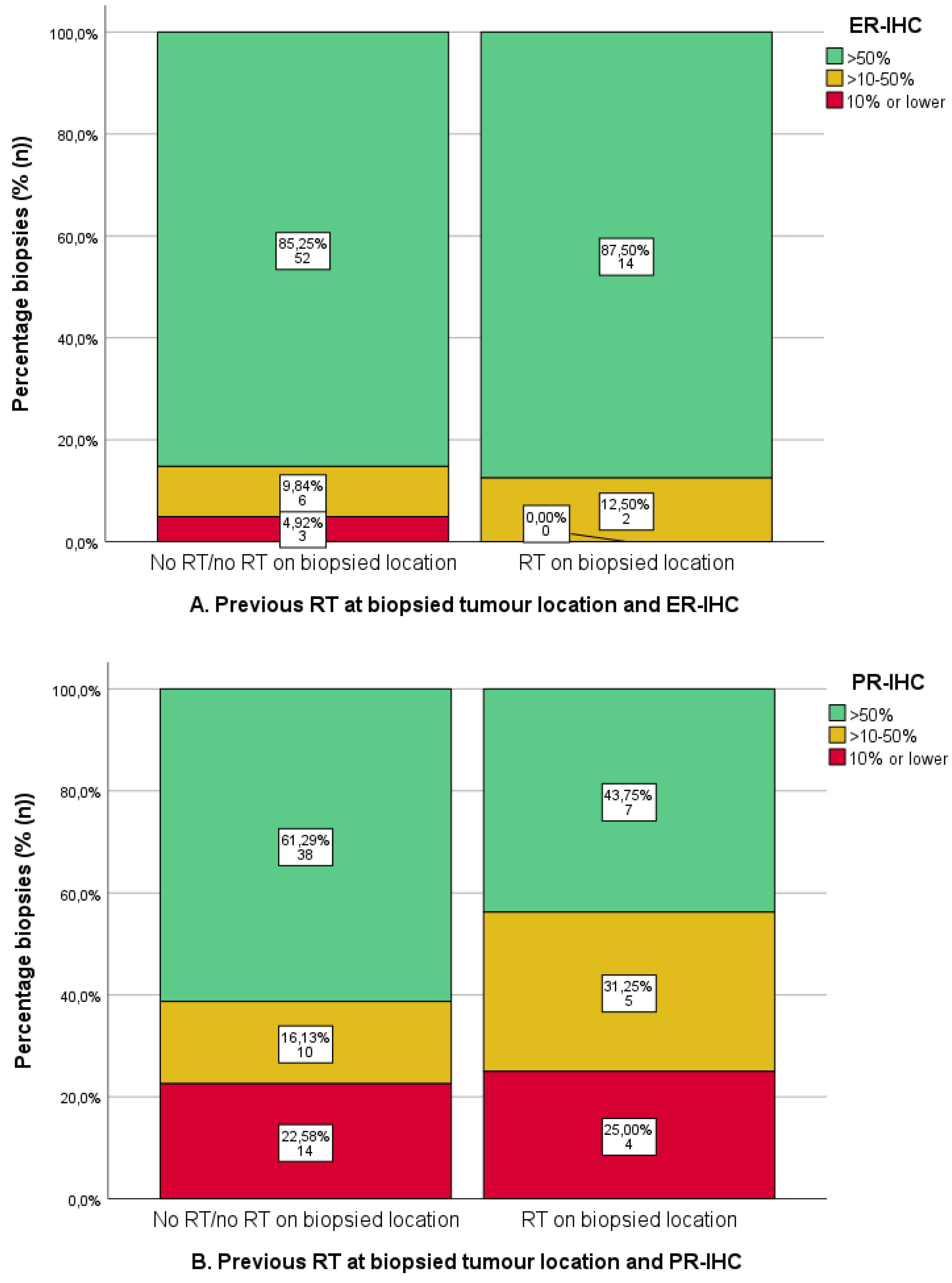
3.5. Previous Radiotherapy and IHC
4. Discussion
4.1. Metastatic Locations and Immunohistochemical Expression
4.2. Radiotherapy and ER/PR-IHC and ERPAS
4.3. Strengths and Weaknesses
4.4. Clinical and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Uterine Cancer. Surveillance, Epidemiology, and End Results Program Cancer Statistics Review 2014–2020. Available online: https://seer.cancer.gov/statfacts/html/corp.html#.Xx63P5vaNyU.link (accessed on 27 May 2024).
- Trovik, J.; Wik, E.; Werner, H.M.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- Van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef] [PubMed]
- Rütten, H.; Verhoef, C.; van Weelden, W.J.; Smits, A.; Dhanis, J.; Ottevanger, N.; Pijnenborg, J.M.A. Recurrent Endometrial Cancer: Local and Systemic Treatment Options. Cancers 2021, 13, 6275. [Google Scholar] [CrossRef]
- Tronconi, F.; Nero, C.; Giudice, E.; Salutari, V.; Musacchio, L.; Ricci, C.; Carbone, M.V.; Ghizzoni, V.; Perri, M.T.; Camarda, F.; et al. Advanced and recurrent endometrial cancer: State of the art and future perspectives. Crit. Rev. Oncol. Hematol. 2022, 180, 103851. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Radiother. Oncol. 2015, 117, 559–581. [Google Scholar] [CrossRef]
- Hoskins, P.J.; Swenerton, K.D.; Pike, J.A.; Wong, F.; Lim, P.; Acquino-Parsons, C.; Lee, N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase II study. J. Clin. Oncol. 2001, 19, 4048–4053. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Pectasides, D.; Xiros, N.; Papaxoinis, G.; Pectasides, E.; Sykiotis, C.; Koumarianou, A.; Psyrri, A.; Gaglia, A.; Kassanos, D.; Gouveris, P.; et al. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol. Oncol. 2008, 109, 250–254. [Google Scholar] [PubMed]
- Arimoto, T.; Nakagawa, S.; Yasugi, T.; Yoshikawa, H.; Kawana, K.; Yano, T.; Taketani, Y. Treatment with paclitaxel plus carboplatin, alone or with irradiation, of advanced or recurrent endometrial carcinoma. Gynecol. Oncol. 2007, 104, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Lentz, S.S.; Brady, M.F.; Major, F.J.; Reid, G.C.; Soper, J.T. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 1996, 14, 357–361. [Google Scholar] [CrossRef]
- Thigpen, J.T.; Brady, M.F.; Alvarez, R.D.; Adelson, M.D.; Homesley, H.D.; Manetta, A.; Soper, J.T.; Given, F.T. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J. Clin. Oncol. 1999, 17, 1736–1744. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.N.; Amir, E.; MacKay, H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 158–166. [Google Scholar] [CrossRef]
- Mahdi, H.; Ray-Coquard, I.; Lorusso, D.; Mirza, M.R.; Monk, B.J.; Slomovitz, B. Evolving treatment paradigms in metastatic or recurrent low-grade endometrial cancer: When is hormonal-based therapy the preferred option? Int. J. Gynecol. Cancer 2023, 33, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A.; ENITEC. Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, W.J.; Lalisang, R.I.; Bulten, J.; Lindemann, K.; van Beekhuizen, H.J.; Trum, H.; Boll, D.; Werner, H.M.J.; van Lonkhuijzen, L.R.C.W.; Yigit, R.; et al. Impact of hormonal biomarkers on response to hormonal therapy in advanced and recurrent endometrial cancer. Am. J. Obstet. Gynecol. 2021, 225, 407.e1-407.e16. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, T.; Brady, M.F.; Homesley, H.D.; Soper, J.T.; Bell, J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2001, 19, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Decruze, S.B.; Green, J.A. Hormone therapy in advanced and recurrent endometrial cancer: A systematic review. Int. J. Gynecol. Cancer. 2007, 17, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Verhaegh, W.; van Ooijen, H.; Inda, M.A.; Hatzis, P.; Versteeg, R.; Smid, M.; Martens, J.; Foekens, J.; van de Wiel, P.; Clevers, H.; et al. Selection of personalized patient therapy through the use of knowledge-based computational models that identify tumor-driving signal transduction pathways. Cancer Res. 2014, 74, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, W.J.; van der Putten, L.J.M.; Inda, M.A.; van Brussel, A.; Snijders, M.; Schriever, L.M.M.; Bulten, J.; Massuger, L.F.A.G.; van de Stolpe, A.; Pijnenborg, J.M.A. Oestrogen receptor pathway activity is associated with outcome in endometrial cancer. Br. J. Cancer 2020, 123, 785–792. [Google Scholar] [PubMed]
- Singh, M.; Zaino, R.J.; Filiaci, V.J.; Leslie, K.K. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2007, 106, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Sommeijer, D.W.; Sjoquist, K.M.; Friedlander, M. Hormonal treatment in recurrent and metastatic gynaecological cancers: A review of the current literature. Curr. Oncol. Rep. 2013, 15, 541–548. [Google Scholar] [CrossRef]
- Kokka, F.; Brockbank, E.; Oram, D.; Gallagher, C.; Bryant, A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2010, 12, CD007926. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, J.M.A.; van Weelden, W.J.; Reijnen, C.; Xanthoulea, S.; Romano, A. Redefining the Position of Hormonal Therapy in Endometrial Cancer in the Era of Molecular Classification. J. Clin. Oncol. 2024, 42, 8–12. [Google Scholar] [CrossRef]
- Soslow, R.A.; Wethington, S.L.; Cesari, M.; Chiappetta, D.; Olvera, N.; Shia, J.; Levine, D.A. Clinicopathologic analysis of matched primary and recurrent endometrial carcinoma. Am. J. Surg. Pathol. 2012, 36, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Tangen, I.L.; Werner, H.M.; Berg, A.; Halle, M.K.; Kusonmano, K.; Trovik, J.; Hoivik, E.A.; Mills, G.B.; Krakstad, C.; Salvesen, H.B. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur. J. Cancer 2014, 50, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Monteiro-Reis, S.; Vieira, R.; Pereira, A.; Rodrigues, M.; Jeronimo, C.; Lopes, J.M. Endometrial Endometrioid Carcinoma Metastases Show Decreased ER-Alpha and PR-A Expression Compared to Matched Primary Tumors. PLoS ONE 2015, 10, e0134969. [Google Scholar]
- Runowicz, C.D.; Nuchtern, L.M.; Braunstein, J.D.; Jones, J.G. Heterogeneity in hormone receptor status in primary and metastatic endometrial cancer. Gynecol. Oncol. 1990, 38, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.J.; Hoivik, E.A.; Halle, M.K.; Taylor-Weiner, A.; Cherniack, A.D.; Berg, A.; Holst, F.; Zack, T.I.; Werner, H.M.; Staby, K.M.; et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat. Genet. 2016, 48, 848–855. [Google Scholar] [PubMed]
- Slomovitz, B.M.; Filiaci, V.L.; Walker, J.L.; Taub, M.C.; Finkelstein, K.A.; Moroney, J.W.; Fleury, A.C.; Muller, C.Y.; Holman, L.L.; Copeland, L.J.; et al. A randomized phase II trial of everolimus and letrozole or hormonal therapy in women with advanced, persistent or recurrent endometrial carcinoma: A GOG Foundation study. Gynecol. Oncol. 2022, 164, 481–491. [Google Scholar]
- Kurra, V.; Krajewski, K.M.; Jagannathan, J.; Giardino, A.; Berlin, S.; Ramaiya, N. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging 2013, 13, 113–122. [Google Scholar] [CrossRef]
- FEDERA. Human Tissue and Medical Research: Code of Conduct for Responsible Use 2011. Available online: https://www.coreon.org/wp-content/uploads/2020/04/coreon-code-of-conduct-english.pdf (accessed on 31 July 2020).
- Geels, Y.P.; van der Putten, L.J.M.; van Tilborg, A.A.G.; Nienhaus, B.E.C.; van den Berg-van Erp, S.H.; Snijders, M.P.L.M.; van der Wurff, A.; Massuger, L.F.A.G.; Bulten, J.; Pijnenborg, J.M.A. Immunohistochemical Profiles of Endometrioid Endometrial Carcinomas with and Without Metastatic Disease. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 173–179. [Google Scholar] [CrossRef]
- Van der Putten, L.J.M.; Visser, N.C.M.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Added Value of Estrogen Receptor, Progesterone Receptor, and L1 Cell Adhesion Molecule Expression to Histology-Based Endometrial Carcinoma Recurrence Prediction Models: An ENITEC Collaboration Study. Int. J. Gynecol. Cancer 2018, 28, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Makker, A.; Goel, M.M. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: An update. Endocr. Relat. Cancer 2016, 23, R85–R111. [Google Scholar] [CrossRef]
- van der Horst, P.H.; Wang, Y.; Vandenput, I.; Kuhne, L.C.; Ewing, P.C.; van Ijcken, W.F.; van der Zee, M.; Amant, F.; Burger, C.W.; Blok, L.J. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS ONE 2012, 7, e30840. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.A.; Blok, E.J.; Kuppen, P.J.K.; Charehbili, A.; den Biezen-Timmermans, E.C.; van Brussel, A.; Fruytier, S.E.; Meershoek-Klein Kranenbarg, E.; Kloet, S.; van der Burg, B.; et al. Estrogen Receptor Pathway Activity Score to Predict Clinical Response or Resistance to Neoadjuvant Endocrine Therapy in Primary Breast Cancer. Mol. Cancer Ther. 2020, 19, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Pras, E.; Hollema, H.; van der Zee, A.G.; Bogchelman, D.H.; Mourits, M.J. Presentations of endometrial activity after curative radiotherapy for cervical cancer. Maturitas 2005, 51, 172–176. [Google Scholar] [CrossRef]




| Total no of Cases n = 80 | Uterine * n = 12 (15.0%) | Hematogenous n = 14 (17.5%) | Lymphogenic n = 12 (15.0%) | Intra-Abdominal n = 9 (11.3%) | Port-Site n = 5 (6.3%) | Vaginal Vault n = 28 (35.0%) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Age (CI) | 71.9 (70.0–74.1) | 73.7 (65.6–81.7) | 73.1 (67.0–79.2) | 70.8 (64.3–77.2) | 67.2 (60.2–74.2) | 71.0 (55.1–86.9) | 72.6 (69.9–75.3) | 0.650 |
| BMI (CI) | 30.4 (28.4–32.3) | 29.3 (22.4–36.2) | 32.8 (26.2–39.5) | 29.2 (25.4–32.9) | 33.5 (27.1–39.9) | 30.2 (19.0–41.3) | 28.7 (25.1–32.4) | 0.639 |
| Tumor type | ||||||||
| Recurrence | 54 (67.5) | 0 (0) | 11 (78.6) | 3 (25.0) | 7 (77.8) | 5 (100) | 28 (100) | <0.001 |
| Advanced | 26 (32.5) | 12 (100) | 3 (21.4) | 9 (75.0) | 2 (22.2) | 0 (0) | 0 (0) | |
| Grade a | ||||||||
| Grade 1–2 (%) | 61 (76.3) | 7 (58.3) | 12 (85.7) | 7 (58.3) | 7 (77.8) | 3 (60.0) | 25 (89.3) | 0.118 |
| Grade 3 (%) | 13 (16.3) | 4 (33.3) | 1 (7.1) | 4 (33.3) | 0 (0) | 1 (20.0) | 3 (10.7) | |
| Unknown (%) | 6 (7.5) | 1 (8.3) | 1 (7.1) | 1 (8.3) | 2 (22.2) | 1 (20.0) | 0 (0) | |
| Histology | ||||||||
| EEC (%) | 76 (93.8) | 11 (91.7) | 14 (100) | 10 (83.3) | 9 (100) | 5 (100) | 26 (92.9) | 0.379 |
| NEEC (%) | 4 (5.0) | 1 (8.3) | 0 (0) | 2 (16.7) | 0 (0) | 0 (0) | 1 (3.6) | |
| Mixed type (%) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.6) | |
| Previous therapy | ||||||||
| Radiotherapy | 33 (42.3) | 0 (0) | 10 (76.9) | 2 (16.7) | 2 (22.2) | 3 (75.0) | 16 (57.1) | <0.001 b |
| Chemotherapy | 6 (7.6) | 0 (0) | 0 (0) | 1 (8.3) | 2 (22.2) | 0 (0) | 3 (10.7) | 0.346 c |
| Drug type | ||||||||
| Progestin | 61 (76.3) | 9 (75.0) | 13 (92.9) | 9 (75.0) | 6 (66.7) | 2 (40.0) | 22 (78.6) | 0.622 d |
| Tamoxifen | 8 (10.0) | 1 (8.3) | 0 (0) | 2 (16.7) | 2 (22.2) | 1 (20.0) | 2 (7.1) | |
| Aromatase inhibitor | 8 (10.0) | 2 (16.7) | 1 (7.7) | 1 (8.3) | 0 (0) | 0 (0) | 4 (14.3) | |
| Unknown | 3 (3.8) | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) | 2 (40.0) | 0 (0) | |
| ER expression (mean %, SD) | 77.3 (24.8) | 78.5 (23.2) | 80.0 (29.2) | 63.8 (31.7) | 76.4 (30.0) | 83.8 (16.0) | 80.9 (17.7) | 0.556 |
| PR expression (mean %, SD) | 49.5 (33.5) | 54.2 (30.5) | 52.9 (35.1) | 34.3 (36.5) | 63.9 (28.6) | 37.5 (33.0) | 49.5 (34.0) | 0.578 |
| ERPAS (mean %, SD) | 16.2 (11.7) | 20.3 (12.1) | 16.3 (12.3) | 11.0 (8.7) | 20.7 (9.2) | 17.2 (14.6) | 15.2 (12.4) | 0.406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luijten, M.M.W.; van Weelden, W.J.; Lalisang, R.I.; Bulten, J.; Lindemann, K.; van Beekhuizen, H.J.; Trum, H.; Boll, D.; Werner, H.M.J.; van Lonkhuijzen, L.R.C.W.; et al. Hormone Receptor Expression and Activity for Different Tumour Locations in Patients with Advanced and Recurrent Endometrial Carcinoma. Cancers 2024, 16, 2084. https://doi.org/10.3390/cancers16112084
Luijten MMW, van Weelden WJ, Lalisang RI, Bulten J, Lindemann K, van Beekhuizen HJ, Trum H, Boll D, Werner HMJ, van Lonkhuijzen LRCW, et al. Hormone Receptor Expression and Activity for Different Tumour Locations in Patients with Advanced and Recurrent Endometrial Carcinoma. Cancers. 2024; 16(11):2084. https://doi.org/10.3390/cancers16112084
Chicago/Turabian StyleLuijten, Maartje M. W., Willem Jan van Weelden, Roy I. Lalisang, Johan Bulten, Kristina Lindemann, Heleen J. van Beekhuizen, Hans Trum, Dorry Boll, Henrica M. J. Werner, Luc R. C. W. van Lonkhuijzen, and et al. 2024. "Hormone Receptor Expression and Activity for Different Tumour Locations in Patients with Advanced and Recurrent Endometrial Carcinoma" Cancers 16, no. 11: 2084. https://doi.org/10.3390/cancers16112084
APA StyleLuijten, M. M. W., van Weelden, W. J., Lalisang, R. I., Bulten, J., Lindemann, K., van Beekhuizen, H. J., Trum, H., Boll, D., Werner, H. M. J., van Lonkhuijzen, L. R. C. W., Yigit, R., Krakstad, C., Witteveen, P. O., Galaal, K., van Ginkel, A. A., Bignotti, E., Weinberger, V., Sweegers, S., Eriksson, A. G. Z., ... European Network for Individualized Treatment in Endometrial Cancer. (2024). Hormone Receptor Expression and Activity for Different Tumour Locations in Patients with Advanced and Recurrent Endometrial Carcinoma. Cancers, 16(11), 2084. https://doi.org/10.3390/cancers16112084








