Current Status and Future Directions of Proton Therapy for Head and Neck Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Toxicity Reduction: Nasopharyngeal Cancer
3.2. Toxicity Reduction: Oropharyngeal Cancer
3.3. Reduced Second Primary Malignancies
4. New Insights
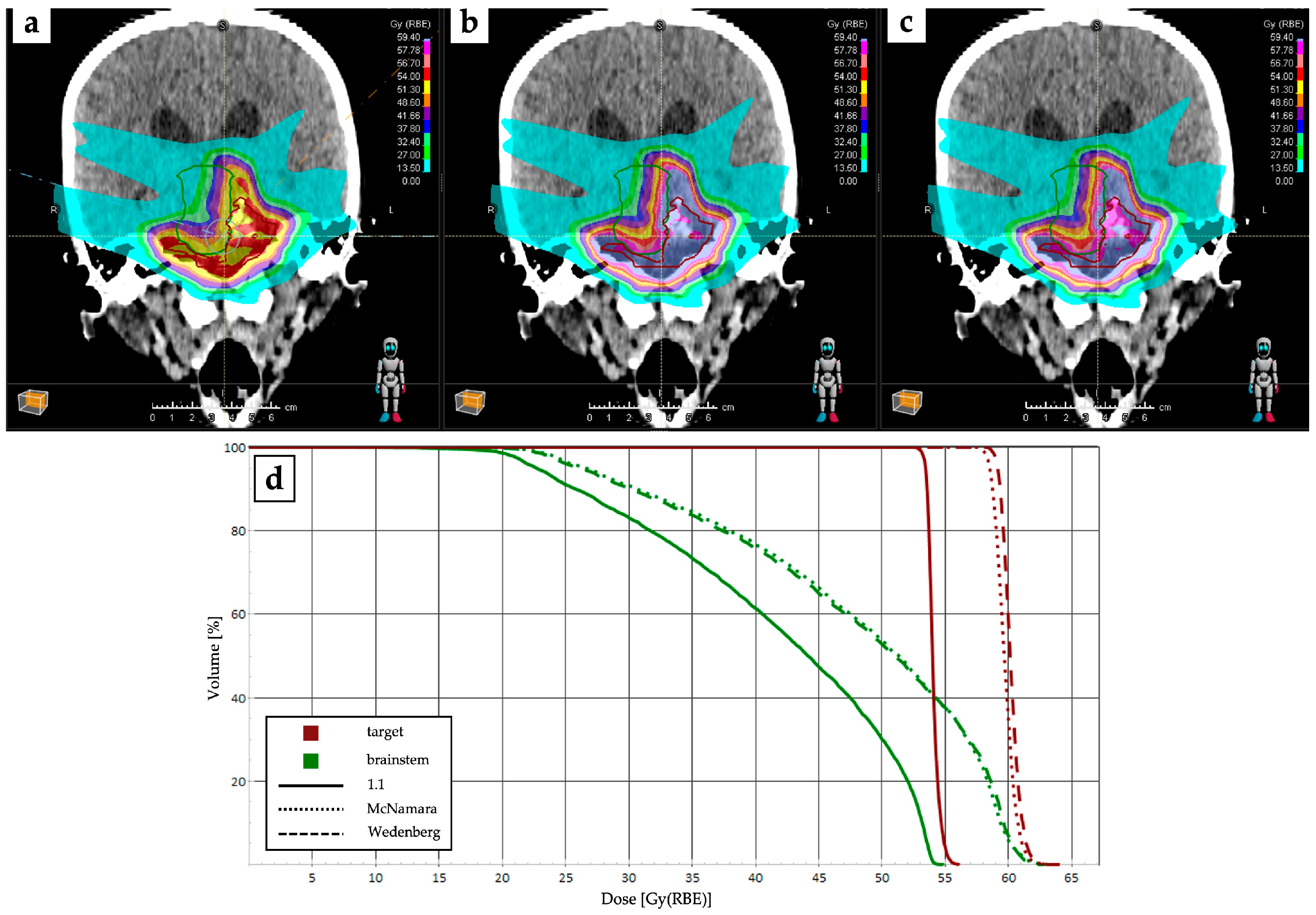
4.1. RBE and LET Optimization and Robustness Improvement
4.2. Multi-Ion Radiotherapy and Proton Minibeam Radiation Therapy
4.3. Favorable Biological Properties and Immunogenic Effects
4.4. De-Escalation Strategy
5. Limitations and Future Directions
- -
- Advancements in preclinical models, both in vitro and in vivo, are essential for unveiling the underlying mechanisms of PT and its interactions with other treatment modalities. By fostering collaborations across physics, medicine, and radiobiology, we can refine our understanding of the biological effects of protons and optimize treatment strategies.
- -
- Defining appropriate endpoints for preclinical and clinical studies is paramount for accurately assessing the effectiveness of PT. By establishing standardized endpoints, consistency across studies can be ensured and data comparison can be facilitated, ultimately driving evidence-based decision making in clinical practice.
- -
- Enhancing the methodology and quality control measures of clinical studies is essential for ensuring the validity and reliability of research findings. By standardizing protocols, implementing rigorous quality assurance procedures, and continuously monitoring data integrity, we can strengthen the credibility of PT research and foster greater confidence in its clinical utility.
- -
- Integrating advanced imaging techniques, omic sciences (e.g., proteomics, genomics, metabolomics, transcriptomics, and radiomics), and individual patients’ biomarkers into the real-time assessment of tumor response is crucial for optimizing PT delivery and detecting resistance patterns. By leveraging these tools, we can monitor treatment response more accurately, enabling timely adjustments to therapy and improving patient outcomes.
- -
- Establishing networks between hadrontherapy centers regionally, nationally, and internationally facilitates the exchange of knowledge, resources, and best practices. Through collaborative efforts, centers can streamline processes, share expertise, and collectively address the challenge of improving patient care.
- -
- Leveraging the existing hadrontherapy facilities to launch larger multinational trials targeting common cancers, including HNC. By pooling resources and collaborating, we can conduct trials with sufficient statistical power to assess the effectiveness of PT.
- -
- Collaborating with the pharmaceutical industry is crucial for identifying and prioritizing new combinations of PT with emerging therapeutic agents. By fostering partnerships, we can accelerate the development of novel treatment regimens that enhance the efficacy of protons while minimizing adverse effects.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Machiels, J.-P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V.; EHNS Executive Board; ESMO Guidelines Committee; ESTRO Executive Board. Squamous Cell Carcinoma of the Oral Cavity, Larynx, Oropharynx and Hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Chan, A.T.; Licitra, L.; Trama, A.; Orlandi, E.; Hui, E.P.; Halámková, J.; Mattheis, S.; Baujat, B.; Hardillo, J.; et al. Nasopharyngeal Carcinoma: ESMO-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wang, T.; Yang, K.; Zhang, S.; Zhang, T.; Li, Q.; Han, J.; Wu, G. A Prospective, Randomized Study Comparing Outcomes and Toxicities of Intensity-Modulated Radiotherapy vs. Conventional Two-Dimensional Radiotherapy for the Treatment of Nasopharyngeal Carcinoma. Radiother. Oncol. 2012, 104, 286–293. [Google Scholar] [CrossRef] [PubMed]
- AIOM-Registri Tumori Italiani-SIAPEC-PASSI-PASSI D’ARGENTO-ONS-Fondazione AIOM. I Numeri Del Cancro in Italia 2022. Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf (accessed on 13 April 2024).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ringash, J. Survivorship and Quality of Life in Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Locati, L.D.; Ronchi, S.; Thariat, J.; Orlandi, E. Quality of Life and Financial Toxicity after (Chemo)Radiation Therapy in Head and Neck Cancer: Are There Any Sex- or Gender-Related Differences? Tumori J. 2022, 108, 522–525. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.; Corry, J.; Ringash, J.; Rischin, D. Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Front. Oncol. 2020, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Beddok, A.; Vela, A.; Calugaru, V.; Tessonnier, T.; Kubes, J.; Dutheil, P.; Gerard, A.; Vidal, M.; Goudjil, F.; Florescu, C.; et al. Proton Therapy for Head and Neck Squamous Cell Carcinomas: A Review of the Physical and Clinical Challenges. Radiother. Oncol. 2020, 147, 30–39. [Google Scholar] [CrossRef]
- Tambas, M.; Van Der Laan, H.P.; Steenbakkers, R.J.H.M.; Doyen, J.; Timmermann, B.; Orlandi, E.; Hoyer, M.; Haustermans, K.; Georg, P.; Burnet, N.G.; et al. Current Practice in Proton Therapy Delivery in Adult Cancer Patients across Europe. Radiother. Oncol. 2022, 167, 7–13. [Google Scholar] [CrossRef]
- Gaikwad, U.; Bajpai, J.; Jalali, R. Combinatorial Approach of Immuno-proton Therapy in Cancer: Rationale and Potential Impact. Asia Pac. J. Clin. Oncol. 2023, 20, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kitpanit, S.; Chilov, M.; Langendijk, J.A.; Lu, J.; Lee, N.Y. A Systematic Review of Proton Therapy for the Management of Nasopharyngeal Cancer. Int. J. Part. Ther. 2021, 8, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kitpanit, S.; Lee, A.; Mah, D.; Sine, K.; Sherman, E.J.; Dunn, L.A.; Michel, L.S.; Fetten, J.; Zakeri, K.; et al. Toxicity Profiles and Survival Outcomes Among Patients With Nonmetastatic Nasopharyngeal Carcinoma Treated With Intensity-Modulated Proton Therapy vs Intensity-Modulated Radiation Therapy. JAMA Netw. Open 2021, 4, e2113205. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-W.; Huang, C.-C.; Lee, Y.-S.; Chou, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Huang, B.-S.; Yang, S.-W.; Huang, C.-C.; Chang, P.-H.; et al. Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy. Cancers 2022, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Holliday, E.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Beadle, B.M.; Zhu, X.R.; Zhang, X.; et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int. J. Part. Ther. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- McDonald, M.W.; Liu, Y.; Moore, M.G.; Johnstone, P.A.S. Acute Toxicity in Comprehensive Head and Neck Radiation for Nasopharynx and Paranasal Sinus Cancers: Cohort Comparison of 3D Conformal Proton Therapy and Intensity Modulated Radiation Therapy. Radiat. Oncol. 2016, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Hung, T.-M.; Huang, B.-S.; Chang, K.-P.; Kang, C.-J.; Huang, S.-F.; Chang, P.-H.; Hsu, C.-L.; et al. Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers 2021, 13, 3555. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.M.; Parvathaneni, U.; Laramore, G.E.; Aljabab, S.; Wong, T.P.; Liao, J.J. Intensity-Modulated Proton Therapy for Nasopharynx Cancer: 2-Year Outcomes from a Single Institution. Int. J. Part. Ther. 2021, 8, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Lin, C.-Y.; Huang, B.-S.; Wei, Y.-C.; Chang, T.-Y.; Yeh, C.-H.; Sung, P.-S.; Jiang, J.-L.; Lin, L.-Y.; Chang, J.T.-C.; et al. Risk of Temporal Lobe Necrosis between Proton Beam and Volumetric Modulated Arc Therapies in Patients with Different Head and Neck Cancers. Radiat. Oncol. 2023, 18, 155. [Google Scholar] [CrossRef]
- Cozzi, L.; Fogliata, A.; Lomax, A.; Bolsi, A. A Treatment Planning Comparison of 3D Conformal Therapy, Intensity Modulated Photon Therapy and Proton Therapy for Treatment of Advanced Head and Neck Tumours. Radiother. Oncol. 2001, 61, 287–297. [Google Scholar] [CrossRef]
- Van De Water, T.A.; Lomax, A.J.; Bijl, H.P.; De Jong, M.E.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Potential Benefits of Scanned Intensity-Modulated Proton Therapy Versus Advanced Photon Therapy With Regard to Sparing of the Salivary Glands in Oropharyngeal Cancer. Int. J. Radiat. Oncol. 2011, 79, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, T.A.; Lomax, A.J.; Bijl, H.P.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Using a Reduced Spot Size for Intensity-Modulated Proton Therapy Potentially Improves Salivary Gland-Sparing in Oropharyngeal Cancer. Int. J. Radiat. Oncol. 2012, 82, e313–e319. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Ye, R.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Phan, J.; Sturgis, E.M.; Ferrarotto, R.; et al. Xerostomia-Related Quality of Life for Patients with Oropharyngeal Carcinoma Treated with Proton Therapy. Radiother. Oncol. 2020, 142, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hutcheson, K.A.; Garden, A.S.; Mott, F.E.; Lu, C.; Goepfert, R.P.; Fuller, C.D.; Lai, S.Y.; Gunn, G.B.; Chambers, M.S.; et al. Determinants of Patient-reported Xerostomia among Long-term Oropharyngeal Cancer Survivors. Cancer 2021, 127, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, X.; Jiang, B.; Chen, J.; Wang, X.; Wang, L.; Sahoo, N.; Zhu, X.R.; Ye, R.; Blanchard, P.; et al. Intensity-Modulated Proton Therapy for Oropharyngeal Cancer Reduces Rates of Late Xerostomia. Radiother. Oncol. 2021, 160, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Morrison, W.H.; Hernandez, M.; Crutison, J.; Lee, J.J.; Ye, R.; Fuller, C.D.; et al. Intensity-Modulated Proton Beam Therapy (IMPT) versus Intensity-Modulated Photon Therapy (IMRT) for Patients with Oropharynx Cancer—A Case Matched Analysis. Radiother. Oncol. 2016, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Manzar, G.S.; Lester, S.C.; Routman, D.M.; Harmsen, W.S.; Petersen, M.M.; Sloan, J.A.; Mundy, D.W.; Hunzeker, A.E.; Amundson, A.C.; Anderson, J.L.; et al. Comparative Analysis of Acute Toxicities and Patient Reported Outcomes between Intensity-Modulated Proton Therapy (IMPT) and Volumetric Modulated Arc Therapy (VMAT) for the Treatment of Oropharyngeal Cancer. Radiother. Oncol. 2020, 147, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Yang, P.; Blanchard, P.; Garden, A.S.; Gunn, B.; Fuller, C.D.; Chambers, M.; Hutcheson, K.A.; Ye, R.; et al. Intensity-Modulated Proton Therapy and Osteoradionecrosis in Oropharyngeal Cancer. Radiother. Oncol. 2017, 123, 401–405. [Google Scholar] [CrossRef]
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the Jaw Following Proton Radiation Therapy for Patients With Head and Neck Cancer. JAMA Otolaryngol. Neck Surg. 2023, 149, 151. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review. Cancers 2023, 15, 2252. [Google Scholar] [CrossRef]
- Sio, T.T.; Lin, H.-K.; Shi, Q.; Gunn, G.B.; Cleeland, C.S.; Lee, J.J.; Hernandez, M.; Blanchard, P.; Thaker, N.G.; Phan, J.; et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int. J. Radiat. Oncol. 2016, 95, 1107–1114. [Google Scholar] [CrossRef]
- Sharma, S.; Zhou, O.; Thompson, R.; Gabriel, P.; Chalian, A.; Rassekh, C.; Weinstein, G.S.; O’Malley, B.W.; Aggarwal, C.; Bauml, J.; et al. Quality of Life of Postoperative Photon versus Proton Radiation Therapy for Oropharynx Cancer. Int. J. Part. Ther. 2018, 5, 11–17. [Google Scholar] [CrossRef]
- Smith, G.L.; Fu, S.; Ning, M.S.; Nguyen, D.-K.; Busse, P.M.; Foote, R.L.; Garden, A.S.; Gunn, G.B.; Fuller, C.D.; Morrison, W.H.; et al. Work Outcomes after Intensity-Modulated Proton Therapy (IMPT) versus Intensity-Modulated Photon Therapy (IMRT) for Oropharyngeal Cancer. Int. J. Part. Ther. 2021, 8, 319–327. [Google Scholar] [CrossRef]
- Ng, S.P.; Pollard, C.; Kamal, M.; Ayoub, Z.; Garden, A.S.; Bahig, H.; Gunn, G.B.; Frank, S.J.; Skinner, H.D.; Phan, J.; et al. Risk of Second Primary Malignancies in Head and Neck Cancer Patients Treated with Definitive Radiotherapy. NPJ Precis. Oncol. 2019, 3, 22. [Google Scholar] [CrossRef]
- Jain, V.; Irmen, P.; O’Reilly, S.; Vogel, J.H.; Lin, L.; Lin, A. Predicted Secondary Malignancies Following Proton versus Photon Radiation for Oropharyngeal Cancers. Int. J. Part. Ther. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second Cancer Risk after Primary Cancer Treatment with Three-Dimensional Conformal, Intensity-Modulated, or Proton Beam Radiation Therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]
- Underwood, T.S.A.; McNamara, A.L.; Appelt, A.; Haviland, J.S.; Sørensen, B.S.; Troost, E.G.C. A Systematic Review of Clinical Studies on Variable Proton Relative Biological Effectiveness (RBE). Radiother. Oncol. 2022, 175, 79–92. [Google Scholar] [CrossRef]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the Relative Biological Effectiveness of Proton Beams in Radiation Therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef]
- Niemierko, A.; Schuemann, J.; Niyazi, M.; Giantsoudi, D.; Maquilan, G.; Shih, H.A.; Paganetti, H. Brain Necrosis in Adult Patients After Proton Therapy: Is There Evidence for Dependency on Linear Energy Transfer? Int. J. Radiat. Oncol. 2021, 109, 109–119. [Google Scholar] [CrossRef]
- Fossum, C.C.; Beltran, C.J.; Whitaker, T.J.; Ma, D.J.; Foote, R.L. Biological Model for Predicting Toxicity in Head and Neck Cancer Patients Receiving Proton Therapy. Int. J. Part. Ther. 2017, 4, 18–25. [Google Scholar] [CrossRef]
- Wagenaar, D.; Schuit, E.; Van Der Schaaf, A.; Langendijk, J.A.; Both, S. Can the Mean Linear Energy Transfer of Organs Be Directly Related to Patient Toxicities for Current Head and Neck Cancer Intensity-Modulated Proton Therapy Practice? Radiother. Oncol. 2021, 165, 159–165. [Google Scholar] [CrossRef]
- Traneus, E.; Ödén, J. Introducing Proton Track-End Objectives in Intensity Modulated Proton Therapy Optimization to Reduce Linear Energy Transfer and Relative Biological Effectiveness in Critical Structures. Int. J. Radiat. Oncol. 2019, 103, 747–757. [Google Scholar] [CrossRef]
- Mou, B.; Beltran, C.J.; Park, S.S.; Olivier, K.R.; Furutani, K.M. Feasibility of Proton Transmission-Beam Stereotactic Ablative Radiotherapy versus Photon Stereotactic Ablative Radiotherapy for Lung Tumors: A Dosimetric and Feasibility Study. PLoS ONE 2014, 9, e98621. [Google Scholar] [CrossRef]
- Van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W. Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions. Cancers 2021, 13, 1859. [Google Scholar] [CrossRef]
- Van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W.F.A.R. Bringing FLASH to the Clinic: Treatment Planning Considerations for Ultrahigh Dose-Rate Proton Beams. Int. J. Radiat. Oncol. 2020, 106, 621–629. [Google Scholar] [CrossRef]
- Ebner, D.K.; Frank, S.J.; Inaniwa, T.; Yamada, S.; Shirai, T. The Emerging Potential of Multi-Ion Radiotherapy. Front. Oncol. 2021, 11, 624786. [Google Scholar] [CrossRef]
- Ortiz, R.; De Marzi, L.; Prezado, Y. Preclinical Dosimetry in Proton Minibeam Radiation Therapy: Robustness Analysis and Guidelines. Med. Phys. 2022, 49, 5551–5561. [Google Scholar] [CrossRef]
- Reaz, F.; Sitarz, M.K.; Traneus, E.; Bassler, N. Parameters for Proton Minibeam Radiotherapy Using a Clinical Scanning Beam System. Acta Oncol. 2023, 62, 1561–1565. [Google Scholar] [CrossRef]
- Girst, S.; Greubel, C.; Reindl, J.; Siebenwirth, C.; Zlobinskaya, O.; Walsh, D.W.M.; Ilicic, K.; Aichler, M.; Walch, A.; Wilkens, J.J.; et al. Proton Minibeam Radiation Therapy Reduces Side Effects in an In Vivo Mouse Ear Model. Int. J. Radiat. Oncol. 2016, 95, 234–241. [Google Scholar] [CrossRef]
- Prezado, Y.; Jouvion, G.; Guardiola, C.; Gonzalez, W.; Juchaux, M.; Bergs, J.; Nauraye, C.; Labiod, D.; De Marzi, L.; Pouzoulet, F.; et al. Tumor Control in RG2 Glioma-Bearing Rats: A Comparison Between Proton Minibeam Therapy and Standard Proton Therapy. Int. J. Radiat. Oncol. 2019, 104, 266–271. [Google Scholar] [CrossRef]
- Reaz, F.; Traneus, E.; Bassler, N. Tuning Spatially Fractionated Radiotherapy Dose Profiles Using the Moiré Effect. Sci. Rep. 2024, 14, 8468. [Google Scholar] [CrossRef]
- Ortiz, R.; Belshi, R.; De Marzi, L.; Prezado, Y. Proton Minibeam Radiation Therapy for Treating Metastases: A Treatment Plan Study. Med. Phys. 2023, 50, 2463–2473. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Grassberger, C.; Trofimov, A.; Lomax, A.; Paganetti, H. Variations in Linear Energy Transfer Within Clinical Proton Therapy Fields and the Potential for Biological Treatment Planning. Int. J. Radiat. Oncol. 2011, 80, 1559–1566. [Google Scholar] [CrossRef]
- Paganetti, H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef]
- Lupu-Plesu, M.; Claren, A.; Martial, S.; N’Diaye, P.-D.; Lebrigand, K.; Pons, N.; Ambrosetti, D.; Peyrottes, I.; Feuillade, J.; Hérault, J.; et al. Effects of Proton versus Photon Irradiation on (Lymph)Angiogenic, Inflammatory, Proliferative and Anti-Tumor Immune Responses in Head and Neck Squamous Cell Carcinoma. Oncogenesis 2017, 6, e354. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Han, S.; Zhu, J.; Li, Y.; Wang, Z.; Fan, Y.; Lin, E.; Zhang, R.; Sahoo, N.; et al. Patterns of Protein Expression in Human Head and Neck Cancer Cell Lines Differ after Proton vs Photon Radiotherapy. Head Neck 2020, 42, 289–301. [Google Scholar] [CrossRef]
- Ogata, T.; Teshima, T.; Kagawa, K.; Hishikawa, Y.; Takahashi, Y.; Kawaguchi, A.; Suzumoto, Y.; Nojima, K.; Furusawa, Y.; Matsuura, N. Particle Irradiation Suppresses Metastatic Potential of Cancer Cells. Cancer Res. 2005, 65, 113–120. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Zhu, J.; Wang, X.; Li, Y.; Wang, Z.; Lin, E.; Wang, X.; Molkentine, D.P.; Blanchard, P.; et al. Proton versus Photon Radiation–Induced Cell Death in Head and Neck Cancer Cells. Head Neck 2019, 41, 46–55. [Google Scholar] [CrossRef]
- Fang, P.; Shiraishi, Y.; Verma, V.; Jiang, W.; Song, J.; Hobbs, B.P.; Lin, S.H. Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int. J. Part. Ther. 2017, 4, 23–32. [Google Scholar] [CrossRef]
- Liu, L.-T.; Chen, Q.-Y.; Tang, L.-Q.; Guo, S.-S.; Guo, L.; Mo, H.-Y.; Chen, M.-Y.; Zhao, C.; Guo, X.; Qian, C.-N.; et al. The Prognostic Value of Treatment-Related Lymphopenia in Nasopharyngeal Carcinoma Patients. Cancer Res. Treat. 2018, 50, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Chimote, A.A.; Lehn, M.A.; Bhati, J.; Mascia, A.E.; Sertorio, M.; Lamba, M.A.; Ionascu, D.; Tang, A.L.; Langevin, S.M.; Khodoun, M.V.; et al. Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma. Cancers 2024, 16, 1008. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Formenti, S. Harnessing Radiation to Improve Immunotherapy: Better with Particles? Br. J. Radiol. 2020, 93, 20190224. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Malamas, A.S.; Bernstein, M.B.; Tsang, K.Y.; Vassantachart, A.; Sahoo, N.; Tailor, R.; Pidikiti, R.; Guha, C.P.; Hahn, S.M.; et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell–Mediated Killing. Int. J. Radiat. Oncol. 2016, 95, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Licitra, L. The Day after De-ESCALaTE and RTOG 1016 Trials Results. Future Oncol. 2019, 15, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Prisman, E.; Berthelet, E.; Tran, E.; Hamilton, S.; Wu, J.; Eskander, A.; Higgins, K.; Karam, I.; Poon, I.; et al. Assessment of Toxic Effects and Survival in Treatment Deescalation With Radiotherapy vs Transoral Surgery for HPV-Associated Oropharyngeal Squamous Cell Carcinoma: The ORATOR2 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov (accessed on 13 April 2024).
- Jellema, A.P.; Slotman, B.J.; Doornaert, P.; Leemans, C.R.; Langendijk, J.A. Unilateral versus Bilateral Irradiation in Squamous Cell Head and Neck Cancer in Relation to Patient-Rated Xerostomia and Sticky Saliva. Radiother. Oncol. 2007, 85, 83–89. [Google Scholar] [CrossRef]
- Jensen, K.; Overgaard, M.; Grau, C. Morbidity after Ipsilateral Radiotherapy for Oropharyngeal Cancer. Radiother. Oncol. 2007, 85, 90–97. [Google Scholar] [CrossRef]
- Razavian, N.B.; D’Agostino, R.B.; Steber, C.R.; Helis, C.A.; Hughes, R.T. Association of Unilateral Radiotherapy With Contralateral Lymph Node Failure Among Patients With Squamous Cell Carcinoma of the Tonsil: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2255209. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton Beam Radiation Therapy Results in Significantly Reduced Toxicity Compared with Intensity-Modulated Radiation Therapy for Head and Neck Tumors That Require Ipsilateral Radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Press, R.H.; Bakst, R.L.; Sharma, S.; Kabarriti, R.; Garg, M.K.; Yeh, B.; Gelbum, D.Y.; Hasan, S.; Choi, J.I.; Barker, C.A.; et al. Clinical Review of Proton Therapy in the Treatment of Unilateral Head and Neck Cancers. Int. J. Part. Ther. 2021, 8, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Mumaw, D.A.; Hazy, A.J.; Vayntraub, A.; Quinn, T.J.; Salari, K.; Chang, J.H.; Kalman, N.; Katz, S.; Urbanic, J.; Press, R.H.; et al. Low Contralateral Failure Rate with Unilateral Proton Beam Radiotherapy for Oropharyngeal Squamous Cell Carcinoma: A Multi-Institutional Prospective Study from the Proton Collaborative Group. Radiother. Oncol. 2024, 190, 109977. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.C.; Tham, I.W.; Earnest, A.; Lee, K.M.; Lu, J.J. Patterns of Regional Lymph Node Metastasis of Nasopharyngeal Carcinoma: A Meta-Analysis of Clinical Evidence. BMC Cancer 2012, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, L.; Sun, Y.; Mao, Y.; Li, W.; Guo, R.; Liu, L.; Li, L.; Lin, A.; Ma, J. Treatment Outcomes and Feasibility of Partial Neck Irradiation for Patients with Nasopharyngeal Carcinoma with Only Retropharyngeal Lymph Node Metastasis after Intensity-modulated Radiotherapy. Head Neck 2014, 36, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, G.; Guan, X.; Wang, X.; Hu, C. The Feasibility of Omitting Irradiation to the Contralateral Lower Neck in Stage N1 Nasopharyngeal Carcinoma Patients. Radiat. Oncol. 2013, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-L.; Huang, C.-L.; Zhang, N.; Jiang, W.; Wu, Y.-S.; Huang, S.H.; Mao, Y.-P.; Liu, Q.; Li, J.-B.; Liang, S.-Q.; et al. Elective Upper-Neck versus Whole-Neck Irradiation of the Uninvolved Neck in Patients with Nasopharyngeal Carcinoma: An Open-Label, Non-Inferiority, Multicentre, Randomised Phase 3 Trial. Lancet Oncol. 2022, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Marchetti, C.; Serpone, M.; Camarda, A.; Vischioni, B.; Ingargiola, R.; Musio, D.; Orlandi, E. Upper-Neck Irradiation versus Standard Whole-Neck Irradiation in Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Tumori J. 2023, 109, 529–536. [Google Scholar] [CrossRef]
- De Felice, F.; Vai, A.; Camarda, A.M.; Iacovelli, N.A.; Orlandi, E. Lower-Neck Sparing Using Proton Therapy in Patients with Uninvolved Neck Nasopharyngeal Carcinoma: Is It Safe? J. Clin. Med. 2022, 11, 3297. [Google Scholar] [CrossRef]
- Ning, M.S.; Gomez, D.R.; Shah, A.K.; Kim, C.R.; Palmer, M.B.; Thaker, N.G.; Grosshans, D.R.; Liao, Z.; Chapman, B.V.; Brooks, E.D.; et al. The Insurance Approval Process for Proton Radiation Therapy: A Significant Barrier to Patient Care. Int. J. Radiat. Oncol. 2019, 104, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Lin, K.-C.; Chen, W.-M.; Shia, B.-C.; Wu, S.-Y. Comparing the Oncologic Outcomes of Proton Therapy and Intensity-Modulated Radiation Therapy for Head and Neck Squamous Cell Carcinoma. Radiother. Oncol. 2024, 190, 109971. [Google Scholar] [CrossRef]
- Verma, V.; Mishra, M.V.; Mehta, M.P. A Systematic Review of the Cost and Cost-effectiveness Studies of Proton Radiotherapy. Cancer 2016, 122, 1483–1501. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.J.; Tishler, R.B.; Pham, N.-L.; Punglia, R.S. Cost-Effectiveness Analysis of Intensity Modulated Radiation Therapy Versus Proton Therapy for Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2018, 101, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, R.P.; Ms, A.; Kamath, A. A Systematic Review of the Economic Burden of Proton Therapy in Head and Neck Cancer. Asian Pac. J. Cancer Prev. 2023, 24, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xia, Y.; Huang, Y.; Okat, D.; Qiu, B.; Doyen, J.; Bondiau, P.; Benezery, K.; Gao, J.; Qian, C. Intensity-modulated Proton Radiation Therapy as a Radical Treatment Modality for Nasopharyngeal Carcinoma in China: Cost-effectiveness Analysis. Head Neck 2022, 44, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kang, J.; Yu, Y.; McBride, S.; Riaz, N.; Cohen, M.; Sherman, E.; Michel, L.; Lee, N.; Tsai, C.J. Trends and Disparities of Proton Therapy Use among Patients with Head and Neck Cancer: Analysis from the National Cancer Database (2005–14). Int. J. Part. Ther. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCall, N.S.; Liu, Y.; Janopaul-Naylor, J.; Bates, J.E.; Remick, J.S.; Rudra, S.; Karam, S.D.; Amini, A.; McDonald, M.W.; Stokes, W.A. Standard but Not Equal: Disparities in Advanced Radiotherapy Techniques for Head and Neck Cancer in the United States. Int. J. Radiat. Oncol. 2022, 112, e11–e12. [Google Scholar] [CrossRef]
- McDonald, M.W.; Bates, J.E.; McCall, N.S.; Goyal, S.; Liu, Y.; Rudra, S.; Remick, J.S.; Tian, S.; El-Deiry, M.W.; Saba, N.F.; et al. Insurance Authorization and Access to Proton Therapy for Patients With Head and Neck Cancers. Int. J. Radiat. Oncol. 2023, 116, 404–412. [Google Scholar] [CrossRef]
| Acronym/ClinicalTrials.gov ID | Country | Study Population | Intervention | Primary Endpoints | Secondary Endpoints | Study Type | Study Start |
|---|---|---|---|---|---|---|---|
| NCT01893307 | United States | Stage III–IVB oropharyngeal squamous cell carcinoma (AJCC v7) | IMRT vs. IMPT with concurrent chemotherapy | -Grade 3–5 late toxicity (CTCAE v4.0) -3-year PFS | Disease-related and patient-reported outcomes (including QALY and cost–benefit economic analysis) | Randomized phase II/III trial | August 2013 |
| NCT02923570 | United States | HNSCC requiring ipsilateral radiation, salivary gland cancer, skin cancer, and melanoma | Standard dose of 60–66 Gy of IMRT vs. PT | Grade ≥ 2 acute mucositis (CTCAE v4.0) | n.a. | Randomized phase II trial | October 2016 |
| ARTSCAN V/NCT03829033 | Sweden | Early squamous cell carcinoma of the tonsil | Photon RT vs. PT | Acute and late toxicity (CTCAE v4.0) | n.a. | Randomized phase II trial | January 2019 |
| NCT04528394 | China | HNSCC (nasopharynx) | Photon RT combined with CIRT vs. PT combined with CIRT< | Grade ≥ 2 xerostomia (CTCAE v4.03) | -OS, PFS, LRC -acute and late toxicities (CTCAE v4.03) | Randomized phase II trial | April 2019 |
| TORPEdO | United Kingdom | HNSCC (locally advanced oropharynx) | 70 Gy/56 Gy in 33 fractions using an SIB technique of IMRT vs. IMPT with concurrent chemotherapy | -UW-QoL v4.0 -gastrostomy dependence or grade 3 weight loss (CTCAE v5.0) | -validate a NTCP model -pattern of health-related quality of life -tube feeding status -weight loss >10% from baseline -acute and late toxicity (CTCAE v5.0) -clinician-rated swallowing function assessment -PSS-HN -LRC, OS -cost-effectiveness | Phase III, multicenter, open-label, randomized controlled trial | February 2020 |
| DAHANCA 35/NCT04607694 | Denmark | HNSCC (pharynx or larynx) | 66–68 Gy in 33–34 fractions of photon RT vs. PT with concurrent chemotherapy | -Grade ≥ 2 late dysphagia (DAHANCA score) -Grade ≥ 4 xerostomia (EORTC QLQ-HN35) | -LRC, OS, DFS, DSS -acute and late toxicity -EORTC QLQ-C30, MD Anderson Dysphagia Index, EQ-5D | Two parallel randomized studies | October 2020 |
| PRO-IMMUNO/NCT06016699 | Netherlands | HNSCC | Photon RT vs. PT with concurrent chemotherapy | Difference in antigen-specific immunity | -Differences in composition and function of circulating immune cells -Immune infiltrate composition within the primary tumor tissue | Observational | September 2021 |
| HYDRA/NCT05364411 | Netherlands | HNSCC | Mean dose of 59 Gy in 20 fractions of photon RT vs. PT | Grade 3–4 late toxicity (CTCAE v5.0) | -Objective response (RECIST v1.1) -In-field and nodal elective field tumor control -Immune profile | Two parallel non-comparative phase-I trials | October 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lillo, S.; Mirandola, A.; Vai, A.; Camarda, A.M.; Ronchi, S.; Bonora, M.; Ingargiola, R.; Vischioni, B.; Orlandi, E. Current Status and Future Directions of Proton Therapy for Head and Neck Carcinoma. Cancers 2024, 16, 2085. https://doi.org/10.3390/cancers16112085
Lillo S, Mirandola A, Vai A, Camarda AM, Ronchi S, Bonora M, Ingargiola R, Vischioni B, Orlandi E. Current Status and Future Directions of Proton Therapy for Head and Neck Carcinoma. Cancers. 2024; 16(11):2085. https://doi.org/10.3390/cancers16112085
Chicago/Turabian StyleLillo, Sara, Alfredo Mirandola, Alessandro Vai, Anna Maria Camarda, Sara Ronchi, Maria Bonora, Rossana Ingargiola, Barbara Vischioni, and Ester Orlandi. 2024. "Current Status and Future Directions of Proton Therapy for Head and Neck Carcinoma" Cancers 16, no. 11: 2085. https://doi.org/10.3390/cancers16112085
APA StyleLillo, S., Mirandola, A., Vai, A., Camarda, A. M., Ronchi, S., Bonora, M., Ingargiola, R., Vischioni, B., & Orlandi, E. (2024). Current Status and Future Directions of Proton Therapy for Head and Neck Carcinoma. Cancers, 16(11), 2085. https://doi.org/10.3390/cancers16112085






