Adjuvant Therapy with Immune Checkpoint Inhibitors after Carbon Ion Radiotherapy for Mucosal Melanoma of the Head and Neck: A Case-Control Study
Abstract
Simple Summary
Abstract
1. Introduction
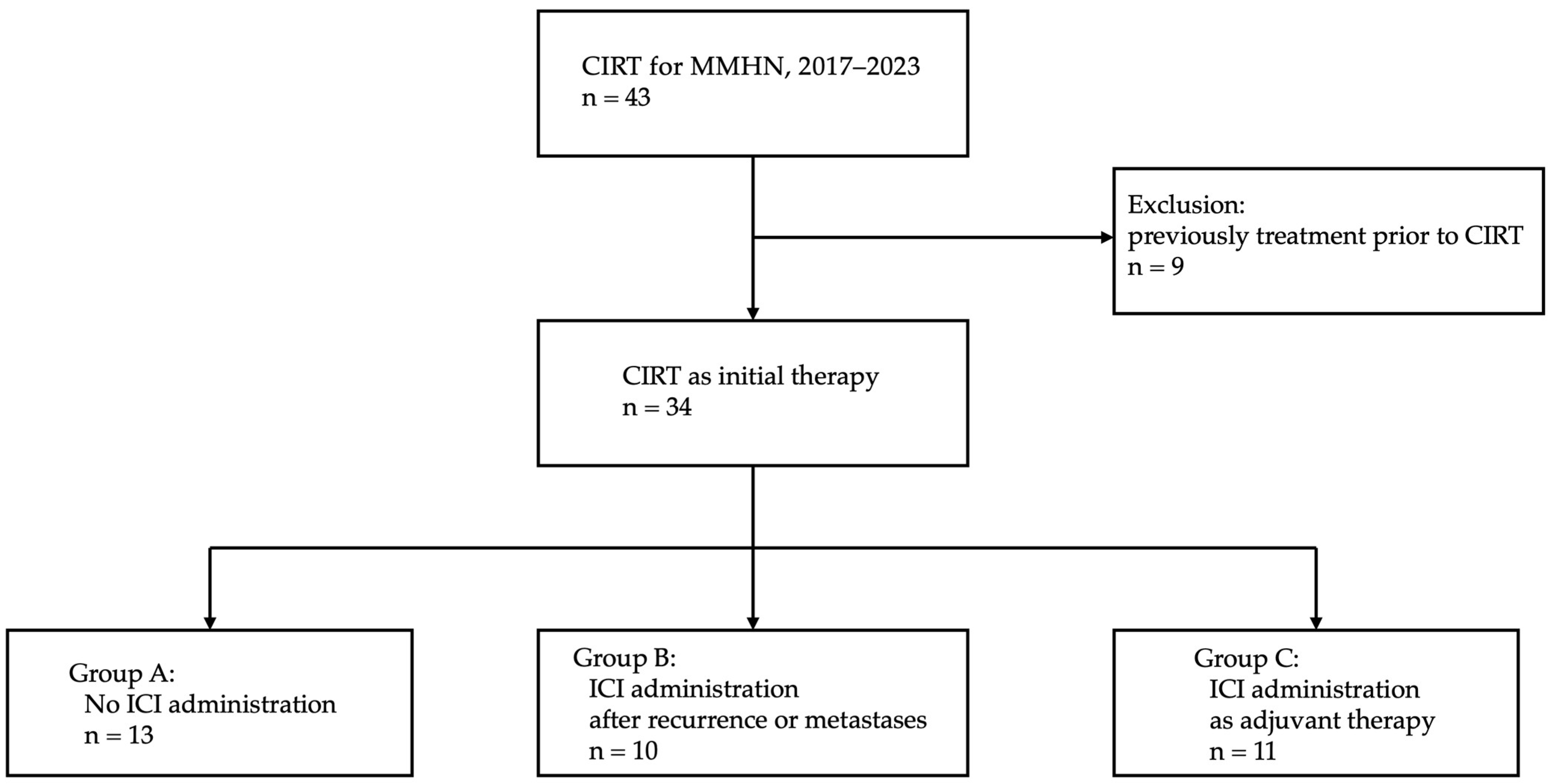
2. Materials and Methods
2.1. Patients and Methods
2.2. CIRT
2.3. ICI Therapy
2.4. Evaluation
2.5. QOL Analyses
2.6. Cost Parameters
2.7. Statistical Analysis
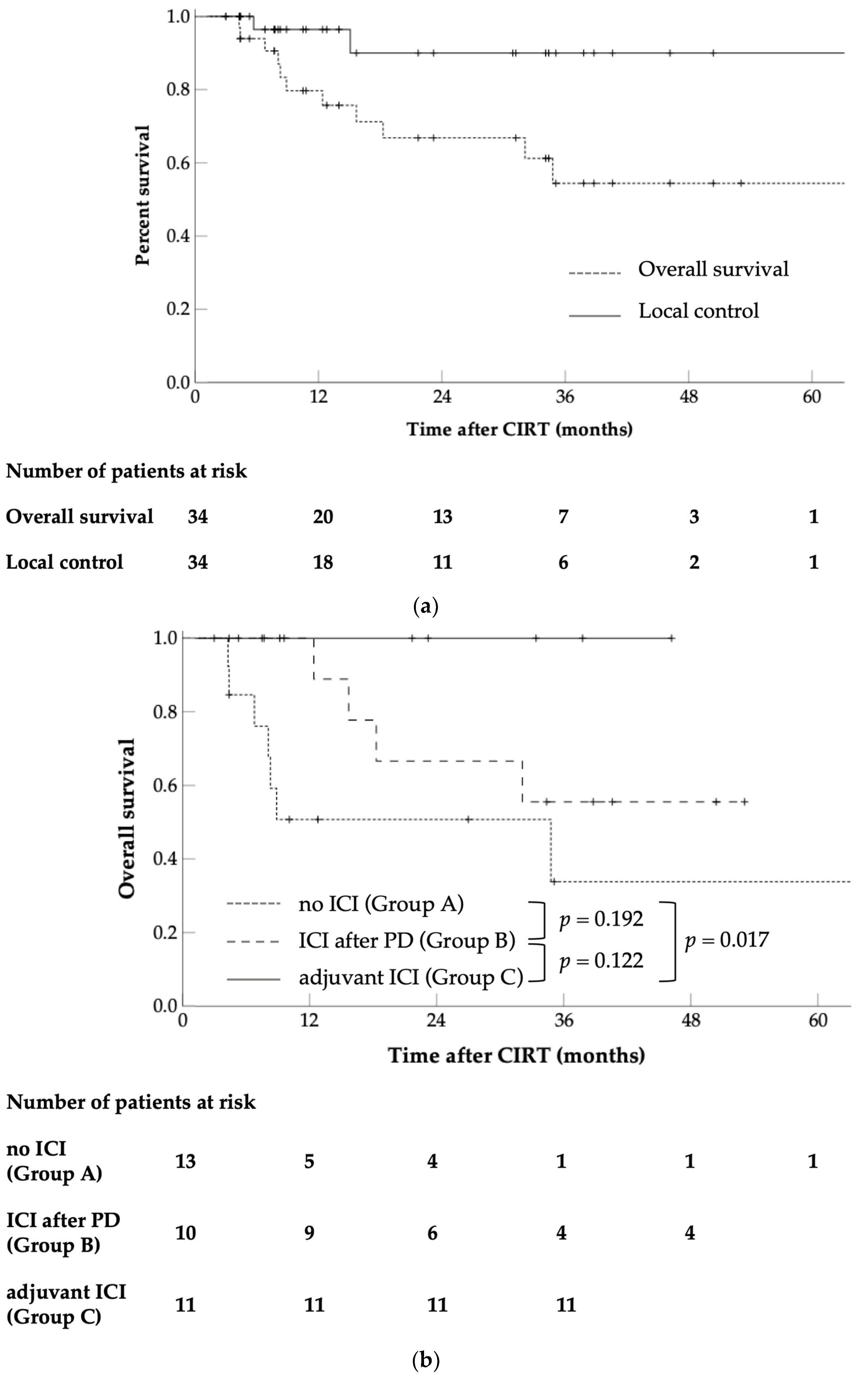
3. Results
3.1. OS
3.2. LC
3.3. DMFS and PFS
3.4. Univariate Analysis of OS
3.5. Acute and Late Adverse Events
3.6. QOL Analysis
3.7. Cost-Effectiveness Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal melanoma: A literature review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P.; et al. mucosal melanoma of the head and neck. Crit. Rev. Oncol. Hematol. 2017, 112, 136–152. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Lourenco, S.V.; Fernandes, J.D.; Hsieh, R.; Coutinho-Camillo, C.M.; Bologna, S.; Sangueza, M.; Nico, M.M. Head and neck mucosal melanoma: A review. Am. J. Dermatopathol. 2014, 36, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.; Gupta, V.; Hu, K.; Harrison, L.B.; Bakst, R. Mucosal melanoma of the head and neck: A systematic review of the literature. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Marcus, D.M.; Marcus, R.P.; Prabhu, R.S.; Owonikoko, T.K.; Lawson, D.H.; Switchenko, J.; Beitler, J.J. Rising incidence of mucosal melanoma of the head and neck in the United States. J. Skin Cancer 2012, 2012, 231693. [Google Scholar] [CrossRef] [PubMed]
- Jethanamest, D.; Vila, P.M.; Sikora, A.G.; Morris, L.G. Predictors of survival in mucosal melanoma of the head and neck. Ann. Surg. Oncol. 2011, 18, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Rodrigo, J.P.; Cardesa, A.; Triantafyllou, A.; Devaney, K.O.; Mendenhall, W.M.; Haigentz, M., Jr.; Strojan, P.; Pellitteri, P.K.; Bradford, C.R.; et al. Update on primary head and neck mucosal melanoma. Head Neck 2016, 38, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Levyn, H.; Valero, C.; Adilbay, D.; Eagan, A.; Zheng, J.; Gonen, M.; Cohen, M.; Patel, S.; Ganly, I.; et al. Skull base surgery for malignant tumors: The 2nd international collaborative study (1995–2015). Head Neck 2024. [Google Scholar] [CrossRef]
- Nenclares, P.; Ap Dafydd, D.; Bagwan, I.; Begg, D.; Kerawala, C.; King, E.; Lingley, K.; Paleri, V.; Paterson, G.; Payne, M.; et al. Head and neck mucosal melanoma: The United Kingdom national guidelines. Eur. J. Cancer 2020, 138, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guidelines, N. Head and Neck Cancers Version 3.2024. Available online: https://www.nccn.org (accessed on 18 May 2024).
- Owens, J.M.; Roberts, D.B.; Myers, J.N. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Temam, S.; Mamelle, G.; Marandas, P.; Wibault, P.; Avril, M.F.; Janot, F.; Julieron, M.; Schwaab, G.; Luboinski, B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005, 103, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Meleti, M.; Leemans, C.R.; De Bree, R.; Vescovi, P.; Sesenna, E.; Van Der Waal, I. Head and neck mucosal melanoma: Experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck 2008, 30, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Roberts, D.B.; Kupferman, M.E.; Demonte, F.; El-Naggar, A.K.; Williams, M.; Rosenthal, D.S.; Hanna, E.Y. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson cancer center. Cancer 2010, 116, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Benlyazid, A.; Thariat, J.; Temam, S.; Malard, O.; Florescu, C.; Choussy, O.; Makeieff, M.; Poissonnet, G.; Penel, N.; Righini, C.; et al. Postoperative radiotherapy in head and neck mucosal melanoma: A GETTEC study. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, X.; Gao, L.; Zhang, Y.; Luo, J.; Zhang, S.; Wang, K.; Qu, Y.; Wu, R.; Liu, Q.; et al. Long-term treatment outcomes and prognosis of mucosal melanoma of the head and neck: 161 cases from a single institution. Oral Oncol. 2017, 74, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gal, T.J.; Silver, N.; Huang, B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope 2011, 121, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Ziai, H.; Cardemil, F.; Huang, S.H.; Su, J.; Goldstein, D.P.; Gilbert, R.; Hosni, A.; Hope, A.; Waldron, J.; et al. Importance of margins, radiotherapy, and systemic therapy in mucosal melanoma of the head and neck. Laryngoscope 2021, 131, 2269–2276. [Google Scholar] [CrossRef]
- Torabi, S.J.; Benchetrit, L.; Spock, T.; Cheraghlou, S.; Judson, B.L. Clinically node-negative head and neck mucosal melanoma: An analysis of current treatment guidelines & outcomes. Oral. Oncol. 2019, 92, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Tam, S.; Abdelmeguid, A.S.; Kupferman, M.E.; Su, S.Y.; Raza, S.M.; DeMonte, F.; Hanna, E.Y. Patterns of treatment failure in patients with sinonasal mucosal melanoma. Ann. Surg. Oncol. 2018, 25, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Fernandez, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 5-year efficacy and biomarker results from CheckMate 238. Clin. Cancer Res. 2023, 29, 3352–3361. [Google Scholar] [CrossRef]
- Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Nemoto, K.; et al. Multicenter study of carbon-ion radiation therapy for mucosal melanoma of the head and neck: Subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Shiroiwa, T.; Ikeda, S.; Noto, S.; Igarashi, A.; Fukuda, T.; Saito, S.; Shimozuma, K. Comparison of value set based on DCE and/or TTO data: Scoring for EQ-5D-5L health states in Japan. Value Health 2016, 19, 648–654. [Google Scholar] [CrossRef]
- van Hout, B.; Janssen, M.F.; Feng, Y.S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life. Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Krengli, M.; Jereczek-Fossa, B.A.; Kaanders, J.H.; Masini, L.; Beldi, D.; Orecchia, R. What is the role of radiotherapy in the treatment of mucosal melanoma of the head and neck? Crit. Rev. Oncol. Hematol. 2008, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Shimizu, K.T.; Tran, L.M.; Juillard, G.; Calcaterra, T.C. Mucosal melanoma of the head and neck: The impact of local control on survival. Laryngoscope 1994, 104, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Smart, A.C.; Giobbie-Hurder, A.; Desai, V.; Xing, J.L.; Lukens, J.N.; Taunk, N.K.; Sullivan, R.J.; Mooradian, M.J.; Hsu, C.C.; Buchbinder, E.I.; et al. Multicenter evaluation of radiation and immune checkpoint inhibitor therapy in mucosal melanoma and review of recent literature. Adv. Radiat. Oncol. 2024, 9, 101310. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.K.; McKeown, J.; Grover, P.; Johnson, D.B.; Zaremba, A.; Dimitriou, F.; Weiser, R.; Farid, M.; Namikawa, K.; Sullivan, R.J.; et al. Outcomes of patients with resected stage III/IV acral or mucosal melanoma, treated with adjuvant anti-PD-1 based therapy. Eur. J. Cancer 2024, 199, 113563. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Takahashi, Y.; Turri-Zanoni, M.; Ferrari, M.; Liu, J.; Counsell, N.; Mattavelli, D.; Rampinelli, V.; Vermi, W.; Lombardi, D.; et al. International multicenter study of clinical outcomes of sinonasal melanoma shows survival benefit for patients treated with immune checkpoint inhibitors and potential improvements to the current TNM staging system. J. Neurol. Surg. B Skull Base 2023, 84, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Teterycz, P.; Czarnecka, A.M.; Indini, A.; Spalek, M.J.; Labianca, A.; Rogala, P.; Cybulska-Stopa, B.; Quaglino, P.; Ricardi, U.; Badellino, S.; et al. Multimodal treatment of advanced mucosal melanoma in the era of modern immunotherapy. Cancers 2020, 12, 3131. [Google Scholar] [CrossRef] [PubMed]
- Nenclares, P.; Harrington, K.J. Management of head and neck mucosal melanoma. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Wahl, A.; Tveit, K.M.; Monge, O.R.; Brustad, T. Survival curves after X-ray and heat treatments for melanoma cells derived directly from surgical specimens of tumours in man. Radiother. Oncol. 1985, 4, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Barranco, S.C.; Romsdahl, M.M.; Humphrey, R.M. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971, 31, 830–833. [Google Scholar] [PubMed]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Working Group for Pancreas, C. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kamada, T.; Ebner, D.K.; Shinoto, M.; Terashima, K.; Isozaki, Y.; Yasuda, S.; Makishima, H.; Tsuji, H.; Tsujii, H.; et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.S.; Demizu, Y.; Koto, M.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Ohno, T.; Shioyama, Y.; Okimoto, T.; Daimon, T.; et al. Multicenter study of carbon-ion radiation therapy for adenoid cystic carcinoma of the head and neck: Subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.R.; Lehrer, E.J.; Hsieh, K.; Hotca, A.; Jones, B.M.; Powers, A.; Sharma, S.; Liu, J.; Gupta, V.; Mell, L.; et al. Management of older adults with locally advanced head and neck cancer. Cancers 2022, 14, 2809. [Google Scholar] [CrossRef] [PubMed]
- Sprave, T.; Gkika, E.; Verma, V.; Grosu, A.L.; Stoian, R. Patient reported outcomes based on EQ-5D-5L questionnaires in head and neck cancer patients: A real-world study. BMC Cancer 2022, 22, 1236. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.; Smit, L.; Grunhagen, D.J.; Verhoef, C.; Sleijfer, S.; van der Veldt, A.A.M.; Uyl-de Groot, C.A. Cost-effectiveness of adjuvant systemic therapies for patients with high-risk melanoma in Europe: A model-based economic evaluation. ESMO Open 2021, 6, 100303. [Google Scholar] [CrossRef] [PubMed]
- Mojtahed, S.A.; Boyer, N.R.; Rao, S.A.; Gajewski, T.F.; Tseng, J.; Turaga, K.K. Cost-effectiveness analysis of adjuvant therapy for BRAF-mutant resected stage III melanoma in Medicare patients. Ann. Surg. Oncol. 2021, 28, 9039–9047. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Exellence-NICE Health Technology Evaluations: The Manual. Available online: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed on 15 July 2024).
- Vanness, D.J.; Lomas, J.; Ahn, H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann. Intern. Med. 2021, 174, 25–32. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Report: 2002, Reducing Risks, Promoting Healthy Life. 2002. Available online: https://www.who.int/publications/i/item/9241562072 (accessed on 15 July 2024).
- Department of Economic and Social Affairs, United Nations. National Accounts-Analysis of Main Aggregates (AMA). Available online: https://unstats.un.org/UNSDWebsite/ (accessed on 15 July 2024).
- UMIN-CTR Clinical Trial. Available online: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000048210 (accessed on 15 July 2024).
| All Cases | Group A * | Group B * | Group C * | p Value | ||
|---|---|---|---|---|---|---|
| 34 | 13 | 10 | 11 | |||
| Age (years) | Median (range) | 72 (49–94) | 82 (54–94) | 71 (55–79) | 68 (49–83) | 0.009 |
| Sex | Male | 11 | 4 | 4 | 3 | 0.814 |
| Female | 23 | 9 | 6 | 8 | ||
| ECOG PS | 0 | 11 | 7 | 3 | 1 | 0.164 |
| 1 | 22 | 6 | 6 | 10 | ||
| 2 | 1 | 0 | 1 | 0 | ||
| Tumor location | Nasal cavity | 24 | 10 | 7 | 7 | 0.614 |
| Paranasal | 8 | 2 | 3 | 3 | ||
| Oral cavity | 1 | 1 | 0 | 0 | ||
| Pharynx | 1 | 0 | 0 | 1 | ||
| Tumor stage (UICC 8th) | T3 | 10 | 4 | 2 | 4 | 0.707 |
| T4 | 24 | 9 | 8 | 7 | ||
| Nodal stage (UICC 8th) | N0 | 32 | 12 | 10 | 10 | 0.636 |
| N1 | 2 | 1 | 0 | 1 | ||
| Tumor diameter (mm) | Median (range) | 52 (12–75) | 54 (12–72) | 61 (31–67) | 50 (35–75) | 0.139 |
| PD-L1 expression status | ≥5% | 9 | 2 | 3 | 4 | 0.165 |
| <5% | 7 | 1 | 4 | 2 | ||
| Unknown | 18 | 10 | 3 | 5 | ||
| BRAF mutation status | Wild-type | 15 | 3 | 5 | 7 | 0.134 |
| Mutation | 1 | 0 | 1 | 0 | ||
| Unknown | 18 | 10 | 4 | 4 | ||
| Operability | Yes | 12 | 3 | 2 | 7 | 0.057 |
| No | 22 | 10 | 8 | 4 | ||
| PTV volume (mL) | Median (range) | 156.0 (87.5–212.7) | 151.2 (87.5–197.4) | 153.8 (111.2–189.9) | 156.2 (121.0–212.7) | 0.920 |
| All Cases | Group A * | Group B * | Group C * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 2-yr OS (%) | p Value | 2-yr OS (%) | p Value | 2-yr OS (%) | p Value | 2-yr OS (%) | p Value | ||
| Age | <72 | 16 | 91.7 | ref. | 100 | ref. | 83.3 | ref. | 100 | ref. |
| ≥72 | 18 | 42.0 | 0.004 | 40.9 | 0.078 | 33.3 | 0.266 | 100 | - | |
| Sex | Male | 11 | 79.5 | ref. | 50.0 | ref. | 100 | ref. | 100 | ref. |
| Female | 23 | 60.3 | 0.316 | 50.8 | 0.866 | 50.0 | 0.093 | 100 | - | |
| ECOG PS | 0 | 23 | 70.0 | ref. | 53.6 | ref. | 60.0 | ref. | 100 | ref. |
| 1/2 | 11 | 62.3 | 0.202 | 50.0 | 0.714 | 75.0 | 0.899 | 100 | - | |
| Tumor location | Nasal cavity | 24 | 66.7 | ref. | 67.5 | ref. | 57.1 | ref. | 100 | ref. |
| others | 10 | 64.3 | 0.908 | 0.0 | 0.090 | 100 | 0.219 | 100 | - | |
| Tumor stage | T3 | 10 | 78.8 | ref. | 75.0 | ref. | 50.0 | ref. | 100 | ref. |
| T4a/T4b | 24 | 59.5 | 0.115 | 38.1 | 0.164 | 71.4 | 0.928 | 100 | - | |
| Tumor diameter | <52 mm | 17 | 76.4 | ref. | 62.5 | ref. | 50.0 | ref. | 100 | ref. |
| ≥52 mm | 17 | 60.2 | 0.227 | 42.9 | 0.400 | 71.4 | 0.642 | 100 | - | |
| PD-L1 status | ≥5% | 9 | 65.6 | ref. | 50.0 | ref. | 50.0 | ref. | 100 | ref. |
| <5% | 7 | 83.3 | 0.177 | - | 0.676 | 75.0 | 0.351 | 100 | - | |
| unknown | 18 | 51.4 | 45.0 | 33.3 | 100 | |||||
| BRAF mutation | Yes | 1 | 100 | ref. | - | ref. | - | ref. | - | ref. |
| No | 15 | 92.9 | 0.086 | 66.7 | 0.728 | 100 | 0.156 | 100 | - | |
| unknown | 18 | 60.7 | 45.0 | 25.0 | 100 | |||||
| Operability | Resectable | 12 | 87.5 | ref. | 100 | ref. | 50.0 | ref. | 100 | ref. |
| Unresectable | 12 | 56.4 | 0.033 | 34.3 | 0.035 | 71.4 | 0.928 | 100 | - | |
| PTV volume | <156 mL | 17 | 46.3 | ref. | 28.6 | ref. | 25.0 | ref. | 100 | ref. |
| ≥156 mL | 17 | 92.3 | 0.095 | 80.0 | 0.149 | 100 | 0.057 | 100 | - | |
| Acute Adverse Event | Late Adverse Event | |||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade | Grades 1–2 | Grade 3 | Grade 4+ | Any Grade | Grades 1–2 | Grade 3 | Grade 4+ | |
| Mucositis | 33 (97%) | 31 (91%) | 2 (6%) | 0 | 2 (6%) | 2 (6%) # | 0 | 0 |
| Dermatitis | 27 (79%) | 27 (79%) | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Dry mouth | 8 (24%) | 8 (24%) | 0 | 0 | 2 (6%) | 2 (6%) | 0 | 0 |
| Dysgeusia | 6 (18%) | 6 (18%) | 0 | 0 | 2 (6%) | 2 (6%) | 0 | 0 |
| Tumor hemorrhage | 2 (6%) | 0 | 2 (6%) | 0 | 1 (3%) | 0 | 1 (3%) | 0 |
| Pneumonitis | 1 (3%) | 0 | 1 (3%) * | 0 | 0 | 0 | 0 | 0 |
| Optic nerve disorder | 0 | 0 | 0 | 0 | 3 (9%) | 3 (9%) | 0 | 0 |
| Uveitis | 0 | 0 | 0 | 0 | 2 (6%) | 2 (6%) * | 0 | 0 |
| Cataract | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 1 (3%) | 0 |
| Keratitis | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Photophobia | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Watering eyes | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Trismus | 0 | 0 | 0 | 0 | 2 (6%) | 2 (6%) | 0 | 0 |
| Trigeminal nerve disorder | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 1 (3%) | 0 |
| Tinnitus | 0 | 0 | 0 | 0 | 2 (6%) | 2 (6%) | 0 | 0 |
| Otitis media | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Hearing impaired | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 1 (3%) | 0 |
| Oral cavity fistula | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) | 0 | 0 |
| Hypothyroidism | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) * | 0 | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) * | 0 | 0 |
| Soft tissue infection | 0 | 0 | 0 | 0 | 1 (3%) | 0 | 1 (3%) * | 0 |
| Eczema | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) * | 0 | 0 |
| Arthralgia | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) * | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 | 1 (3%) | 1 (3%) * | 0 | 0 |
| EQ-5D-5L | EQ VAS | |||||
|---|---|---|---|---|---|---|
| Mean | SD | p Value | Mean | SD | p Value | |
| Baseline | 0.867 | 0.101 | ref. | 70.0 | 16.425 | ref. |
| 1 M | 0.895 | 0.124 | 0.794 | 75.0 | 16.251 | 0.242 |
| 3 M | 0.881 | 0.175 | 0.537 | 72.5 | 19.260 | 0.536 |
| 6 M | 0.889 | 0.163 | 0.972 | 70.0 | 14.573 | 0.081 |
| 9 M | 0.894 | 0.172 | 0.208 | 72.5 | 14.577 | 0.326 |
| 12 M | 0.867 | 0.176 | 0.310 | 80.0 | 19.656 | 0.779 |
| 15 M | 0.895 | 0.132 | 0.753 | 70.0 | 10.670 | 0.609 |
| 18 M | 0.895 | 0.105 | 0.825 | 80.0 | 16.073 | 0.786 |
| 21 M | 0.895 | 0.077 | 0.715 | 80.0 | 14.142 | 0.578 |
| 24 M | 1.000 | 0.106 | 0.415 | 80.0 | 12.392 | 0.680 |
| Treatment | Total Costs (JPY) | Incremental Cost (JPY) | QALYs | Incremental QALYs | ICER (JPY/QALY) |
|---|---|---|---|---|---|
| No ICI (Group A) | 2,732,870 | ref. | 0.642 | reference | ref. |
| ICI administration | 7,293,875 | 4,589,010 | 2.295 | 1.652 | 2,777,404 |
| ICI after PD (Group B) | 6,687,248 | 3,954,378 | 2.490 | 1.847 | 2,140,557 |
| Adjuvant ICI (Group C) | 7,600,205 | 4,867,335 | 1.716 | 1.073 | 4,534,904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, N.; Kano, K.; Okuda, T.; Koge, H.; Shima, S.; Tsuchida, K.; Takakusagi, Y.; Kawashiro, S.; Yoshida, M.; Kitani, Y.; et al. Adjuvant Therapy with Immune Checkpoint Inhibitors after Carbon Ion Radiotherapy for Mucosal Melanoma of the Head and Neck: A Case-Control Study. Cancers 2024, 16, 2625. https://doi.org/10.3390/cancers16152625
Mizoguchi N, Kano K, Okuda T, Koge H, Shima S, Tsuchida K, Takakusagi Y, Kawashiro S, Yoshida M, Kitani Y, et al. Adjuvant Therapy with Immune Checkpoint Inhibitors after Carbon Ion Radiotherapy for Mucosal Melanoma of the Head and Neck: A Case-Control Study. Cancers. 2024; 16(15):2625. https://doi.org/10.3390/cancers16152625
Chicago/Turabian StyleMizoguchi, Nobutaka, Kio Kano, Tatsuya Okuda, Hiroaki Koge, Satoshi Shima, Keisuke Tsuchida, Yosuke Takakusagi, Shohei Kawashiro, Manatsu Yoshida, Yuka Kitani, and et al. 2024. "Adjuvant Therapy with Immune Checkpoint Inhibitors after Carbon Ion Radiotherapy for Mucosal Melanoma of the Head and Neck: A Case-Control Study" Cancers 16, no. 15: 2625. https://doi.org/10.3390/cancers16152625
APA StyleMizoguchi, N., Kano, K., Okuda, T., Koge, H., Shima, S., Tsuchida, K., Takakusagi, Y., Kawashiro, S., Yoshida, M., Kitani, Y., Hashimoto, K., Furukawa, M., Shirai, K., Kamada, T., Yoshida, D., & Katoh, H. (2024). Adjuvant Therapy with Immune Checkpoint Inhibitors after Carbon Ion Radiotherapy for Mucosal Melanoma of the Head and Neck: A Case-Control Study. Cancers, 16(15), 2625. https://doi.org/10.3390/cancers16152625





