Simple Summary
Breast MRI is a valuable imaging modality that plays a critical role in the detection, diagnosis, and treatment of breast cancer. The superior sensitivity of breast MRI allows for more accurate evaluation of the extent of disease for breast cancer, which is important in an era where de-escalation of treatment is being considered and offered. In this review, we provided a detailed overview of breast MRI and its clinical utility in the management of breast cancer as a foundation for its use in the field of radiation oncology. Specifically, we describe breast MRI technique, anatomy, common breast MR imaging findings, and the role of breast MRI in assessing extent of disease and tumor response to neoadjuvant therapy. There has been increased interest in the use of MRI during radiotherapy treatment planning and delivery for patients with early-stage breast cancer as it may allow for more personalized radiotherapy.
Abstract
Contrast-enhanced breast MRI has an established role in aiding in the detection, evaluation, and management of breast cancer. This article discusses MRI sequences, the clinical utility of MRI, and how MRI has been evaluated for use in breast radiotherapy treatment planning. We highlight the contribution of MRI in the decision-making regarding selecting appropriate candidates for breast conservation therapy and review the emerging role of MRI-guided breast radiotherapy.
1. Introduction
1.1. Breast Cancer Overview
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer death among women in the United States. In 2024, an estimated 310,720 new cases of invasive breast cancer will be diagnosed, and approximately 42,250 women will die of the disease [1]. An important strategy in reducing breast cancer mortality is breast cancer screening [2]. When breast cancer is diagnosed, accurate staging is essential in selecting optimal treatment strategies and techniques. The addition of contrast-enhanced breast MRI in appropriate situations can aid in the further detection, evaluation, and management of breast cancer.
1.2. Diagnostic Value of Breast MRI
Since the introduction of breast MRI in the 1980s, multiple studies have evaluated its sensitivity and cancer detection rate compared to mammography alone. The average sensitivity of digital mammography alone is approximately 80–90% in nondense breasts and approximately 50–60% in dense breasts [3,4]. In comparison, breast MRI has a sensitivity of 90–100% irrespective of breast density [5,6,7,8,9,10].
Multiple trials have demonstrated that breast MRI detects cancers that are mammographically, sonographically, and clinically occult [4,5,7,8,9,10,11,12,13,14,15,16]. The high incremental cancer detection rate seen with breast MRI is observed irrespective of breast density and menopausal status [17]. Furthermore, while breast MRI has a lower perceived positive predictive value (PPV), more recent published studies demonstrate the PPV of breast MRI to be similar to that of mammography [18,19]. Importantly, breast MRI has also been shown to preferentially detect intermediate and high-grade cancers, which are more likely to be biologically significant [20,21]. While the routine use of breast MRI for preoperative staging remains controversial given a lack of data, demonstrating that it leads to definitive improvement in patient outcomes [22,23], the superior diagnostic accuracy of breast MRI has resulted in the rapid adoption of breast MRI into clinical practice (Table 1).

Table 1.
Summary of common indications for breast MRI.
2. Breast MRI Technique and Interpretation
2.1. Breast MRI Protocol and Positioning
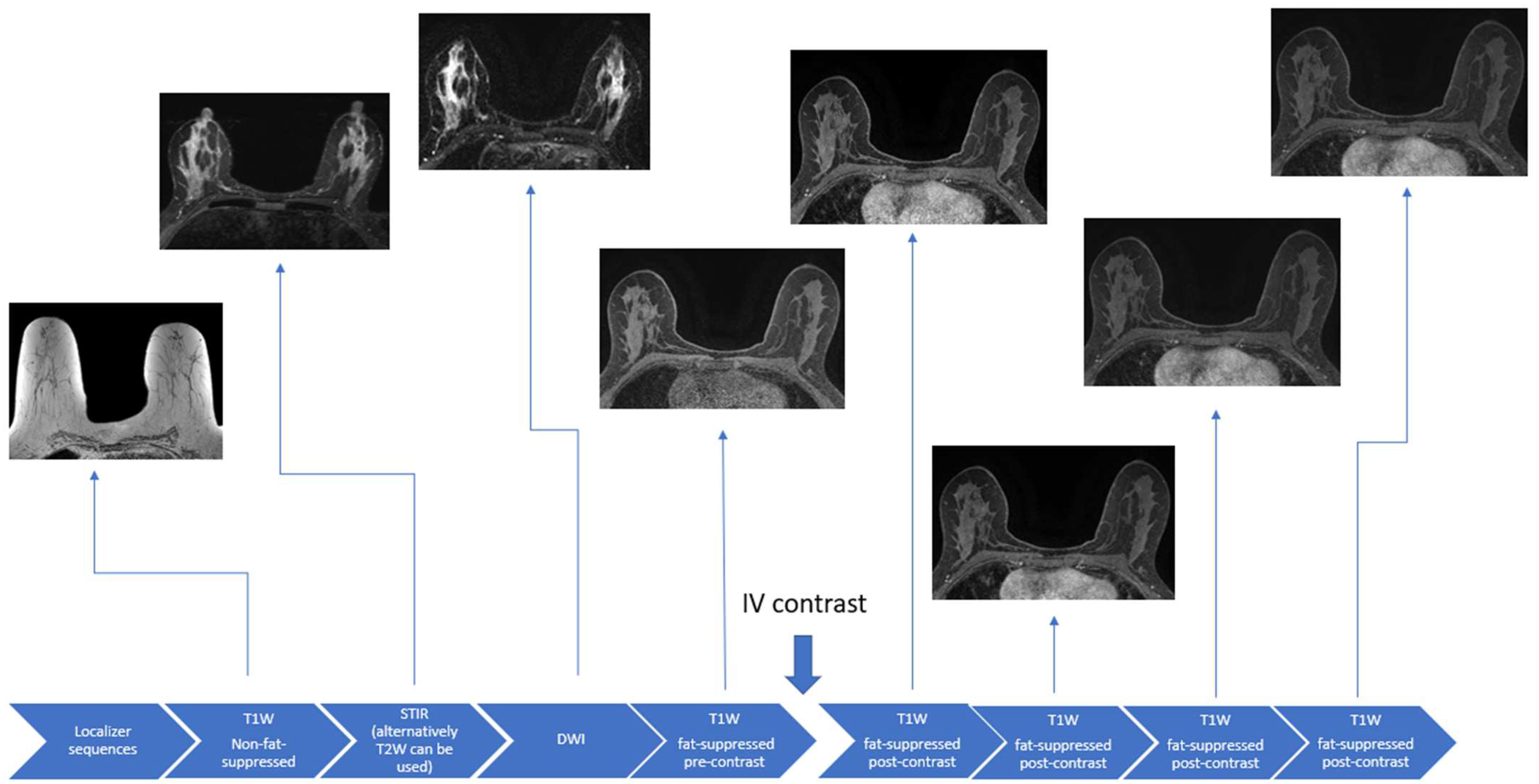
During the breast MRI examination, patients lie prone with the breasts suspended in a dedicated radiofrequency breast coil. Not only does this position allow for the optimized evaluation of the breast tissues, but it can minimize artifacts due to respiratory and cardiac motion [24,25]. Contrast administration is critical in the evaluation of the breast parenchyma for diagnosis and the extent of breast cancer but is not required for exams carried out to solely assess breast implant integrity. Conventional breast MRI sequences include T1-weighted, T2-weighted, or Short Tau Inversion Recovery (STIR), and diffusion-weighted imaging (DWI) sequences (Figure 1).
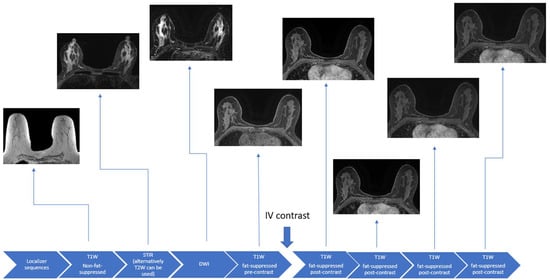
Figure 1.
Schematic example of a full diagnostic breast MRI protocol.
2.2. Primary Sequences for Breast MRI
2.2.1. T1-Weighted Sequences
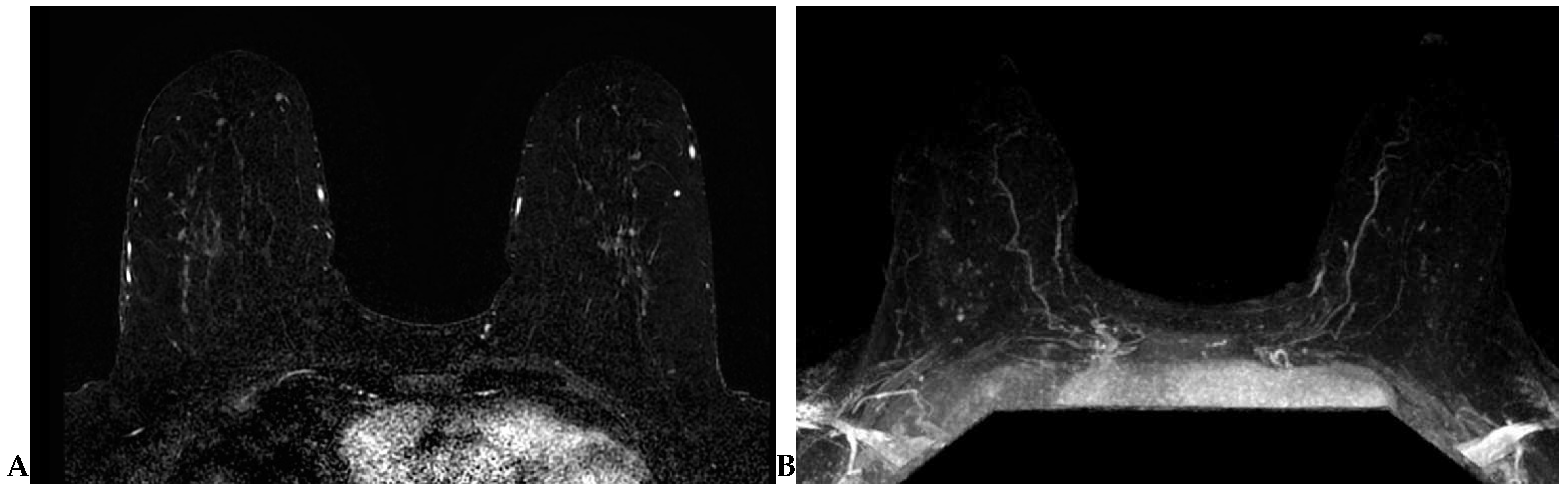
Lesion detection is largely performed on T1-weighted dynamic contrast-enhanced (DCE) sequence images. Prior to DCE sequences, a T1-weighted non-fat-suppressed sequence is typically acquired. Subsequently, pre- and post-contrast T1-weighted fat-suppressed dynamic contrast-enhanced (DCE) sequences are obtained at multiple time points. The creation of subtraction images—a post-processing technique whereby the pre-contrast sequence is “subtracted” from the post-contrast sequences—is required for DCE imaging obtained without fat suppression (Figure 2A). A maximum-intensity projection (MIP) can also be generated from these subtraction images (Figure 2B) and can assist in efficient lesion detection [26]. It is important to note that artifacts (motion, chemical shift, poor fat suppression) may degrade these post-processed sequences.
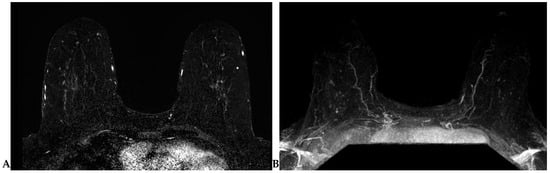
Figure 2.
(A) Axial subtraction image. The pre-contrast T1W sequence is “subtracted” from each post-contrast T1W sequence to generate the subtraction images. (B) Axial maximum-intensity projection (MIP). A MIP can be generated from the subtraction images.
2.2.2. T2-Weighted or STIR Sequences
The standard breast MRI protocol includes a fluid-sensitive sequence, most commonly either an axial T2-weighted (with or without fat suppression) or a short-tau inversion recovery (STIR) sequence. Although some cancers may demonstrate high signal intensity on fluid-sensitive sequences (necrotic cancers, mucinous carcinomas, and metaplastic carcinomas), most masses with high signal intensity on a fluid-sensitive sequence are benign (cysts, seromas, lymph nodes, and fibroadenomas). Studies have demonstrated that fluid-sensitive imaging increases the specificity in distinguishing benign and malignant lesions [27,28,29,30,31,32].
2.2.3. Diffusion Weighted Imaging (DWI)
DWI supplements DCE imaging to improve specificity while maintaining similar rates of sensitivity [33]. Although there is increasing use of DWI, it still remains an area of study. DWI offers lower spatial resolution when compared to the DCE-MR sequences. A small malignancy may not be noticeable on DWI without the corresponding DCE-MR images for correlation. DWI sequences are also more prone to MR artifacts. A consensus statement published by the EUSOBI International Breast Diffusion-Weighted Imaging working group [34] offers a basis for the standardization of DWI and is an important step in promoting DWI in clinical practice and garnering wider application and acceptance.
2.3. Breast Anatomy with MRI
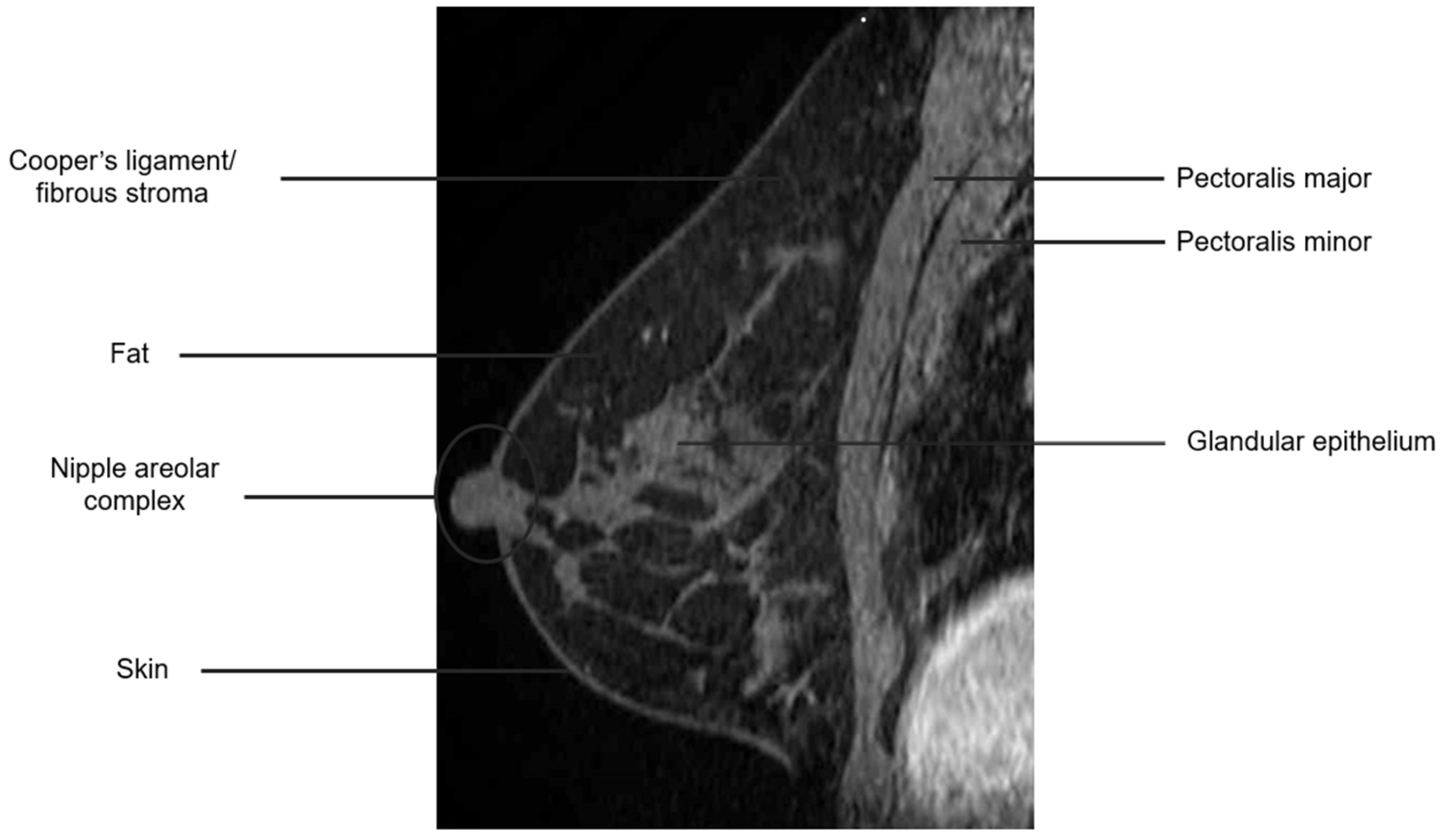
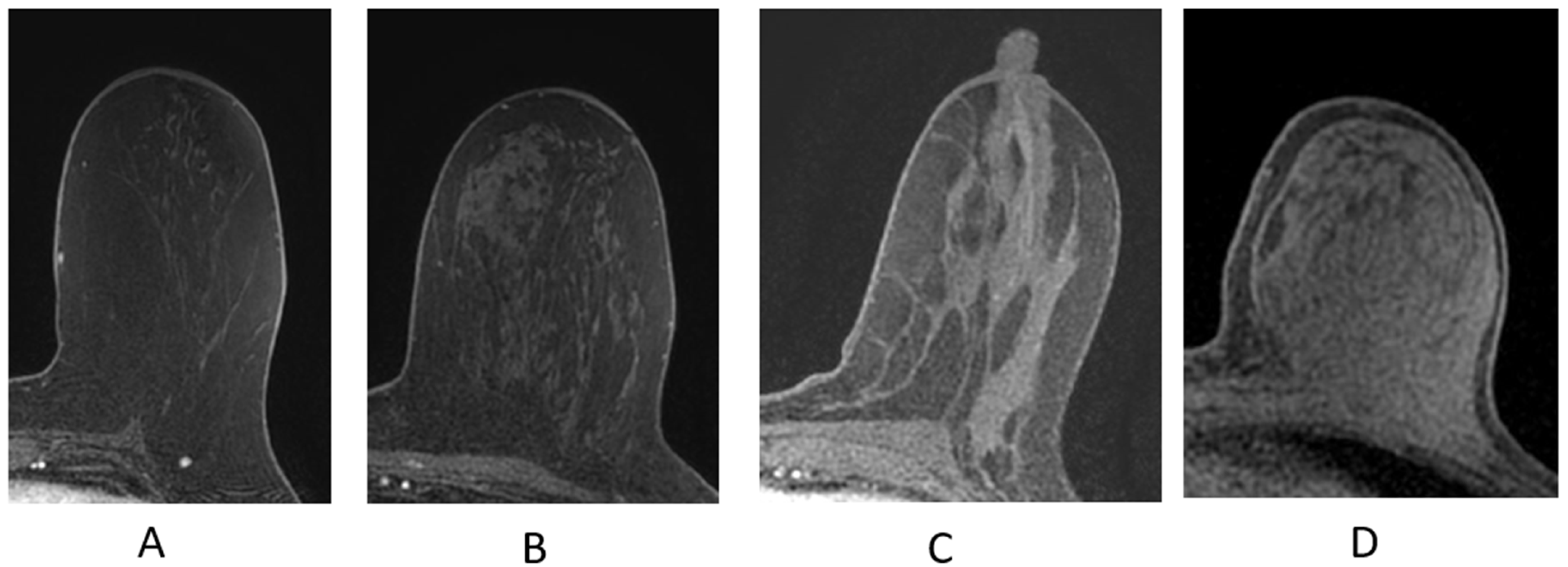
The breast is composed of glandular epithelium, fibrous stroma, and fat. The glandular epithelium in the breast consists of two main building blocks: ducts and lobules. Together, the ducts and lobules form ductal-lobular units, which coalesce to form approximately 15–20 lobes in a breast. Each lobe leads to a duct that widens to form a lactiferous sinus under the nipple–areolar complex prior to exiting the nipple. The fibrous stroma—frequently referred to as Cooper’s ligaments—is composed of bands of connective tissue that traverse the breast and insert into the dermis. These basic structures are seen at breast MRI (Figure 3). The amount of fibroglandular tissue (FGT) is evaluated on T1-weighted (either on the fat-saturated or non-fat-saturated) breast MR imaging sequences (Figure 4) [35].
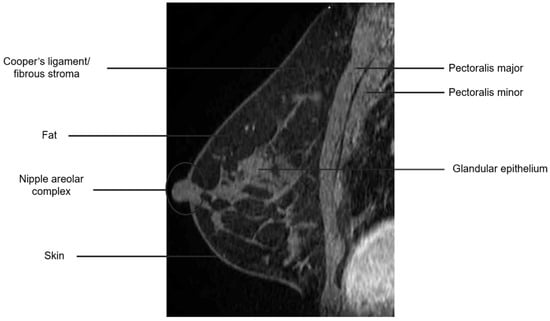
Figure 3.
Anatomy of the breast on a sagittal T1W fat-suppressed post-contrast breast MRI.
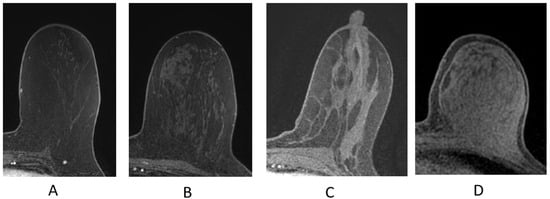
Figure 4.
Fibroglandular tissue (FGT) depicted n axial T1W fat-suppressed breast MRI. (A) Almost entirely fat; (B) scattered fibroglandular tissue; (C) heterogeneous fibroglandular tissue; (D) extreme fibroglanduar tissue.
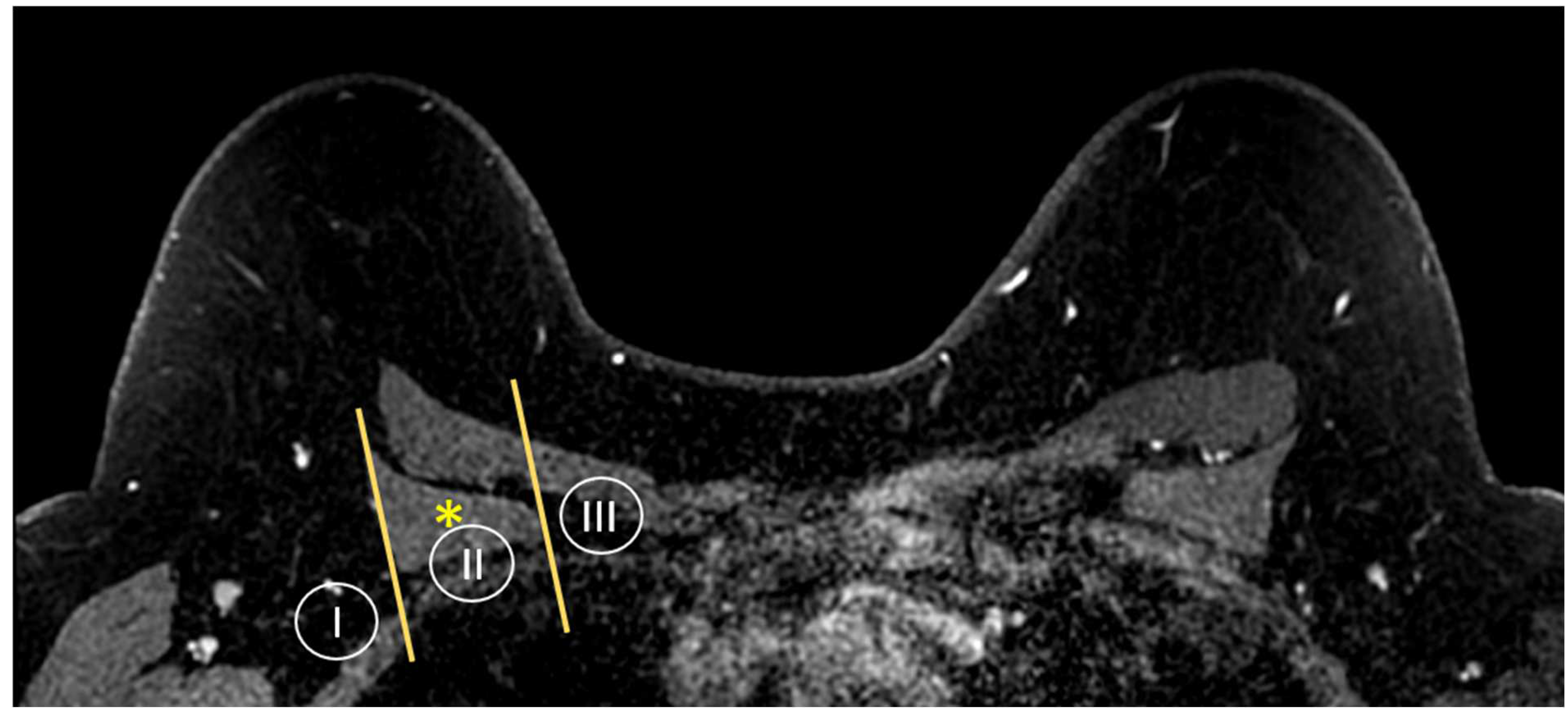
MR imaging is also able to provide a global view of the axillary and internal mammary nodal stations (Figure 5 and Figure 6). Axillary lymph nodes are divided into levels I, II, and III. The levels are defined by the relationship of the lymph node to the pectoralis minor muscle. Level I lymph nodes are inferolateral to the pectoralis minor; level II lymph nodes are posterior to and between the lateral and medial borders of the pectoralis minor; and level III lymph nodes are superomedial to the pectoralis minor [36]. Internal mammary lymph nodes (IMLN) are typically visualized in the first through sixth intercostal spaces.
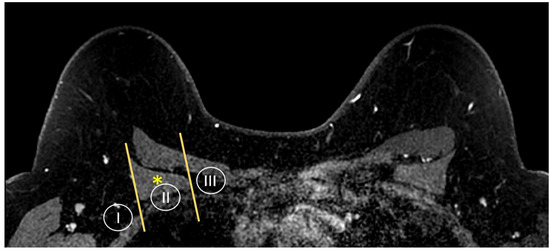
Figure 5.
Anatomy of the axillary nodal stations on axial T1W fat-suppressed post-contrast breast MRI. Levels are defined by the relationship of the lymph node to the pectoralis minor muscle (asterisk).
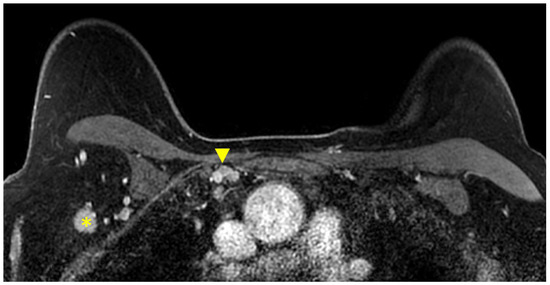
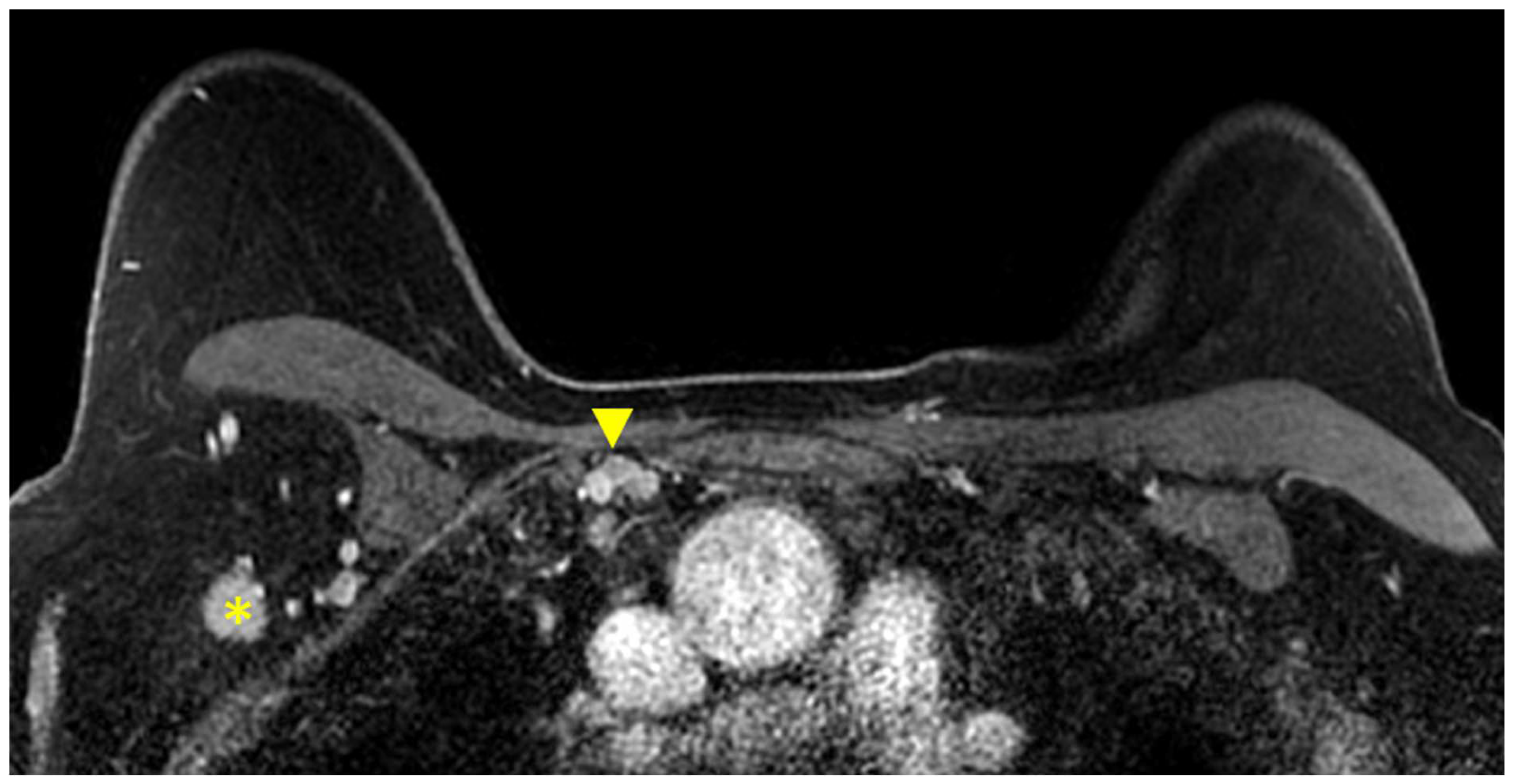
Figure 6.
Axial T1W fat-suppressed post-contrast breast MRI provides a global view of the axillary and internal mammary nodal basins. In this case, level I axillary adenopathy (asterisk) and internal mammary adenopathy (arrowhead) are present.
2.4. Findings in the Breast on MRI
2.4.1. Focus
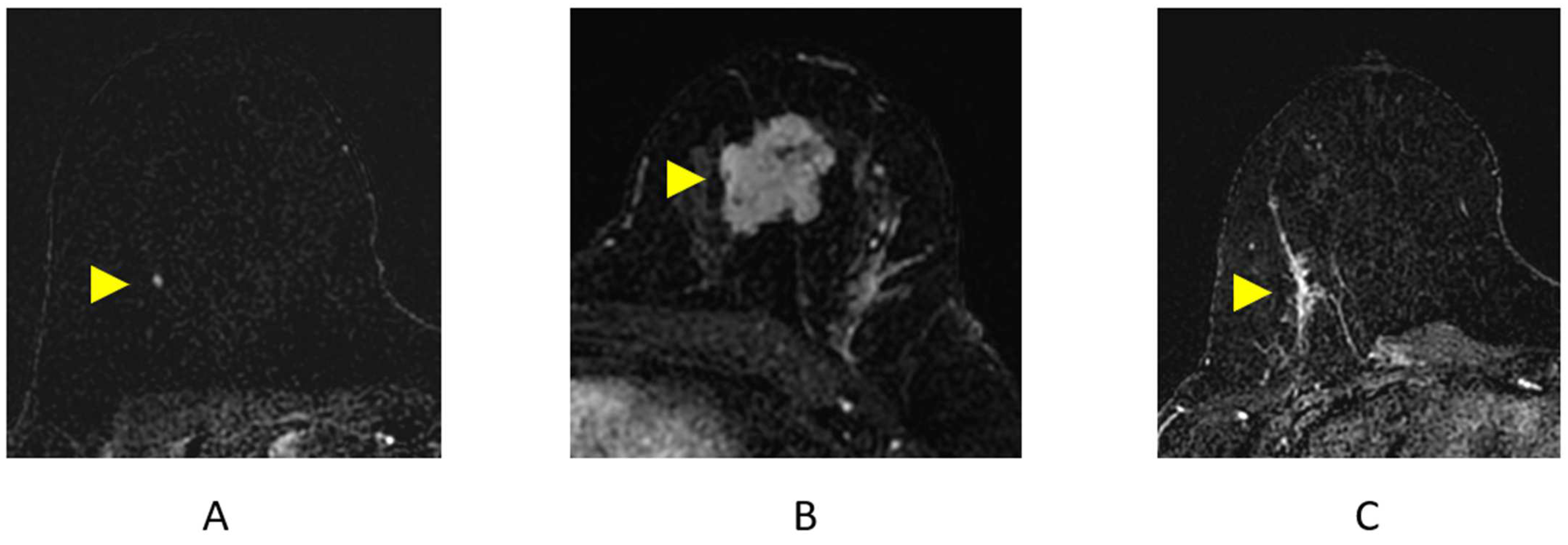
A focus is a small “dot” of enhancement that is so small, the shape and margins cannot be further characterized (Figure 7A) [35]. In clinical practice, the difference between a focus and a small subcentimeter mass is judged by the interpreting radiologist. Almost all foci identified at breast MRI are benign. In rare cases, a focus may represent an early malignancy [37,38].
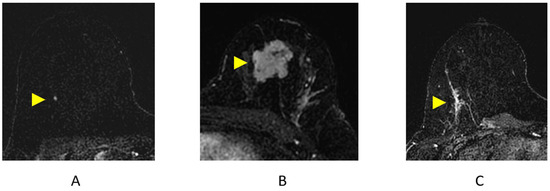
Figure 7.
Breast MRI Findings on axial post-contrast subtraction breast MRI (arrowheads). (A) Focus; (B) mass; (C) non-mass enhancement (NME).
2.4.2. Mass (Figure 7B)
In contrast to a focus, a mass is large enough for shape, margin, and internal enhancement characteristics to be depicted [35]. A spiculated margin has the highest likelihood of malignancy—with a PPV of 84–91%—compared to other shape and margin descriptors [38,39,40].
2.4.3. Non-Mass Enhancement (NME)
NME is a term used to describe an area of enhancement distinct from background parenchymal enhancement (BPE) that is not a space-occupying mass (Figure 7C). NME typically conforms to the FGT planes and may not have a pre-contrast correlate on the T2-weighted/STIR MRI sequences [41]. NME can be further characterized by internal enhancement patterns and distribution inside the breast. For example, a segmental or linear distribution of NME carries a higher likelihood of malignancy [38,42,43,44,45]. Studies have also demonstrated that a clustered ring internal enhancement pattern has a 77–100% PPV for malignancy [43,44,45] and that clumped internal enhancement has an 88% PPV for malignancy [43]. In clinical practice, ductal carcinoma in situ (DCIS) and diffuse invasive breast cancers (particularly lobular cancers) are the most common malignant causes of NME [41].
2.4.4. Background Parenchymal Enhancement (BPE)
Normal FGT can enhance on breast MRI and is termed BPE. BPE should be assessed on the first post-contrast sequence on breast MRI (Figure 8). When BPE is bilateral and symmetric, the likelihood of a false-positive interpretation is low [46]. However, when BPE presents in an asymmetric focal or regional fashion, it may be difficult to differentiate from NME [41,46], and follow-up or biopsy may be recommended. Additionally, a clinical history of whole-breast radiation therapy can explain asymmetric BPE, as the irradiated breast will have significantly less BPE compared to the non-treated breast [41,46,47]. Several studies performed in the diagnostic setting have shown that BPE may impact the accuracy in the extent of disease staging in newly diagnosed breast cancer patients, particularly in cancers presenting as NME [48,49].
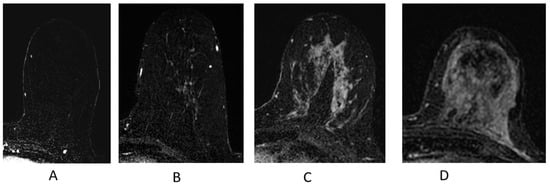
Figure 8.
Background parenchymal enhancement (BPE). (A) Minimal; (B) mild; (C) moderate; (D) marked.
2.4.5. Kinetic Curve Assessment
After an enhancing breast finding has been identified and characterized, the evaluation of lesion-enhancement kinetics is performed. The assessment of the kinetic curves of a breast lesion provides insight into pathophysiology by depicting the enhancement pattern of a lesion over time following contrast administration. These curves reflect the underlying vascular properties and microenvironment of a lesion. A classically suspicious kinetic curve includes early rapid enhancement with delayed washout, as malignant lesions tend to have increased angiogenesis, vascular permeability, and enlarged interstitial spaces, leading to the rapid uptake and washout of the contrast agent. However, the characterization of breast findings on the basis of kinetic assessment alone is not recommended due to the variability of kinetic curves in both benign and malignant pathologies. Morphologic characteristics of the breast finding should take precedence over the kinetic features. Indeed, when both morphologic and kinetic features are combined, the specificity of breast MRI interpretation improves [50].
2.4.6. Identifying Cancers
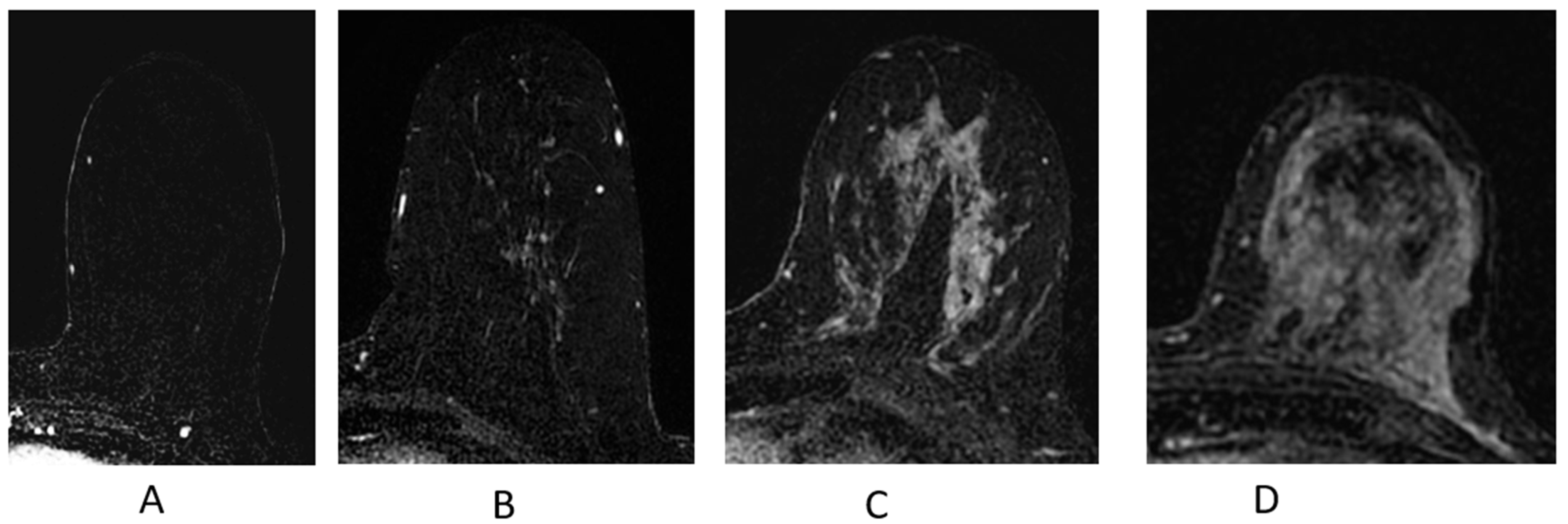
The majority of enhancing breast cancers will be conspicuous on the first post-contrast T1-weighted sequence, particularly on the post-processed subtraction image (Figure 9A). The “source” images—the post-contrast T1-weighted fat-suppressed sequence—will demonstrate the enhancing cancer and provide information on the parenchymal contour and nearby anatomic landmarks (Figure 9B); these are the best sequences in the evaluation and contouring of a breast malignancy.
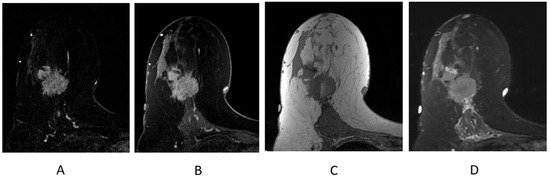
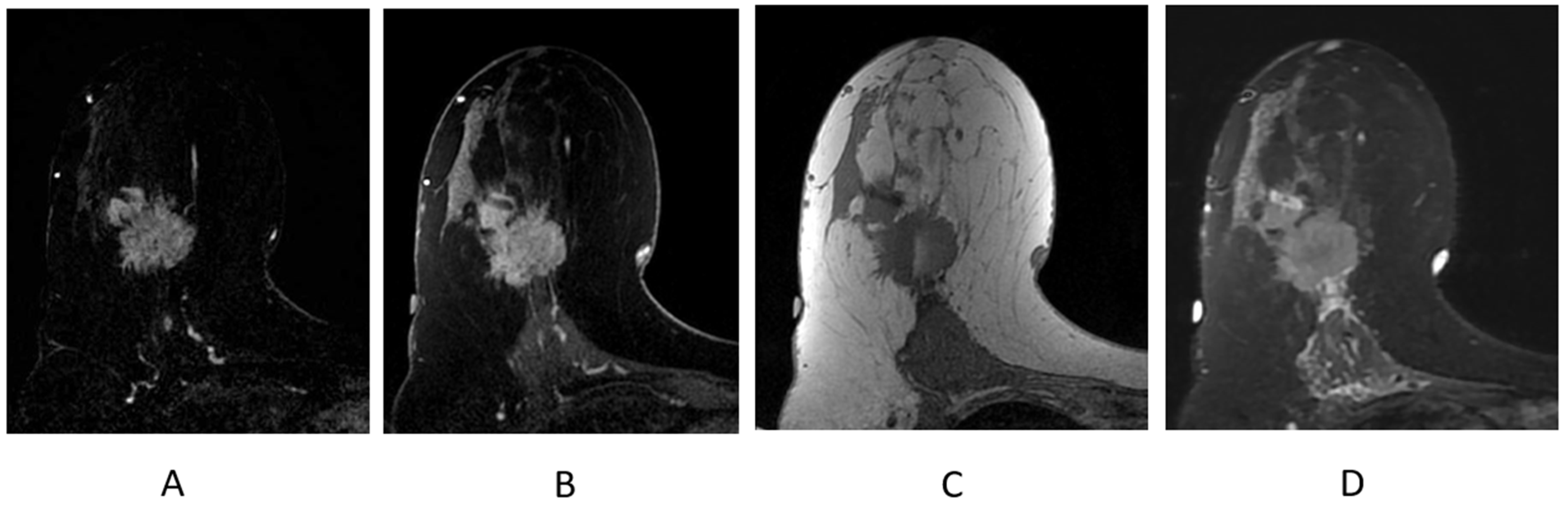
Figure 9.
Invasive ductal carcinoma presenting as an irregular mass on breast MRI. (A) Axial post-contrast subtraction, first time point; (B) axial post-contrast T1-weighted fat-suppressed sequence; (C) axial T1-weighted non-fat-suppressed sequence; (D) axial STIR sequence.
2.4.7. Identifying Lymph Nodes
The cortex of a lymph node has decreased signal intensity on T1-weighted sequences and increased signal intensity on T2-weighted and STIR sequences [36]. Both benign and metastatic lymph nodes can enhance rapidly and homogeneously with washout kinetics at DCE MR imaging [51,52,53]. Although lymph nodes are hyperintense on a T2 or STIR sequence (Figure 10A), lymph node morphology and margins may be characterized on the post-contrast T1-weighted fat-suppressed sequences (Figure 10B). The post-contrast T1-weighted sequences will also depict muscles, vessels, and other anatomic landmarks.
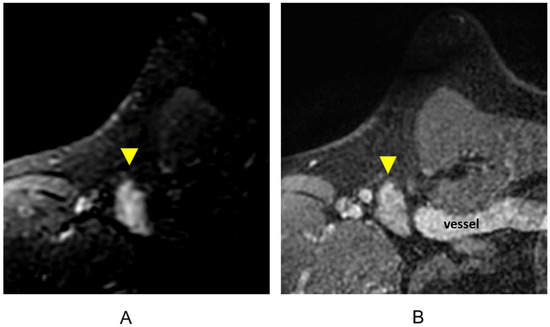
Figure 10.
Lymph node (arrowheads). (A) Axial STIR sequence; (B) axial post-contrast T1-weighted fat-suppressed sequence.
2.5. Breast Imaging Reporting and Data System
The Breast Imaging Reporting and Data System (BI-RADS) was designed by the American College of Radiology (ACR) to standardize breast imaging reporting, with the goal of facilitating communication between radiologists, referring physicians, and patients. The use of standardized BI-RADS terminology is required in breast imaging reports and allows for clear and concise communication of assessment and management recommendations. A final BI-RADS assessment with an accompanying management recommendation should be given for all breast imaging studies performed. The final BI-RADS assessment categories are displayed in Table 2.

Table 2.
BI-RADS final assessment categories.
3. Clinical Utility of MRI for Breast Cancer
There are significant advantages and some limitations to MRI in the detection, diagnosis and assessment of treatment response to systemic therapy as summarized in Table 3 and outlined in the below scections.
3.1. Identification of Cancer in the Breast and Axilla on MRI
3.1.1. Tumor Size Estimation and Factors Contributing to MRI-Pathology Concordance
Several studies have demonstrated that breast MRI is the most accurate imaging modality in tumor size estimation and extent of disease [55,56,57,58,59]. For instance, one study reported that tumor size on pathology was not significantly different from that seen at MR imaging but was underestimated by 14% at mammography and 18% on US [56]. That said, some studies have shown that MRI can overestimate tumor size in 9–37% of cases and also underestimate tumor size in 5–18% of cases [60,61,62,63]. Certain factors have been identified as influencing MRI–pathology size discordance, such as the presence of NME or DCIS, which can result in overestimation of tumor size by MRI [60,61,62].
3.1.2. Evaluation of the Pectoralis Musculature, Chest Wall, and Nipple–Areolar Complex
The involvement of the pectoralis major or minor muscle alone is not considered chest wall involvement by American Joint Committee on Cancer staging but can impact surgical planning. MRI has been shown to reliably demonstrate pectoralis muscle and chest wall invasion, as indicated by abnormal enhancement of these structures [64,65]. In the absence of muscle enhancement, obliteration of the fat plane between the tumor and muscle does not indicate muscle involvement [64,65]. MRI is also able to detect malignant invasion of the nipple–areolar complex with a reported sensitivity of 94% and a specificity of 86% [66], which can aid in patient selection for nipple-sparing mastectomy.
3.1.3. Multifocal/Multicentric Disease
Pre-operative breast MRI is particularly useful in the detection of multifocal and multicentric disease, as multifocality/multicentricity is often underestimated in both mammography and US [67]. A meta-analysis evaluating 19 studies demonstrated that MRI detects additional multicentric or multifocal disease in 16% of women with breast cancer [68]. This study found that for every three women with additional suspicious MRI findings, approximately two will have cancer and one will have a false-positive finding (TP:FP ratio = 1.9). This study also demonstrated a conversion from wide local excision (WLE) to mastectomy or to more extensive surgery in 8.1% and 11.3% of multifocal/multicentric cases, respectively. Further, in women who did not have additional malignancy on final histology, MRI-detected lesions resulted in a conversion from WLE to mastectomy in 1.1% of cases and from WLE to more extensive surgery in 5.5% of cases [68]. This highlights the importance of obtaining biopsy proof of suspicious lesions found upon MR imaging prior to proceeding to more extensive surgery.
3.1.4. Contralateral Disease
Studies have shown that breast MRI can detect a mammographically occult malignancy in the contralateral breast in 3–5% of patients with newly diagnosed breast cancer [17,69,70,71,72,73]. This also applies to women with dense breast tissue where a recently published study found the use of preoperative breast MRI reduced metachronous contralateral cancers [74].
3.1.5. Evaluation of Axillary Lymph Nodes
While US is the first-line modality for axillary imaging as it allows the visualization and image-guided sampling of abnormal level I and II axillary lymph nodes to assist in accurate pre-treatment staging [75,76], MRI is able to provide a global view of the axillae, allowing for comparison of both sides and enhancing the detection of a focally abnormal lymph node [36]. In addition, breast MRI can provide the assessment of the level III and internal mammary nodal stations.
The criteria used for sonographic evaluation of lymph nodes can also be applied at MR imaging. Specifically, the complete or partial replacement of a node with an ill-defined or irregular mass, complete or partial effacement of the fatty hilum, and abnormal morphology have been shown to have a high specificity for nodal metastasis in the setting of invasive breast cancer [36,52,77,78,79]. In a small study evaluating breast cancer patients, two additional MRI-specific nodal imaging features were reported as having potential in predicting nodal metastases–(1) “rim enhancement”, defined as increased signal intensity at the periphery of a node on DCE MR images and (2) “perifocal edema”, defined as increased T2/STIR signal in the fat surrounding a node [77]. These two findings had a 100% predictive value for the detection of axillary nodal metastases in the 56 breast cancer patients evaluated.
Lymph node size should not be used as a criterion for assessment since the overall size of a node has poor diagnostic accuracy for predicting metastatic nodal disease on imaging modalities [36,52,53,80,81,82]. In fact, axillary lymph nodes that demonstrate an oval morphology, smooth margins, and a uniform cortical thickness measuring ≤ 3 mm have a very high negative predictive value (NPV) for excluding metastases, irrespective of overall nodal size [82,83]. Symmetric bilateral axillary lymph nodes with smooth cortices have a high NPV for excluding metastasis [77]. MRI can also be valuable in identifying lymph nodes missed by an operator-dependent US exam, as seen in one study which found that MRI detected metastatic nodal disease in 15% of patients who had false-negative axillary US [84]. Given that the modalities have similar sensitivities (US 99.1% vs. MRI 97.4%) and specificities (US 15.4% vs. MRI 15.4%), the routine use of axillary US following a normal axillary MRI is not warranted if the entire axilla is included in the imaging field of view (FOV) without obscuration by (an) artifact(s) [85].
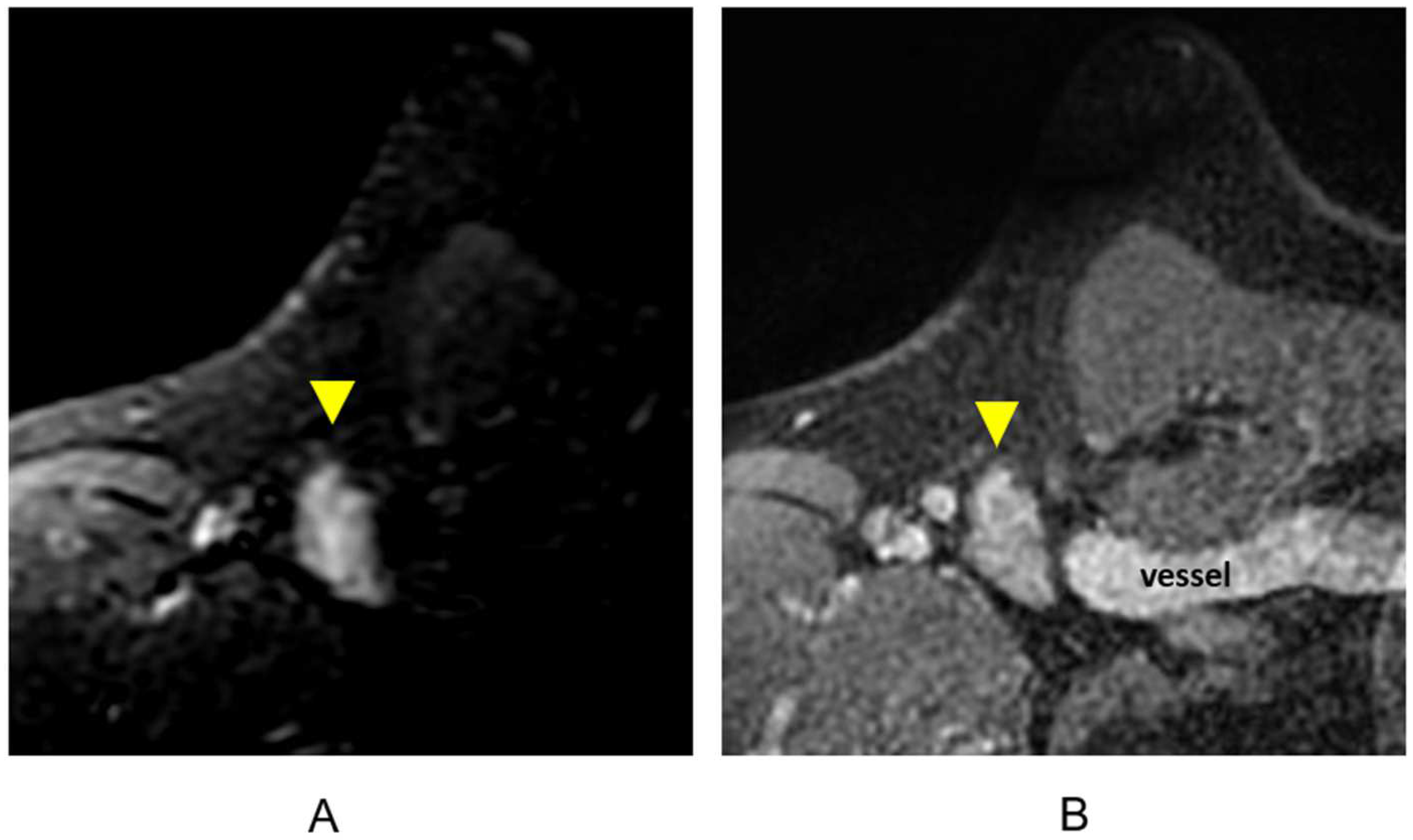
3.1.6. Evaluation of Internal Mammary Lymph Nodes (IMLNs)
Breast cancer involving IMLNs is associated with a worse prognosis, including decreased overall survival and higher rates of distant metastatic disease within three years following treatment [86,87,88]. IMLN metastases usually occur with concurrent axillary metastases; however, isolated IMLN metastases can occur in 1–5% of breast cancers [36,89]. Tumors with involved IMLNs are characteristically associated with a deep or medial lesion [36,90]. A retrospective study showed that the MRI identification of tumor contact with an internal mammary perforator vessel had high accuracy in predicting ipsilateral and contralateral IMLN involvement [91]. As the surgical dissection of IMLN metastases has not shown a survival benefit, surgical treatment of IMLN metastases is not typically performed, particularly given the associated morbidity [90]. Regional nodal irradiation that includes the IMLNs has demonstrated a 10-year reduction in distant metastases and improvement in disease-free survival [92,93]. For this reason, it is essential that involved IMLNs are identified at the time of staging imaging. Despite this, no clear guidelines or established size criteria exist for IMLN evaluation and reporting.
In studies assessing the presence of IMLNs in asymptomatic high-risk women presenting for screening breast MRI, normal IMLNs measuring 2–10 mm were detected in approximately half of the women screened [94,95]. In the setting of a recently diagnosed breast malignancy, however, subcentimeter IMLNs can contain metastatic disease [88,89,96,97]. The level of clinical suspicion is higher when the primary tumor is located in the deep or medial aspect of the breast. In equivocal cases, further evaluation with PET/CT can be helpful as studies have demonstrated that PET/CT is superior to conventional imaging for the detection of IMLN metastases [98,99,100,101].
3.2. The Impact of MRI on Decisions for Breast Conservation Therapy
3.2.1. Breast Conservation Therapy
Breast conservation therapy (BCT) with lumpectomy and whole-breast radiation therapy is a well-established treatment for women with early-stage breast cancer. The primary objective of BCT is to achieve equivalent outcomes as mastectomy while allowing women to maintain a cosmetically acceptable and sensate breast. Whole-breast radiation therapy after breast-conserving surgery (BCS) is meant to eradicate potential microscopic residual disease, which can lead to approximately a 20–60% recurrence rate, with risk of recurrence influenced by patient and tumor characteristics such as age, tumor grade, tumor size, and hormone receptor status [102]. While the absolute benefit of radiation after BCS varies according to patient and tumor characteristics, the proportional decrease in disease recurrence and reduction in breast cancer death varies little [102]. Several prospective randomized trials have shown long-term equivalence between BCT and mastectomy in terms of local control and overall survival [103,104]. More recent population-based studies meant to reflect use of more modern therapies have continued to support the use of breast conservation therapy compared to mastectomy [105,106].
Historically, adjuvant whole-breast radiotherapy has been delivered over the course of 5 weeks. However, a 3-week hypofractionated course has been encouraged in light of studies showing similar tumor control and cosmetic outcome between the regimens [107,108]. An even shorter treatment alternative, whole-breast radiotherapy for women with early stage 1 hormone-sensitive low-risk breast cancer, is accelerated partial breast irradiation (APBI). APBI targets the lumpectomy cavity (LC) with a margin while largely sparing normal surrounding breast tissue from high-dose radiation. APBI takes advantage of the fact that ipsilateral breast tumor recurrence after BCS occurs at or near the location of the initial breast cancer in approximately 75–90% of cases regardless of whether the patient receives adjuvant whole-breast radiation [109]. APBI can be delivered using external bream radiotherapy, low- or high-dose rate brachytherapy, and intraoperative radiotherapy. The 10-year results of NRG NSABP B39-RTOG 0413 and the RAPID trial reported a difference in local control between whole breast radiotherapy and APBI of < 1% [110,111]. The most recently published APBI guidelines from ASTRO expanded the criteria to include more women, such as those with low-risk DCIS, as defined in RTOG 9804 and women as young as 50 years old [59]. With growing data showing excellent ipsilateral breast tumor control in a larger patient population, it is reasonable to expect an increase in the utilization of APBI within the field of breast radiotherapy.
3.2.2. The Use of MRI for Preoperative Staging and Surgical Planning
Despite the enhanced sensitivity of breast MRI, the routine use of breast MRI for preoperative staging remains controversial. MRI has been shown to change surgical management most often to a more radical surgery without an indication that it improves surgical care or prognosis [112,113]. Two randomized trials, the United Kingdom COMICE trial and the Dutch MONET trial demonstrated that preoperative MRI did not reduce re-excision rates for BCS [22,114]. Furthermore, a meta-analysis of four studies including 3169 patients demonstrated that preoperative MRI did not impact the risk of local recurrence (LR) or recurrence-free survival (RFS) at 8 years [115]. In contrast, other literature supports the idea that MRI reduces re-operation rates [116,117,118,119]. Additionally, a recently published study evaluating 261 patients showed that preoperative breast MRI was associated with reduced LR in women with dense breast tissue, suggesting that this subset of patients may in fact benefit from a preoperative MRI [74]. The results of the ongoing Alliance A011104/ACRIN 6694 trial, which aims to compare the 5-year local-regional recurrence rate following BCT in women at high risk of LR, may shed some light on the relationship between preoperative breast MRI, surgical outcomes, and costs.
3.2.3. Assessment of Tumor Response after Neoadjuvant Therapy
Neoadjuvant therapy (NAT) can allow more patients to undergo breast conservation by reducing the primary tumor size in patients that otherwise would have been ineligible [120,121], and MRI has a significant role in assessing the response to therapy. Several factors have been shown to affect the predictive value and diagnostic accuracy of MR imaging in the assessment of treatment response to NAT, including tumor molecular subtype, tumor imaging phenotype, the chemotherapeutic regimen, and the pathology response criteria utilized for assessment. The most common classification of a pCR is the resolution of all invasive disease with or without a residual in situ disease in the breast and clearance of axillary nodal disease.
Breast MRI is currently the most accurate imaging modality for the assessment of tumor response to NAT [57,58,122,123,124,125]. Overall, the published literature shows good concordance between residual tumor size measured on MRI and tumor size on pathology following surgical excision, particularly when predicting the presence of residual disease at final pathologic examination. Breast MRI to evaluate response to NAT is one of the recommended clinical indications by the American College of Radiology (ACR) and European Society of Breast Imaging, and also in the NCCN guidelines as an optional tool [126,127,128]. The use of contrast-enhanced breast MRI to determine disease response has a sensitivity approaching 90%, a specificity of 60% to 100%, and an accuracy of approximately 91% [57,58,59,70,123,129,130,131,132]. While the PPV of breast MRI following NAT is high at 93%, the NPV is only moderate at 65%, which decreases the overall diagnostic accuracy of breast MRI to 84% [122]. As such, a complete imaging response at breast MRI is not sufficient to allow patients to forgo surgical excision.
3.2.4. MR Imaging Response by Molecular Subtype
Published studies reveal that women who exhibit a pCR to neoadjuvant chemotherapy demonstrate improved disease-free survival [133,134,135,136,137]. The independent association between triple negative/HER2-positive molecular subtype and pCR is well documented [138,139]. Interestingly, breast CE-MRI has greater accuracy in assessing residual tumor size following NAT in triple-negative and HER2-positive/ER-negative tumors [140,141]. These tumors commonly demonstrate concentric shrinkage on post-NAT MR evaluation, and residual disease is easier to measure and quantify. MRI is less accurate in the evaluation of residual disease in luminal subtypes [85,86,135,136,137]. HER2-negative luminal breast cancers are more likely to show residual disease as small foci or scattered cells at the tumor bed (tumor fragmentation) which can often result in an underestimation of residual disease extent at MR imaging [142].
3.2.5. MR Imaging Response by Imaging Phenotype
Several studies have shown that kinetic changes in tumor enhancement on MR imaging during NAT are identified prior to changes in tumor volume [143,144,145]. Imaging phenotypes of breast cancer affect the reliability of breast MRI in the assessment of tumor size and NAT response. For example, MRI can more accurately predict residual disease on post-treatment imaging if the breast cancer initially presents as a mass that has clear margins for measurement or if the mass is more enhanced than the BPE [146]. Breast cancers that present as masses with clearly defined margins tend to demonstrate concentric shrinkage on post-NAT MR imaging, permitting more accurate size measurements. In contrast, breast cancers presenting as NME on pre-treatment MRI are more likely to show residual disease as scattered cells or small foci at the tumor bed on post-NAT MR imaging [146]. This type of response is known as tumor fragmentation and can decrease the accuracy of MRI in predicting residual disease resulting in the underestimation of disease [146,147,148,149,150]. Conversely, MR imaging may overestimate residual disease in cases where reactive inflammation and/or fibrosis are present at the treated tumor bed [150,151].
3.2.6. MRI in Assessing Nodal Response to NAT
The MRI of the axilla is only 61.0% sensitive for the detection of residual nodal disease after neoadjuvant chemotherapy [152,153]. In comparison, the reported sensitivities of axillary US and FDG PET/CT for detection of residual nodal disease are 69.8% and 63.2%, respectively [152,154]. As no imaging test can yet reliably detect residual nodal disease, a surgical axillary assessment remains warranted following the completion of NAT.
3.2.7. Systemic Therapy
Certain chemotherapeutic regimens can impact the diagnostic accuracy of breast MRI. Specifically, taxane-based therapies, antiangiogenic agents, and ER modulators may alter perfusion to the breast. It is hypothesized that anti-vascular effects impact contrast enhancement [146,151]. In these cases, residual disease may be underestimated [146,151].
3.2.8. MRI vs. FDG PET/CT in Assessment of NAT Response
A meta-analysis of 595 patients from 10 studies evaluated the accuracy of MR imaging versus FDG PET/CT imaging in measuring responses to NAT [125]. The study found that the timing of imaging influenced diagnostic accuracy. MRI outperformed FDG PET/CT after the completion of neoadjuvant therapy through its higher sensitivity. However, FDG PET/CT outperformed MRI during neoadjuvant therapy through its higher specificity [125]. This suggests that although MRI may be the superior modality at assessing residual disease after the completion of NAT, FDG PET/CT can be considered when assessing response during therapy in select clinical scenarios.
3.3. The Role of MRI in Assisting with APBI Candidacy
MRI-Based Eligibility for APBI
A meta-analysis of six studies evaluated patient eligibility for ABPI utilizing MRI, in which 6–25% of patients were deemed ineligible, with a pooled event rate of 11% (95% CI: 6–19%) [155]. In this study, the addition of breast MRI reduced inter-study heterogeneity and found that women who were premenopausal, at least pT2, or with invasive lobular carcinoma (ILC) were more likely found to be poor candidates for APBI. Similarly, in a mixed retrospective and prospective study of 521 women who underwent physical exam and mammography with or without US, MRI changed eligibility in 12.9% of patients [156]. In this study, univariate analysis indicated that tumor size ≥ 2 cm, age < 50, diagnosis of ILC, and HER2 positivity were associated with a higher risk of ineligibility after MRI. A scoring index was generated correlating the presence of 0, 1, 2, or 3 of these risk factors with a 2.8%, 13.2%, 38.1%, and 100% risk of MRI identifying occult disease, respectively. Additionally, a retrospective study conducted on a cohort of mainly young or high-risk patients found a PBI ineligibility rate of 38%, mainly due to the presence of multicentric and contralateral disease [157]. Current indications for APBI include those that are low-risk DCIS or stage 1 hormone-sensitive breast cancer in women older than 50 years of age. MRI was not utilized in 3 randomized trials evaluating APBI, which demonstrated that in-breast recurrence was non-inferior to that achieved with whole-breast radiation and event rates of in-breast recurrence were very low in both arms (RAPID, NSABP B39/RTOG 0413, University of Florence). Therefore, the potential utility of MRI for evaluating for APBI eligibility so far is mostly in higher-risk populations for whom APBI is not standardly indicated.
Preoperative MRI may be most useful for women who appear eligible for APBI by less selective APBI guidelines [158], such as those from Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) [158]. MRI-based APBI screening may find that the highest utility in efforts aiming to evaluate a broader patient population and less clinically relevant when evaluating a very low-risk subgroup.
3.4. Use of MRI for Post Breast Conservation Therapy Evaluation
Fat necrosis is commonly encountered following BCT and can present as a wide range of imaging findings on MRI. Enhancements related to fat necrosis should have an associated fat signal and may present as an oil cyst, a rim-enhancing mass, an area of focal enhancement, diffuse enhancement, distortion, or irregular masses. If the associated fat signal is not apparent, the MR imaging appearance may mimic a malignancy, prompting an image-guided biopsy [159].
Following breast radiation therapy, skin and trabecular thickening and edema are expected and present as increased T2/STIR signal in the skin and breast parenchyma. These findings gradually decrease over time but may never entirely resolve. In fact, edema in the post-treated breast can be seen in more than 25% of women six years following radiation therapy [47]. Comparison with prior studies is critical, as breast and skin edema following radiation therapy is expected to decrease or remain stable over time and increasing edema can be a sign of recurrent disease.

Table 3.
Strengths and Limitations of MRI.
Table 3.
Strengths and Limitations of MRI.
| Strengths | Limitations |
|---|---|
| High sensitivity [5,6,7,8,9,10] | Cost |
| Tumor size estimation [55,56,57,58,59] | Accessibility |
| Defining extent of disease | Claustophobia |
| Pectoralis major/minor invasion [64,65] | IV gadolinium contrast allergy (rare) |
| Invasion into the nipple–areolar complex [66] | Presence of a MRI incompatible implantable device |
| Multicentric/multifocal disease [68] | |
| Mammographically occult disease in contralateral breast [17,69,70,71,72,73,74] | |
| Metastatic involvement of axillary or IMN nodes [36,52,77,78,79,91,96,97] | |
| Tumor response to NAT [57,58,59,70,120,121,122,123,127,128,129,130] | |
| Post treatment changes in the breast | |
| Non-ionizing radiation |
4. Breast MRI for Radiation Treatment Planning
4.1. Identification and Sequences for Cavity and Clips
4.1.1. Identifying Seromas and Surgical Cavities
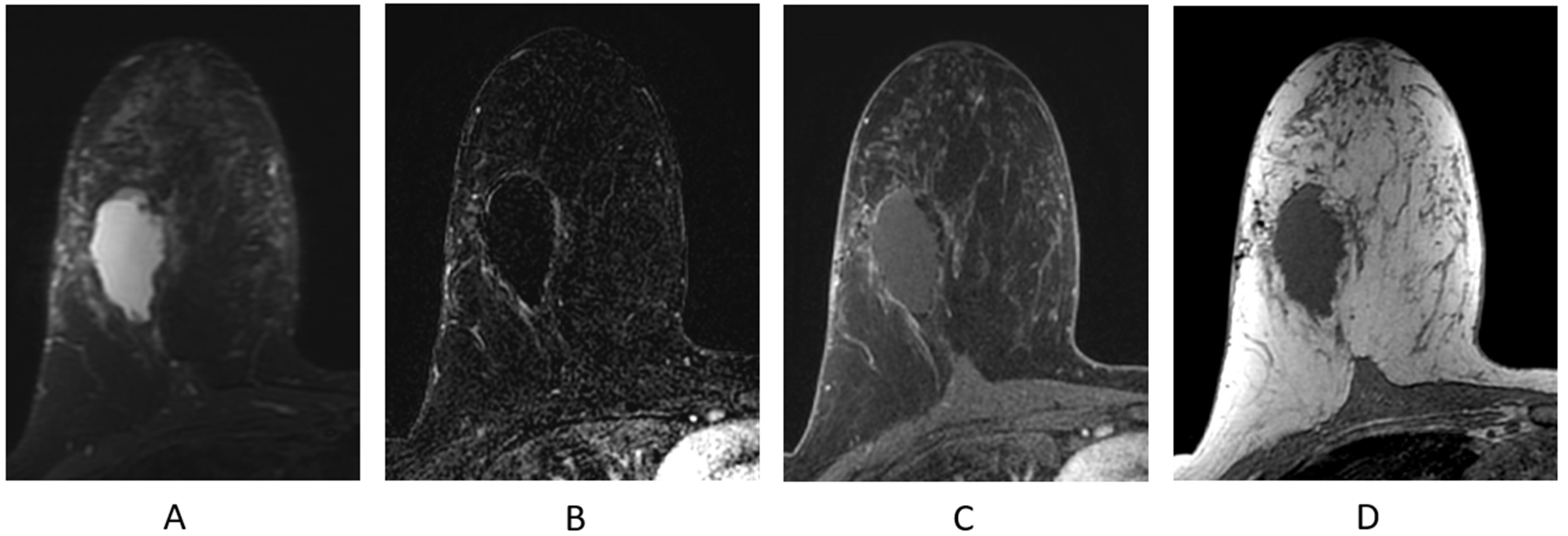
The normal appearance of the breast following partial mastectomy often includes a seroma at the surgical site (Figure 11). Seromas and surgical cavities containing fluid will demonstrate increased T2/STIR signal and be most conspicuous on a fluid-sensitive sequence (Figure 11A). Seromas are T1 hypointense (Figure 11D) and typically non-enhancing, but can demonstrate thin, smooth, rim enhancement on the T1-weighted post-contrast sequences (Figure 11B,C). Most seromas slowly decrease in size over time and resolve, leaving a residual non-enhancing scar on breast MR imaging.
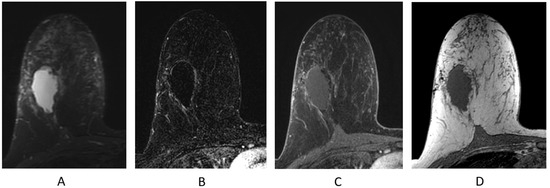
Figure 11.
Seroma/surgical cavity on breast MRI. (A) Axial STIR sequence; (B) axial post-contrast subtraction; (C) axial post-contrast T1-weighted fat-suppressed sequence; (D) axial T1-weighted non-fat-suppressed sequence.
4.1.2. Identifying Surgical/Biopsy Clips
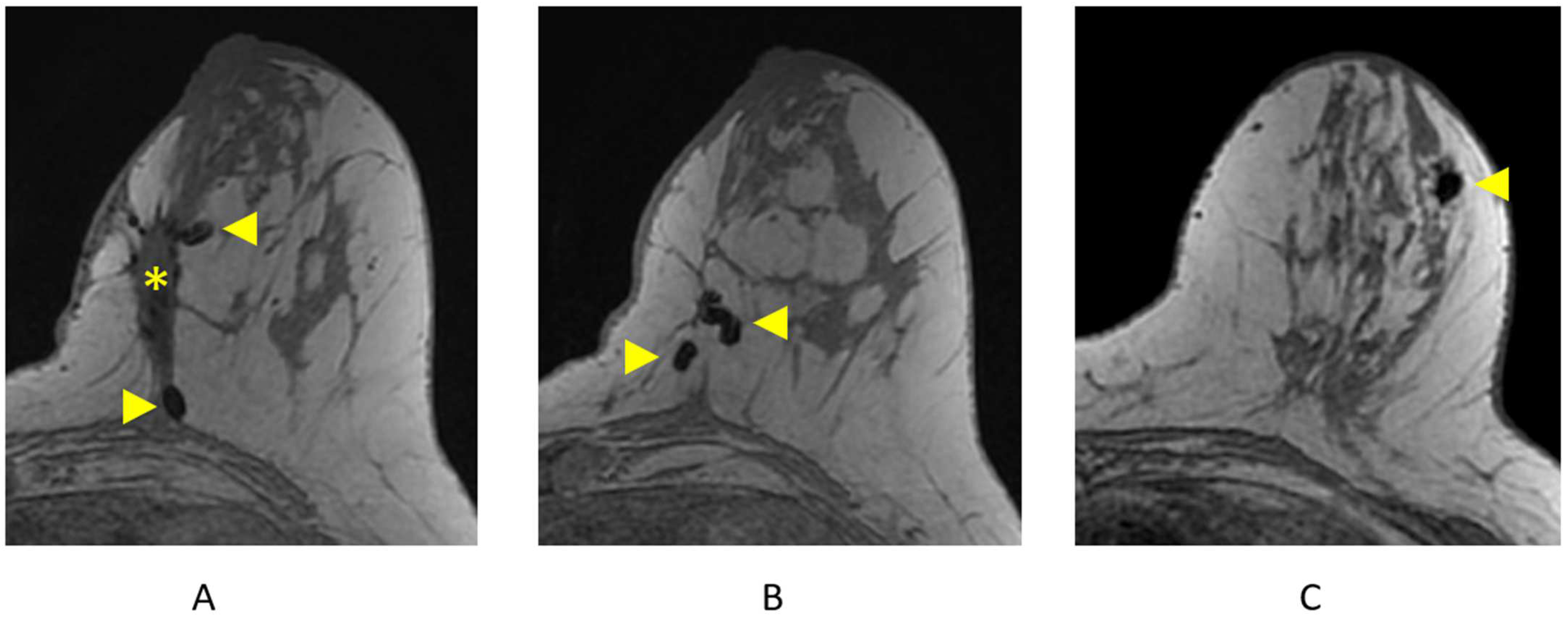
Clips are best visualized on the pre-contrast T1-weighted non-fat-suppressed sequence and are represented as areas of focal susceptibility artifact (“signal void”) (Figure 12). Careful correlation with recent mammography is important to confirm the presence of surgical or biopsy clips, as focal areas of susceptibility artifact may also represent other processes, including hemosiderin deposition.
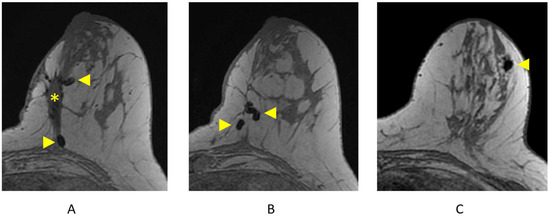
Figure 12.
Surgical and biopsy clips on axial T1-weighted breast MRI. (A) Lumpectomy site (asterisk) and areas of focal susceptibility representing surgical clips (arrowheads); (B) additional surgical clips (arrowheads); (C) focal susceptibility artifact representing a breast biopsy clip/tissue marker (arrowhead).
4.2. Treatment Planning Position and Co-Registration
4.2.1. CT Simulation Position
Breast MRI is not being utilized in routine clinical practice for breast radiotherapy treatment planning but has been investigated in research protocols. One challenge for the routine use of MRI in radiation planning is patient positioning. Breast radiotherapy planning and treatment have been traditionally performed in the supine position with arms overhead, while a clinical breast MRI is acquired in the prone position. Breast MRI in the supine position has been shown to be feasible with thoughtful consideration of imaging coil position, imaging parameters, and motion compensation techniques [25,160,161]. These studies show that supine MRI can have comparable imaging quality; thus, there may be potential future implications in surgical and radiation planning where patients are routinely supine.
A more widely used alternative to supine breast MRI for radiation planning is to perform CT simulation in the prone position. Breast radiotherapy in the prone position is being more commonly utilized and has been shown to have benefits, particularly for women with larger and/or pendulous breast and left-sided breast cancer [162,163,164,165]. The boundaries of the breast CTV volume will vary to some degree between the supine and prone positions. Due to variances in body habitus, breast density, and breast size, the breasts may naturally lie more laterally or inferiorly when supine, which can make it more challenging to limit the dose to organs inside the thoracic cavity compared to a prone setup. The prone position has several advantages for radiation delivery, including a reduction in respiratory motioning, displacing the LC and breast tissue farther from the heart and lungs and improving dose homogeneity throughout the breast [166,167]. Treatment in the prone position can more easily incorporate standard diagnostic MRI with the co-registration of imaging to aid in target and organ-at-risk (OAR) delineation.
4.2.2. CT-MRI Co-Registration
In the process of co-registration, sometimes incorrectly referred to as a fusion, the CT images serve as the primary dataset, and the MR images are matched to the anatomy of the primary data set. Anatomical registration points are used to align the images, such as the nipple, sternal notch, chest wall/ribs, and tip of the scapula. Further refining of the co-registration can occur by aligning the vasculature and breast parenchymal markings, particularly in the area of the LC, given that this is the high-risk target. To reduce co-registration errors, the patient should be scanned in as close to the same position as possible. This includes arm positioning, which can affect breast position and contour. A patient’s arms are positioned overhead for radiation planning, but positioning can vary for a standard diagnostic MRI. Specifically, routine arm position for MRI may differ by institutional protocol and optimal positioning can vary depending on the coil manufacturer or model. Further, MRI with arms down may be preferred in some cases, such as a smaller patient where arms overhead lead to less breast tissue in the coil, or in patients with more mobile breast tissue, where an arm down may allow for a more even distribution of tissue over the image volume [168]. Given the variation in standard MRI protocols, it is important to perform MRI in the treatment with position planning or careful consideration of potential differences in the patient position if a diagnostic clinical MRI is being co-registered for planning purposes.
4.3. Radiation Therapy Target Volumes
4.3.1. Lumpectomy Cavity
When a well-visualized seroma is not present at the time of CT radiation treatment planning, there can be large variations in cavity delineation between observers [169,170,171]. Strategies to identify the cavity and interpret planning CT images include referring to operative notes, surgical clips or fiducials, clinical examination, and pre/postoperative imaging (mammogram, US, MRI). The use of CT findings and surgical clips to identify the tumor bed is considered the current gold standard for radiation planning [172]. Surgical clips are better visualized on CT than on MRI. This is particularly true when clips are close to the breast-tissue–chest-wall interface [173]. Not all institutions routinely place surgical clips or place them in all cases. MRI is better than CT at distinguishing postoperative changes, such as a small seroma, hemorrhage, fibrosis, or edema, from normal breast tissue. Further, CT-based imaging for LC delineation can be particularly challenging in the case of dense breasts, small cavities, and a delay from surgery to RT planning [169,171]. Several studies have explored the feasibility and potential role of MRI in boost or APBI treatment planning.
Al-Hammadi et al. found that the use of supine breast MRI improved the visibility of the cavity and reduced interobserver variability (IOV) compared to CT [174]. Similarly, Jolicoeur et al. also noted a reduction in IOV, as well as a smaller cavity volume, with MRI compared to CT [175]. Further, Jacobsen et al. found in a series where clips were occasionally but not routinely used such that both MRI and CT had low IOV; however, T2-weighted MRI was the best at demarcating the interface between the seroma and chest wall, skin, and dense breast tissue [176]. These studies suggest that MRI may have utility in defining an LC without surgical clips.
On the other hand, when surgical clips are present, there is a lack of evidence that MRI improves the IOV for tumor bed delineation compared to only using a CT/clip-based approach [177,178,179,180,181]. IOV was either similar using MRI compared to CT alone, or the incorporation of MRI increased variability in contours between observers. In general, there are inconsistent conclusions regarding the impact of MRI on determining the cavity volume, the cavity visibility score (as defined in Smith et al. [182]), or the centroid of the cavity [173,177,178,179,180,181]. Studies also vary in the approach to image co-registration, type of MR sequences, image quality, and observer knowledge of MRI and/or breast radiotherapy. Study outcomes are also likely impacted by MRI timing after surgery, which is a factor in clip migration, seroma absorption, and changes from scar tissue formation [183,184]. Furthermore, the type of BCS closure technique can also influence MRI interpretation, with full-thickness closure surgical techniques and oncoplastic procedures being more difficult for visualizing the LC. MRI can lead to an overestimation of the LC size as it provides better visualization of soft tissue abnormalities between clips that otherwise appear as normal breast tissue on CT [173].
In summary, the incorporation of MRI for cavity definition is still under investigation, and there are insufficient data to support the routine incorporation of MRI for radiation treatment planning. The existing data suggest that MRI may be the most useful in assisting with tumor bed definition when clips are not present. In general, clips are good surrogates for the LC; therefore, including MRI sequences (such as T1-weighted sequences without fat suppression or T1-weighted GRE sequences) in which clips are best visualized should be incorporated. LC delineation on MRI may be the most challenging to interpret in the setting of full-thickness closure surgery and oncoplastic procedures due to less of a clear seroma and the degree of peri-cavity postoperative changes. Despite the limitations in the existing literature, it does appear that certain clinical situations may benefit from the use of MRI to better define the LC boundaries, such as cavities that interface with an axillary seroma, where toxicity may be higher from an overestimation of the cavity size [181]. Defining each of those clinical situations that could benefit from the addition of a breast MRI requires further study.
4.3.2. CTV and PTV Expansions
The clinical target volumes (CTVs) for breast radiotherapy include the highest-risk tissue around the LC or tumor bed (lumpectomy CTV) and the breast tissue (breast CTV). The lumpectomy CTV is typically a 10–15 mm expansion on the LC/tumor bed volume and is limited posteriorly similar to the breast CTV. Given the size of this expansion, large variations in the LC volume will be more clinically relevant than small ones. The breast CTV is defined by the glandular and fatty breast tissue seen on CT and the radio-opaque marker demarcating palpable breast tissue placed by the radiation oncologist at the time of CT simulation. The cranial, medial, and lateral borders of the breast are highly variable depending on breast size, patient position, and breast ptosis. Medially, the breast CTV should not cross the midline, and the posterior border excludes the pectoralis and serratus muscles unless clinically warranted by surgical pathology (RTOG Contouring consensus; MA 39 and NSABP B51 protocols). The planning target volume (PTV) is generally a 5–7 mm expansion of each CTV and generally should not cross the midline. The CTV and PTV volumes are limited to 5 mm from the skin surface to account for the dose build-up region.
4.3.3. The Impact of MRI on Whole-Breast Radiotherapy Planning
The role of MR imaging in delineating the breast CTV for radiation planning has been evaluated in several small series. Giezen et al. found that supine MRI-based volumes led to increased glandular breast tissue volumes by 4%, due to an extension of breast tissue further in the cranio-lateral and cranio-medial directions on MRI compared to CT [185]. Pogson et al. also noted a larger breast CTV in the prone vs. supine position [178]. In both studies, IOV by imaging modality was similar. Dundas et al. showed that while whole-breast CTV volumes were smaller on supine vs. prone CT when using MRI, the ultimately target volumes were the same after the CTV was expanded to a PTV and cropped 5 mm inside of the external skin contour [186]. Similarly, Kirby et al. found minimal differences in CT vs. MRI-based breast target volumes when expansion for PTV was taken into account [173]. In summary, MRI does not appear to offer significant advantage over CT for defining whole-breast targets for whole-breast radiotherapy.
4.3.4. Preoperative APBI
Preoperative APBI offers the potential advantages of better target visualization given intact tumor and smaller treatment volumes prior to resection, allowing for greer sparing of uninvolved breast tissue. The correlation between increased partial breast treatment volumes and poor cosmetic outcome makes strategies for limiting the target volume appealing [187,188]. This may be of further interest with the increasing use of oncoplastic procedures, which tend to result in larger post lumpectomy treatment volumes. Additionally, smaller treatment volumes and a strong correlation with pathological tumor size seen on MRI also facilitate dose escalation to a geometrically accurate target [189]. A disadvantage of preoperative APBI, on the other hand, is that the axillary nodal status in unknown, so careful examination of the axilla is recommended. Preoperative APBI delivered by using conformal external beam techniques or stereotactic body radiotherapy (SBRT or SABR) have been explored with and without MRI for treatment planning on a number of small prospective trials [126,190,191,192,193,194,195,196,197].
Optimal MRI sequences for treatment planning in the preoperative setting are CE T1-weighted fat-suppressed sequences for tumor visualization and T2-weighted sequences to differentiate from post-biopsy changes. An advantage of the presence of gross tumor is the visibility on MRI, which can reduce the IOV seen in the postoperative setting. As such, most studies have considered MRI critical for target definition given that not all breast tumors are reliably identified on CT alone and MRI better demonstrates tumor spiculation/irregularity [192,198]. While MRI offers more detailed anatomy, extended tumor spiculae, markers within tumors, blood vessels near tumors, and areas of increased enhancement in glandular breast tissue can lead to increased IOV in tumor delineation [199]. In an effort to reduce the IOV in target volume definition and aid in more comparable outcomes between future clinical trials, consensus-based guidelines for contouring primary breast tumors on MRI in preoperative PBI trials have been developed [199].
It has been hypothesized that definitive ablative radiotherapy could potentially offer an alternative, non-invasive curative approach for patients with early-stage breast cancer. Achieving a pCR with preoperative radiotherapy with or without systemic therapy would introduce the possibility of eliminating surgical resection for early-stage breast cancer. In a systematic review evaluating response and clinical outcomes after preoperative APBI followed by BCS in patients with low-risk breast cancer, recurrence rates were overall low, and three out of six studies reported pCR rates ranging from 9–48% [200]. Pathologic CR rates increased with increasing duration between preoperative radiotherapy and surgical excision. The ROCK trial demonstrated a 9% pCR rate 2 weeks after 21 Gy in a single fraction using Cyberknife in 22 patients [201]. Nicholas et al. showed a 15% pCR rate in 27 patients 3 weeks after 38.5 Gy in 10 fractions delivered twice daily [196]. The highest rates were found in the ABLATIVE trial with a pCR in 33% and 48% of patients 6 and 8 months after 21 Gy in a single fraction, respectively [194]. A preoperative paradigm has been shown to be feasible and can achieve acceptable toxicity and cosmesis with good local control on short-term follow-up; however, larger randomized trials with longer follow-up and larger patient numbers are needed.
Preoperative breast radiotherapy studies also offer a means for assessing the relationship between dose, tumor characteristics, and tumor response rates [194,202]. Quantitative and semi-quantitative analysis of pre- and post-treatment MRI has the potential to create biological imaging correlates and identify predictors of treatment response. This would facilitate appropriate patient selection for preoperative therapy, as well as broader implications for a more personalized medicine-based approach.
5. MRI-Guided Breast Radiotherapy
5.1. MRI-Linac Systems
MR-guided radiotherapy utilizing hybrid MRI-linac (MRL) systems is beginning to take a clinical foothold with its novel ability to provide real-time data on target cavity position and offer daily dosimetry optimization. The MRL combines an MRI scanner, with differing Tesla strength depending on themanufacturer, and an MV-energy photon linac mounted on a ring-shaped gantry, which enables real-time MRI acquisition during radiotherapy to account for anatomical position and motion [203]. Respiratory gating can be performed so that the radiation beam turns off if the target moves out of the pre-specified window [204]. Alternatively, target tracking entails the changing of a beam shape according to the target motion which is detected in a fast feedback loop [205,206]. These techniques can lead to the minimization and possible elimination of planning target volumes, thereby limiting normal tissue irradiation and allowing further dose escalation to target volumes.
5.2. Implications of Intrafraction Monitoring
Intrafraction motion during a diagnostic breast MRI is generally limited to <3 mm, with the exception of rare large body shifts due to setup or deep breaths over 1 cm [207]. Limited intrafraction motion and enhanced visualization of the surgical cavity afforded by MRI have led some investigators to evaluate the reduction of GTV to PTV expansion for APBI from the standard 2–2.5 cm to a 1 cm expansion with MR guidance, which is more consistent with an expansion utilized in brachytherapy planning [208]. The feasibility of reducing or eliminating the PTV expansion for MRL-based APBI has been evaluated in a 30-patient prospective registry study [209]. The lack of PTV resulted in treatment volumes 52% less than the conventional 1 cm CT-based PTV and appeared to be reasonable in most patients with a mean LC displacement (AP and SI directions) of 0.6 mm. However, the largest intrafraction displacement was 6 mm, suggesting that some patients will still benefit from a PTV, and perhaps the cine MR images at the time of simulation can be used to determine PTV expansion [209]. While intrafraction motion is generally small and MRL-guided therapy may allow for smaller PTV volumes, onboard MR image guidance is important due to the heterogeneity of intrafraction and interfraction motion between patients.
Another strategy for motion management that can account for day-to-day anatomical variation is MR-based adaptive planning. Ng et al. demonstrated the feasibility of a real-time MR-guided PBI adaptive planning workflow that incorporates same-day contouring based on the MRI images acquired before treatment and same-day dose optimization with a new tangential intensity-modulated radiotherapy plan [210]. This approach is one way to help mitigate the challenges of daily setup uncertainty and better account for potential changes in the surgical cavity, breast tissue, or cardiac positioning during treatment. An important consideration for this strategy is that more time is required than standard CT-guided treatment, and it requires the presence of a clinician, dosimetrist, physicist, and therapists, making it more resource-intensive.
5.3. Dosimetric Impact of the Magnetic Field on Breast Radiotherapy
One concern about using MRL for breast radiotherapy is the potential effects of the magnetic field on secondary electrons generated by ionizing radiation and their subsequent impact on skin dose. The magnetic field causes a reduction in the build-up distance due to a curved electron path trajectory, which results in a decreased entrance depth in tissue [211]. Additionally, electrons exiting the body are subjected to a phenomenon termed the electron return effect (ERE), where electrons take a circular path in the air and reenter the tissue-depositing dose at tissue–air interfaces [211,212]. The ERE dose increases with increasing magnetic strength and as such lateral ERE is strongest for a 3T and shows no effect at 0.2T [213]. While diagnostic imaging is performed with a 1.5T or 3T MRI, the use of weaker-strength magnetic fields such as a 0.35T MRI has been used successfully to visualize and track volumes during APBI [209]. ERE can be minimized when using multiple beams such as with intensity-modulated radiotherapy (IMRT); however, breast radiotherapy most commonly employs 3D conformal techniques where opposed oblique fields cover a large superficial area. This has led to some investigators lending caution to the use of MRL in whole breast irradiation (WBI). Lastly, the electron stream effect (ESE) has also been described, where electrons generated in the body start spiraling along the magnetic field towards the head and arm. One way to manage ESE is to shield areas outside of the treatment field with the use of bolus to avoid unwanted doses to the skin outside of the field [214].
These dosimetric concerns regarding local skin dose may be minimalized in APBI where treatment volumes are small, resulting in less skin involvement. For example, van Heijst et al. compared a seven-field IMRT APBI, a seven-field IMRT WBI and a two-field tangent WBI plans in varying magnetic strengths (0T, 0.35T and 1.5T) and found an increased skin dose with the whole breast plans that was negligible with APBI [215]. Another small study of palliative patients with intact tumors in close proximity to skin explored the impact of the magnetic field for different geometric beam arrangements (2–3 tangent beam arrangement, 5-beam IMRT and VMAT) used to deliver hypofractionated APBI (40 Gy in 5 fractions) on a 1.5T MRL [216]. In their APBI analysis, the largest variability between the plan configurations were in skin dose (more noticeable for the 3 mm than the 5 mm skin thickness), which was mitigated by adding additional beam angles. Differences seen between studies may be reflected in the difference in the number of beams, the skin thickness used for analysis or treatment planning systems [216]. Additionally, as noted by Kim et al., heart and lung doses were not significantly affected by the magnetic field, although the lung maximum was higher in the magnetic field, possibly due to the tissue–air interface at the chest wall. For higher ablative doses, caution should be taken to avoid overdosing the chest wall, which would increase the risk of chest wall pain or rib fracture [217]. While excess skin dose with the use of the MRL should be minimized for standard breast patients, this feature may be worth further investigation for those patients with cutaneous involvement who may benefit from “magnetic bolusing” [216].
5.4. Patient Position Considerations
The MRI bore is smaller than the CT bore, which can limit patient positioning options. In the supine position, he arm position may be more limited by the need to reduce the elbow span to accommodate the bore size. There is a limitation in the degree of inclination that is feasible, and a small wedge or no inclination may work best [218]. An anterior receiver coil can cause breast deformation while in the supine position, but this can be mitigated with the support of a coil bridge [160,175,181]. In the prone position, patient selection is limited by the space needed for a more pendulous breast to hang without touching the table top and for th placement of a receiver coil to the back of a patient so that a full body contour can be obtained for treatment planning. An addition consideration is patient positioning devices, which are not universally MRI-compatible, so careful selection is required and certain devices may need to be created if not commercially available.
6. Conclusions and Future Directions
In the assessment and treatment of breast cancer, the use of MR imaging has been primarily used to define the extent of initial and residual disease. However, its role is evolving to offer prognostic value and help guide treatment, particularly for APBI techniques. With further study, it may eventually be feasible to omit surgical resection in some patients who demonstrate a pCR to preoperative MRI-guided breast radiotherapy and systemic therapy alone. Another promising area of study is translational field of radiomics, which offers a means for extracting quantitative biomarkers from radiological images to provide information on the subtype, grade, heterogeneity, molecular profile, and genetic signatures. Radiomics enables the prediction of tumor biology, treatment response, and risk of recurrence. MRI-guided preoperative radiotherapy and radiomics provide a means for functional imaging analyses to better understand the radiobiology of breast cancer and facilitate the selection of patients who would be most likely to respond well to this strategy. Additionally, the preoperative radiotherapy approach also allows for the use of biopsy and lumpectomy tissue samples to investigate pre- and post-radiation gene expression [191]. The optimal use of MRI will allow for further personalization of breast cancer therapy.
Author Contributions
Conceptualization, I.W.; methodology, I.W. and D.A.; validation, I.W., D.A., J.W. and R.F.P.; formal analysis, I.W., D.A. and J.W.; investigation, I.W., D.A. and R.F.P.; resources, I.W., R.F.P., D.A. and S.A.R.; data curation, I.W. and D.A.; writing—original draft preparation, I.W., D.A. and R.F.P.; writing—review and editing, I.W., D.A., J.W., R.F.P. and S.A.R.; visualization, I.W. and S.A.R.; supervision, I.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACR | American College of Radiology |
| ADC | apparent diffusion coefficient |
| AJCC | American Joint Committee on Cancer |
| APBI | accelerated partial breast irradiation |
| BCS | breast conserving surgery |
| BCT | breast conservation therapy |
| BI-RADS | Breast Imaging Reporting and Data System |
| BPE | background parenchymal enhancement |
| CTV | clinical target volume |
| DCE | dynamic contrast-enhanced |
| DCIS | ductal carcinoma in situ |
| DWI | diffusion weighted imaging |
| ERE | electron return effect |
| ESE | electron stream effect |
| FDG | fludeoxyglucose |
| FGT | fibroglandular tissue |
| GRE | gradient echo sequence |
| ILC | invasive lobular carcinoma |
| IMLN | internal mammary lymph node |
| IOV | interobserver variability |
| LC | lumpectomy cavity |
| LR | local recurrence |
| MBD | mammographic breast density |
| MIP | maximum intensity projection |
| MRI | Magnetic resonance imaging |
| MRL | MR-linac |
| NAC | neoadjuvant chemotherapy |
| NME | non-mass enhancement |
| NPV | negative predictive value |
| OAR | organ at risk |
| pCR | pathologic complete response |
| PET/CT | positron emission tomography–computed tomography |
| PPV | positive predictive value |
| PTV | planning target volume |
| RECIST | response evaluation criteria in solid tumors |
| RFS | recurrence-free survival |
| SBRT/SABR | stereotactic body radiotherapy |
| SNR | signal-to-noise ratio |
| STIR | Short-tau Inversion Recovery |
| T | Tesla |
| US | Ultrasound |
| WLE | wide local excision |
References
- American Cancer Society. American Cancer Society: Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef] [PubMed]
- Pisano, E.D.; Hendrick, R.E.; Yaffe, M.J.; Baum, J.K.; Acharyya, S.; Cormack, J.B.; Hanna, L.A.; Conant, E.F.; Fajardo, L.L.; Bassett, L.W.; et al. Diagnostic accuracy of digital versus film mammography: Exploratory analysis of selected population subgroups in DMIST. Radiology 2008, 246, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Bohm-Velez, M.; Mahoney, M.C.; Evans, W.P., 3rd; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Podo, F.; Santoro, F.; Manoukian, S.; Bergonzi, S.; Trecate, G.; Vergnaghi, D.; Federico, M.; Cortesi, L.; Corcione, S.; et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): Final results. Investig. Radiol. 2011, 46, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Luft, N.; Bernhart, C.; Weber, M.; Bernathova, M.; Tea, M.K.; Rudas, M.; Singer, C.F.; Helbich, T.H. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J. Clin. Oncol. 2015, 33, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Weigel, S.; Schrading, S.; Arand, B.; Bieling, H.; Konig, R.; Tombach, B.; Leutner, C.; Rieber-Brambs, A.; Nordhoff, D.; et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. J. Clin. Oncol. 2010, 28, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Blume, J.D.; Weatherall, P.; Thickman, D.; Hylton, N.; Warner, E.; Pisano, E.; Schnitt, S.J.; Gatsonis, C.; Schnall, M.; et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 2005, 103, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Liberman, L.; Ballon, D.J.; Robson, M.; Abramson, A.F.; Heerdt, A.; Dershaw, D.D. MRI of occult breast carcinoma in a high-risk population. AJR Am. J. Roentgenol. 2003, 181, 619–626. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef]
- Leach, M.O.; Boggis, C.R.; Dixon, A.K.; Easton, D.F.; Eeles, R.A.; Evans, D.G.; Gilbert, F.J.; Griebsch, I.; Hoff, R.J.; Kessar, P.; et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005, 365, 1769–1778. [Google Scholar] [CrossRef]
- Warner, E.; Plewes, D.B.; Hill, K.A.; Causer, P.A.; Zubovits, J.T.; Jong, R.A.; Cutrara, M.R.; DeBoer, G.; Yaffe, M.J.; Messner, S.J.; et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004, 292, 1317–1325. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Localio, A.R.; Conant, E.F.; Rosen, M.; Thomas, K.M.; Schnall, M.D. Multimodality screening of high-risk women: A prospective cohort study. J. Clin. Oncol. 2009, 27, 6124–6128. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Isaacs, C.; Schnall, M.D.; Pisano, E.D.; Ascher, S.M.; Weatherall, P.T.; Bluemke, D.A.; Bowen, D.J.; Marcom, P.K.; Armstrong, D.K.; et al. Cancer yield of mammography, MR, and US in high-risk women: Prospective multi-institution breast cancer screening study. Radiology 2007, 244, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, A.; Curpen, B.; Warner, E.; Betel, C.; Wright, B.; Jong, R. Breast MRI as an adjunct to mammography for breast cancer screening in high-risk patients: Retrospective review. AJR Am. J. Roentgenol. 2015, 204, 889–897. [Google Scholar] [CrossRef]
- Lehman, C.D.; Gatsonis, C.; Kuhl, C.K.; Hendrick, R.E.; Pisano, E.D.; Hanna, L.; Peacock, S.; Smazal, S.F.; Maki, D.D.; Julian, T.B.; et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N. Engl. J. Med. 2007, 356, 1295–1303. [Google Scholar] [CrossRef]
- Niell, B.L.; Gavenonis, S.C.; Motazedi, T.; Chubiz, J.C.; Halpern, E.P.; Rafferty, E.A.; Lee, J.M. Auditing a breast MRI practice: Performance measures for screening and diagnostic breast MRI. J. Am. Coll. Radiol. 2014, 11, 883–889. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Keulers, A.; Strobel, K.; Schneider, H.; Gaisa, N.; Schrading, S. Not all false positive diagnoses are equal: On the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography/digital tomosynthesis screening. Breast Cancer Res. 2018, 20, 13. [Google Scholar] [CrossRef]
- Sung, J.S.; Stamler, S.; Brooks, J.; Kaplan, J.; Huang, T.; Dershaw, D.D.; Lee, C.H.; Morris, E.A.; Comstock, C.E. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016, 280, 716–722. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Leutner, C.; Schild, H.H.; Schrading, S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017, 283, 361–370. [Google Scholar] [CrossRef]
- Turnbull, L.; Brown, S.; Harvey, I.; Olivier, C.; Drew, P.; Napp, V.; Hanby, A.; Brown, J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet 2010, 375, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Turner, R.; Morrow, M. Preoperative Magnetic Resonance Imaging in Breast Cancer: Meta-Analysis of Surgical Outcomes. Ann. Surg. 2013, 257, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Siegler, P.; Holloway, C.M.; Causer, P.; Thevathasan, G.; Plewes, D.B. Supine breast MRI. J. Magn. Reson. Imaging 2011, 34, 1212–1217. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.E.M.; Ghate, S. Solitary, Well-Circumscribed, T2 Hyperintense Masses on MRI Have Very Low Malignancy Rates. J. Breast Imaging 2019, 1, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, L.; Savelli, S.; Angeletti, M.; Porfiri, L.M.; D’Ambrosio, I.; Maggi, C.; Castro, E.D.; Bennati, P.; Fanelli, G.P.; Vestri, A.R.; et al. Breast MRI: Are T2 IR sequences useful in the evaluation of breast lesions? Eur. J. Radiol. 2009, 71, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Arponen, O.; Masarwah, A.; Sutela, A.; Taina, M.; Kononen, M.; Sironen, R.; Hakumaki, J.; Vanninen, R.; Sudah, M. Incidentally detected enhancing lesions found in breast MRI: Analysis of apparent diffusion coefficient and T2 signal intensity significantly improves specificity. Eur. Radiol. 2016, 26, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Kim, H.J.; Kim, T.H.; Ryeom, H.K.; Lee, J.; Kim, G.C.; Yuk, J.S.; Kim, W.H. Invasive Breast Cancer: Prognostic Value of Peritumoral Edema Identified at Preoperative MR Imaging. Radiology 2018, 287, 68–75. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Watanabe, J. Can T2-weighted 3-T breast MRI predict clinically occult inflammatory breast cancer before pathological examination? A single-center experience. Breast Cancer 2014, 21, 115–121. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Watanabe, J. Is evaluation of the presence of prepectoral edema on T2-weighted with fat-suppression 3 T breast MRI a simple and readily available noninvasive technique for estimation of prognosis in patients with breast cancer? Breast Cancer 2014, 21, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Chauhan, S. Effectivity of combined diffusion-weighted imaging and contrast-enhanced MRI in malignant and benign breast lesions. Pol. J. Radiol. 2018, 83, e82–e93. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2019, 30, 1436–1450. [Google Scholar] [CrossRef]
- Morris, E.A.; Comstock, C.E.; Lee, C.H. ACR BI-RADS® Magnetic Resonance Imaging. In ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Ecanow, J.S.; Abe, H.; Newstead, G.M.; Ecanow, D.B.; Jeske, J.M. Axillary staging of breast cancer: What the radiologist should know. RadioGraphics 2013, 33, 1589–1612. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.; Sung, J.; Lee, C.; Comstock, C.; Wynn, R.; Morris, E. Characteristics and outcome of enhancing foci followed on breast MRI with management implications. Clin. Radiol. 2014, 69, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.; Morris, E.A.; Lee, M.J.; Kaplan, J.B.; LaTrenta, L.R.; Menell, J.H.; Abramson, A.F.; Dashnaw, S.M.; Ballon, D.J.; Dershaw, D.D. Breast lesions detected on MR imaging: Features and positive predictive value. AJR Am. J. Roentgenol. 2002, 179, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.A.; Benndorf, M.; Dietzel, M.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A. False-positive findings at contrast-enhanced breast MRI: A BI-RADS descriptor study. AJR Am. J. Roentgenol. 2010, 194, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.W.; Schnall, M.D.; Orel, S.G. Update of breast MR imaging architectural interpretation model. Radiology 2001, 219, 484–494. [Google Scholar] [CrossRef]
- Giess, C.S.; Raza, S.; Birdwell, R.L. Patterns of nonmasslike enhancement at screening breast MR imaging of high-risk premenopausal women. RadioGraphics 2013, 33, 1343–1360. [Google Scholar] [CrossRef]
- Gity, M.; Ghazi Moghadam, K.; Jalali, A.H.; Shakiba, M. Association of Different MRI BIRADS Descriptors With Malignancy in Non Mass-Like Breast Lesions. Iran. Red. Crescent Med. J. 2014, 16, e26040. [Google Scholar] [CrossRef]
- Tozaki, M.; Fukuda, K. High-spatial-resolution MRI of non-masslike breast lesions: Interpretation model based on BI-RADS MRI descriptors. AJR Am. J. Roentgenol. 2006, 187, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Kasami, M. High-spatial-resolution 3-T breast MRI of nonmasslike enhancement lesions: An analysis of their features as significant predictors of malignancy. AJR Am. J. Roentgenol. 2012, 198, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, M.; Igarashi, T.; Fukuda, K. Breast MRI using the VIBE sequence: Clustered ring enhancement in the differential diagnosis of lesions showing non-masslike enhancement. AJR Am. J. Roentgenol. 2006, 187, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Giess, C.S.; Yeh, E.D.; Raza, S.; Birdwell, R.L. Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. RadioGraphics 2014, 34, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dershaw, D.D.; Lee, C.H.; Joo, S.; Morris, E.A. Breast MRI after conservation therapy: Usual findings in routine follow-up examinations. AJR Am. J. Roentgenol. 2010, 195, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.E.; Kim, S.H.; Lee, A.W. Background parenchymal enhancement in breast MRIs of breast cancer patients: Impact on tumor size estimation. Eur. J. Radiol. 2014, 83, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Preibsch, H.; Beckmann, J.; Pawlowski, J.; Kloth, C.; Hahn, M.; Staebler, A.; Wietek, B.M.; Nikolaou, K.; Wiesinger, B. Accuracy of Breast Magnetic Resonance Imaging Compared to Mammography in the Preoperative Detection and Measurement of Pure Ductal Carcinoma In Situ: A Retrospective Analysis. Acad. Radiol. 2019, 26, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Schnall, M.D.; Rosten, S.; Englander, S.; Orel, S.G.; Nunes, L.W. A combined architectural and kinetic interpretation model for breast MR images. Acad. Radiol. 2001, 8, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.D.; Staff, R.T.; Redpath, T.W.; Gilbert, F.J.; Ah-See, A.K.; Brookes, J.A.; Miller, I.D.; Payne, S. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: Comparison with pathology of excised nodes. Br. J. Radiol. 2002, 75, 220–228. [Google Scholar] [CrossRef]
- Mortellaro, V.E.; Marshall, J.; Singer, L.; Hochwald, S.N.; Chang, M.; Copeland, E.M.; Grobmyer, S.R. Magnetic resonance imaging for axillary staging in patients with breast cancer. J. Magn. Reson. Imaging 2009, 30, 309–312. [Google Scholar] [CrossRef]
- Kvistad, K.A.; Rydland, J.; Smethurst, H.B.; Lundgren, S.; Fjosne, H.E.; Haraldseth, O. Axillary lymph node metastases in breast cancer: Preoperative detection with dynamic contrast-enhanced MRI. Eur. Radiol. 2000, 10, 1464–1471. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Re-porting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef]
- Boetes, C.; Mus, R.D.; Holland, R.; Barentsz, J.O.; Strijk, S.P.; Wobbes, T.; Hendriks, J.H.; Ruys, S.H. Breast tumors: Comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology 1995, 197, 743–747. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Prevos, R.; Smidt, M.; Tjan-Heijnen, V.C.; van Goethem, M.; Schipper, R.; Beets-Tan, R.G.; Wildberger, J.E. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: A systematic review. Insights Imaging 2013, 4, 163–175. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Houssami, N.; Macaskill, P.; Sardanelli, F.; Irwig, L.; Mamounas, E.P.; von Minckwitz, G.; Brennan, M.E.; Ciatto, S. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl. Cancer Inst. 2013, 105, 321–333. [Google Scholar] [CrossRef]
- Yeh, E.; Slanetz, P.; Kopans, D.B.; Rafferty, E.; Georgian-Smith, D.; Moy, L.; Halpern, E.; Moore, R.; Kuter, I.; Taghian, A. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am. J. Roentgenol. 2005, 184, 868–877. [Google Scholar] [CrossRef]
- Jethava, A.; Ali, S.; Wakefield, D.; Crowell, R.; Sporn, J.; Vrendenburgh, J. Diagnostic Accuracy of MRI in Predicting Breast Tumor Size: Comparative Analysis of MRI vs Histopathological Assessed Breast Tumor Size. Conn. Med. 2015, 79, 261–267. [Google Scholar]
- Mennella, S.; Garlaschi, A.; Paparo, F.; Perillo, M.; Celenza, M.; Massa, T.; Rollandi, G.A.; Garlaschi, G. Magnetic resonance imaging of breast cancer: Factors affecting the accuracy of preoperative lesion sizing. Acta Radiol. 2015, 56, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rominger, M.; Berg, D.; Frauenfelder, T.; Ramaswamy, A.; Timmesfeld, N. Which factors influence MRI-pathology concordance of tumour size measurements in breast cancer? Eur. Radiol. 2016, 26, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Breast Imaging; Slanetz, P.J.; Moy, L.; Baron, P.; di Florio, R.M.; Green, E.D.; Heller, S.L.; Holbrook, A.I.; Lee, S.J.; Lewin, A.A.; et al. ACR Appropriateness Criteria((R)) Monitoring Response to Neoadjuvant Systemic Therapy for Breast Cancer. J. Am. Coll. Radiol. 2017, 14, S462–S475. [Google Scholar] [CrossRef]
- Kazama, T.; Nakamura, S.; Doi, O.; Suzuki, K.; Hirose, M.; Ito, H. Prospective evaluation of pectoralis muscle invasion of breast cancer by MR imaging. Breast Cancer 2005, 12, 312–316. [Google Scholar] [CrossRef]
- Morris, E.A.; Schwartz, L.H.; Drotman, M.B.; Kim, S.J.; Tan, L.K.; Liberman, L.; Abramson, A.F.; Van Zee, K.J.; Dershaw, D.D. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast MR images: Early experience. Radiology 2000, 214, 67–72. [Google Scholar] [CrossRef]
- Moon, J.Y.; Chang, Y.W.; Lee, E.H.; Seo, D.Y. Malignant invasion of the nipple-areolar complex of the breast: Usefulness of breast MRI. AJR Am. J. Roentgenol. 2013, 201, 448–455. [Google Scholar] [CrossRef]
- Tardivon, A.A.; Ollivier, L.; El Khoury, C.; Thibault, F. Monitoring therapeutic efficacy in breast carcinomas. Eur. Radiol. 2006, 16, 2549–2558. [Google Scholar] [CrossRef]
- Houssami, N.; Ciatto, S.; Macaskill, P.; Lord, S.J.; Warren, R.M.; Dixon, J.M.; Irwig, L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J. Clin. Oncol. 2008, 26, 3248–3258. [Google Scholar] [CrossRef]
- Fischer, U.; Kopka, L.; Grabbe, E. Breast carcinoma: Effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999, 213, 881–888. [Google Scholar] [CrossRef]
- Hollingsworth, A.B.; Stough, R.G.; O’Dell, C.A.; Brekke, C.E. Breast magnetic resonance imaging for preoperative locoregional staging. Am. J. Surg. 2008, 196, 389–397. [Google Scholar] [CrossRef]
- Liberman, L.; Morris, E.A.; Kim, C.M.; Kaplan, J.B.; Abramson, A.F.; Menell, J.H.; Van Zee, K.J.; Dershaw, D.D. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am. J. Roentgenol. 2003, 180, 333–341. [Google Scholar] [CrossRef]
- Lee, S.G.; Orel, S.G.; Woo, I.J.; Cruz-Jove, E.; Putt, M.E.; Solin, L.J.; Czerniecki, B.J.; Schnall, M.D. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: Preliminary results. Radiology 2003, 226, 773–778. [Google Scholar] [CrossRef]
- Lehman, C.D.; Blume, J.D.; Thickman, D.; Bluemke, D.A.; Pisano, E.; Kuhl, C.; Julian, T.B.; Hylton, N.; Weatherall, P.; O’Loughlin, M.; et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: Results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J. Surg. Oncol. 2005, 92, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Faermann, R.W.J.; Chepelev, L.; Kendal, W.; Verma, R.; Scott-Moncrieff, A.; Peddle, S.; Doherty, G.; Lau, J.; Ramsay, T.; Arnaout, A.; et al. Outcomes after Surgery for Early Stage Breast Cancer in Women Staged With Preoperative Breast Magnetic Resonance Imaging According to Breast Tissue Density. J. Breast Imaging 2019, 1, 115–121. [Google Scholar] [CrossRef]
- Khan, A.; Sabel, M.S.; Nees, A.; Diehl, K.M.; Cimmino, V.M.; Kleer, C.G.; Schott, A.F.; Hayes, D.F.; Chang, A.E.; Newman, L.A. Comprehensive axillary evaluation in neoadjuvant chemotherapy patients with ultrasonography and sentinel lymph node biopsy. Ann. Surg. Oncol. 2005, 12, 697–704. [Google Scholar] [CrossRef]
- Henry-Tillman, R.; Glover-Collins, K.; Preston, M.; Gallagher, K.; Tummel, E.; Robertson, Y.V.; Ochoa, D.; Korourian, S.; Westbrook, K.; Klimberg, V.S. The SAVE review: Sonographic analysis versus excision for axillary staging in breast cancer. J. Am. Coll. Surg. 2015, 220, 560–567. [Google Scholar] [CrossRef]
- Baltzer, P.A.; Dietzel, M.; Burmeister, H.P.; Zoubi, R.; Gajda, M.; Camara, O.; Kaiser, W.A. Application of MR mammography beyond local staging: Is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. AJR Am. J. Roentgenol. 2011, 196, W641–W647. [Google Scholar] [CrossRef]
- Korteweg, M.A.; Zwanenburg, J.J.; Hoogduin, J.M.; van den Bosch, M.A.; van Diest, P.J.; van Hillegersberg, R.; Eijkemans, M.J.; Mali, W.P.; Luijten, P.R.; Veldhuis, W.B. Dissected sentinel lymph nodes of breast cancer patients: Characterization with high-spatial-resolution 7-T MR imaging. Radiology 2011, 261, 127–135. [Google Scholar] [CrossRef]
- Memarsadeghi, M.; Riedl, C.C.; Kaneider, A.; Galid, A.; Rudas, M.; Matzek, W.; Helbich, T.H. Axillary lymph node metastases in patients with breast carcinomas: Assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology 2006, 241, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Schmidt, R.A.; Kulkarni, K.; Sennett, C.A.; Mueller, J.S.; Newstead, G.M. Axillary lymph nodes suspicious for breast cancer metastasis: Sampling with US-guided 14-gauge core-needle biopsy--clinical experience in 100 patients. Radiology 2009, 250, 41–49. [Google Scholar] [CrossRef]
- Alvarez, S.; Anorbe, E.; Alcorta, P.; Lopez, F.; Alonso, I.; Cortes, J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef]
- Bedi, D.G.; Krishnamurthy, R.; Krishnamurthy, S.; Edeiken, B.S.; Le-Petross, H.; Fornage, B.D.; Bassett, R.L., Jr.; Hunt, K.K. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: In vitro sonographic study. AJR Am. J. Roentgenol. 2008, 191, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.H.; Daly, C.P.; Nees, A.V.; Helvie, M.A. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology 2010, 257, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Assing, M.A.; Patel, B.K.; Karamsadkar, N.; Weinfurtner, J.; Usmani, O.; Kiluk, J.V.; Drukteinis, J.S. A comparison of the diagnostic accuracy of magnetic resonance imaging to axillary ultrasound in the detection of axillary nodal metastases in newly diagnosed breast cancer. Breast J. 2017, 23, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Almerey, T.; Villacreses, D.; Li, Z.; Patel, B.; McDonough, M.; Gibson, T.; Maimone, S.; Gray, R.; McLaughlin, S.A. Value of Axillary Ultrasound after Negative Axillary MRI for Evaluating Nodal Status in High-Risk Breast Cancer. J. Am. Coll. Surg. 2019, 228, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jain, P.A.; Jethwa, S.C.; Tripathy, D.; Yamashita, M.W. Radiologist’s role in breast cancer staging: Providing key information for clinicians. RadioGraphics 2014, 34, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Scatarige, J.C.; Boxen, I.; Smathers, R.L. Internal mammary lymphadenopathy: Imaging of a vital lymphatic pathway in breast cancer. RadioGraphics 1990, 10, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ramani, S.K.; Rastogi, A.; Thakur, M.H. Incidence of internal mammary node in locally advanced breast cancer and its correlation with metastatic disease: A retrospective observational study. Br. J. Radiol. 2019, 92, 20190098. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Zheng, K.; Yin, X.D.; Xing, L.; Zhang, A.J.; Shi, Y.; Kong, L.Q.; Li, F.; Ma, B.L.; et al. Predictors of internal mammary lymph nodes (IMLN) metastasis and disease-free survival comparison between IMLN-positive and IMLN-negative breast cancer patients: Results from Western China Clinical Cooperation Group (WCCCG) database (CONSORT). Medicine 2018, 97, e11296. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Leaw, S.; Wang, Z.; Wang, P.; Hu, X.; Chen, J.; Lu, J.; Shao, Z. Internal mammary lymph node recurrence: Rare but characteristic metastasis site in breast cancer. BMC Cancer 2010, 10, 479. [Google Scholar] [CrossRef]
- Behzadi, S.T.; Moser, R.; Kiesl, S.; Nano, J.; Peeken, J.C.; Fischer, J.C.; Fallenberg, E.M.; Huber, T.; Haller, B.; Klein, E.; et al. Tumor Contact With Internal Mammary Perforator Vessels as Risk Factor for Gross Internal Mammary Lymph Node Involvement in Patients With Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024in press. [CrossRef] [PubMed]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef]
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef]
- Mack, M.; Chetlen, A.; Liao, J. Incidental Internal Mammary Lymph Nodes Visualized on Screening Breast MRI. AJR Am. J. Roentgenol. 2015, 205, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.M.; Munir, R.; Wisner, D.J.; Azziz, A.; Holland, B.C.; Kornak, J.; Joe, B.N. Internal mammary lymph nodes as incidental findings at screening breast MRI. Clin. Imaging 2015, 39, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Delikat, A.; Liao, J.; Chetlen, A.L. Pre- and post-magnetic resonance imaging features of suspicious internal mammary lymph nodes in breast cancer patients receiving neo-adjuvant therapy: Are any imaging features predictive of malignancy? Breast J. 2018, 24, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Odagiri, K.; Andoh, K.; Doiuchi, T.; Sugimura, K.; Shiotani, S.; Asaga, T. Evaluation of small internal mammary lymph node metastases in breast cancer by MRI. Radiat. Med. 1999, 17, 189–193. [Google Scholar] [PubMed]
- Seo, M.J.; Lee, J.J.; Kim, H.O.; Chae, S.Y.; Park, S.H.; Ryu, J.S.; Ahn, S.H.; Lee, J.W.; Son, B.H.; Gong, G.Y.; et al. Detection of internal mammary lymph node metastasis with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Eubank, W.B.; Mankoff, D.A.; Takasugi, J.; Vesselle, H.; Eary, J.F.; Shanley, T.J.; Gralow, J.R.; Charlop, A.; Ellis, G.K.; Lindsley, K.L.; et al. 18fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J. Clin. Oncol. 2001, 19, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Segaert, I.; Mottaghy, F.; Ceyssens, S.; De Wever, W.; Stroobants, S.; Van Ongeval, C.; Van Limbergen, E.; Wildiers, H.; Paridaens, R.; Vergote, I.; et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010, 16, 617–624. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Lee, A.W. Comparisons of Positron Emission Tomography/Computed Tomography and Ultrasound Imaging for Detection of Internal Mammary Lymph Node Metastases in Patients With Breast Cancer and Pathologic Correlation by Ultrasound-Guided Biopsy Procedures. J. Ultrasound Med. 2015, 34, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.F.; Quinn, T.J.; Siddiqui, Z.; Jawad, M.S.; Chen, P.Y.; Gustafson, G.S.; Dilworth, J.T. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother. Oncol. 2020, 142, 186–194. [Google Scholar] [CrossRef]
- Christiansen, P.; Mele, M.; Bodilsen, A.; Rocco, N.; Zachariae, R. Breast-Conserving Surgery or Mastectomy?: Impact on Survival. Ann. Surg. Open 2022, 3, e205. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Kavanagh, B.; Bhatnagar, A.; Jacobson, G.; Lutz, S.; Patton, C.; Potters, L.; Steinberg, M. Choosing wisely: The American Society for Radiation Oncology’s top 5 list. Pract. Radiat. Oncol. 2014, 4, 349–355. [Google Scholar] [CrossRef]
- Fisher, E.R.; Dignam, J.; Tan-Chiu, E.; Costantino, J.; Fisher, B.; Paik, S.; Wolmark, N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: Intraductal carcinoma. Cancer 1999, 86, 429–438. [Google Scholar] [CrossRef]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef]
- Houssami, N.; Hayes, D.F. Review of Preoperative Magnetic Resonance Imaging (MRI) in Breast Cancer: Should MRI Be Performed on All Women with Newly Diagnosed, Early Stage Breast Cancer? CA Cancer J. Clin. 2009, 59, 290–302. [Google Scholar] [CrossRef]
- Scomersi, S.; Urbani, M.; Tonutti, M.; Zanconati, F.; Bortul, M. Role of magnetic resonance imaging in managing selected women with newly diagnosed breast cancer. Breast 2010, 19, 115–119. [Google Scholar] [CrossRef]
- Peters, N.H.; van Esser, S.; van den Bosch, M.A.; Storm, R.K.; Plaisier, P.W.; van Dalen, T.; Diepstraten, S.C.; Weits, T.; Westenend, P.J.; Stapper, G.; et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: The MONET–randomised controlled trial. Eur. J. Cancer 2011, 47, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Turner, R.; Macaskill, P.; Turnbull, L.W.; McCready, D.R.; Tuttle, T.M.; Vapiwala, N.; Solin, L.J. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J. Clin. Oncol. 2014, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Waters, J.; Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011, 378, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Wardelmann, E.; Kuhn, W.; Maass, N.; Schrading, S. Impact of Preoperative Breast MR Imaging and MR-guided Surgery on Diagnosis and Surgical Outcome of Women with Invasive Breast Cancer with and without DCIS Component. Radiology 2017, 284, 645–655. [Google Scholar] [CrossRef]
- Vos, E.L.; Voogd, A.C.; Verhoef, C.; Siesling, S.; Obdeijn, I.M.; Koppert, L.B. Benefits of preoperative MRI in breast cancer surgery studied in a large population-based cancer registry. Br. J. Surg. 2015, 102, 1649–1657. [Google Scholar] [CrossRef]
- Obdeijn, I.M.; Tilanus-Linthorst, M.M.; Spronk, S.; van Deurzen, C.H.; de Monye, C.; Hunink, M.G.; Menke, M.B. Preoperative breast MRI can reduce the rate of tumor-positive resection margins and reoperations in patients undergoing breast-conserving surgery. AJR Am. J. Roentgenol. 2013, 200, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B., Jr.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Barbieri, E.; Sagona, A.; Bottini, A.; Canavese, G.; Gentile, D. De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers 2024, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Croshaw, R.; Shapiro-Wright, H.; Svensson, E.; Erb, K.; Julian, T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann. Surg. Oncol. 2011, 18, 3160–3163. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.S.; Liu, S.Y.; Shen, K.W. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: A meta-analysis. AJR Am. J. Roentgenol. 2010, 195, 260–268. [Google Scholar] [CrossRef]
- Wu, L.M.; Hu, J.N.; Gu, H.Y.; Hua, J.; Chen, J.; Xu, J.R. Can diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast Cancer Res. Treat. 2012, 135, 17–28. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Trahan, T.J.; Xiao, J.; Taghipour, M.; Mena, E.; Connolly, R.M.; Subramaniam, R.M. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist 2016, 21, 931–939. [Google Scholar] [CrossRef]
- Palta, M.; Yoo, S.; Adamson, J.D.; Prosnitz, L.R.; Horton, J.K. Preoperative single fraction partial breast radiotherapy for early-stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 37–42. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef]
- Bevers, T.B.; Helvie, M.; Bonaccio, E.; Calhoun, K.E.; Daly, M.B.; Farrar, W.B.; Garber, J.E.; Gray, R.; Greenberg, C.C.; Greenup, R.; et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Hylton, N.M.; Blume, J.D.; Bernreuter, W.K.; Pisano, E.D.; Rosen, M.A.; Morris, E.A.; Weatherall, P.T.; Lehman, C.D.; Newstead, G.M.; Polin, S.; et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—Results from ACRIN 6657/I-SPY TRIAL. Radiology 2012, 263, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Chagpar, A.B.; Middleton, L.P.; Sahin, A.A.; Dempsey, P.; Buzdar, A.U.; Mirza, A.N.; Ames, F.C.; Babiera, G.V.; Feig, B.W.; Hunt, K.K.; et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann. Surg. 2006, 243, 257–264. [Google Scholar] [CrossRef]
- Belli, P.; Costantini, M.; Malaspina, C.; Magistrelli, A.; LaTorre, G.; Bonomo, L. MRI accuracy in residual disease evaluation in breast cancer patients treated with neoadjuvant chemotherapy. Clin. Radiol. 2006, 61, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Semiglazov, V. RECIST for Response (Clinical and Imaging) in Neoadjuvant Clinical Trials in Operable Breast Cancer. J. Natl. Cancer Inst. Monogr. 2015, 2015, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of imaging in neoadjuvant therapy for breast cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Andrade, W.P.; Lima, E.N.; Osorio, C.A.; do Socorro Maciel, M.; Baiocchi, G.; Bitencourt, A.G.; Fanelli, M.F.; Damascena, A.S.; Soares, F.A. Can FDG-PET/CT predict early response to neoadjuvant chemotherapy in breast cancer? Eur. J. Surg. Oncol. 2013, 39, 1358–1363. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Barni, S. Neoadjuvant chemotherapy and concomitant trastuzumab in breast cancer: A pooled analysis of two randomized trials. Anticancer Drugs 2011, 22, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, R.A.; Yau, C.; Rosen, M.; Tandon, V.J.; I-Spy, T.; Investigators, A.; Hylton, N.; Esserman, L.J. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann. Surg. Oncol. 2013, 20, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.P.; Toro-Burguete, J.; Dang, H.; Young, J.; Soran, A.; Zuley, M.; Bhargava, R.; Bonaventura, M.; Johnson, R.; Ahrendt, G. MRI staging after neoadjuvant chemotherapy for breast cancer: Does tumor biology affect accuracy? Ann. Surg. Oncol. 2011, 18, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Bahri, S.; Mehta, R.S.; Kuzucan, A.; Yu, H.J.; Carpenter, P.M.; Feig, S.A.; Lin, M.; Hsiang, D.J.; Lane, K.T.; et al. Breast cancer: Evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology 2011, 261, 735–743. [Google Scholar] [CrossRef]
- Delille, J.P.; Slanetz, P.J.; Yeh, E.D.; Halpern, E.F.; Kopans, D.B.; Garrido, L. Invasive ductal breast carcinoma response to neoadjuvant chemotherapy: Noninvasive monitoring with functional MR imaging pilot study. Radiology 2003, 228, 63–69. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Sardanelli, F.; Ciatto, S.; Mamounas, E.; Brennan, M.; Macaskill, P.; Irwig, L.; von Minckwitz, G.; Houssami, N. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: Systematic review of the accuracy of MRI. Breast 2012, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Hayes, C.; Assersohn, L.; Powles, T.; Makris, A.; Suckling, J.; Leach, M.O.; Husband, J.E. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: Initial clinical results. Radiology 2006, 239, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Chen, J.H.; Mehta, R.S.; Carpenter, P.M.; Nie, K.; Kwon, S.Y.; Yu, H.J.; Nalcioglu, O.; Su, M.Y. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann. Surg. Oncol. 2009, 16, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Im, Y.H.; Han, B.K.; Choi, N.; Lee, J.; Kim, J.H.; Choi, Y.L.; Ahn, J.S.; Nam, S.J.; Park, Y.S.; et al. Accuracy of MRI for estimating residual tumor size after neoadjuvant chemotherapy in locally advanced breast cancer: Relation to response patterns on MRI. Acta Oncol. 2007, 46, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Han, B.K.; Kim, R.B.; Ko, E.Y.; Shin, J.H.; Hahn, S.Y.; Nam, S.J.; Lee, J.E.; Lee, S.K.; Im, Y.H.; et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann. Surg. Oncol. 2013, 20, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Han, H.; Han, B.K.; Kim, S.M.; Kim, R.B.; Lee, G.W.; Park, Y.H.; Nam, S.J. Prognostic Significance of a Complete Response on Breast MRI in Patients Who Received Neoadjuvant Chemotherapy According to the Molecular Subtype. Korean J. Radiol. 2015, 16, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Wasser, K.; Sinn, H.P.; Fink, C.; Klein, S.K.; Junkermann, H.; Lüdemann, H.P.; Zuna, I.; Delorme, S. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur. Radiol. 2003, 13, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Desbiez-Bourcier, A.V.; Chapiron, C.; Arbion, F.; Body, G.; Brunereau, L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur. J. Surg. Oncol. 2004, 30, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Boughey, J.C.; Jones, K.N.; Shah, S.S.; Glazebrook, K.N. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann. Surg. Oncol. 2013, 20, 3199–3204. [Google Scholar] [CrossRef]
- Javid, S.; Segara, D.; Lotfi, P.; Raza, S.; Golshan, M. Can breast MRI predict axillary lymph node metastasis in women undergoing neoadjuvant chemotherapy. Ann. Surg. Oncol. 2010, 17, 1841–1846. [Google Scholar] [CrossRef]
- Alvarado, R.; Yi, M.; Le-Petross, H.; Gilcrease, M.; Mittendorf, E.A.; Bedrosian, I.; Hwang, R.F.; Caudle, A.S.; Babiera, G.V.; Akins, J.S.; et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann. Surg. Oncol. 2012, 19, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, G.; Trimboli, R.M.; Benedek, A.; Jereczek-Fossa, B.A.; Fossati, P.; Leonardi, M.C.; Carbonaro, L.A.; Orecchia, R.; Sardanelli, F. MR Imaging for Selection of Patients for Partial Breast Irradiation: A Systematic Review and Meta-Analysis. Radiology 2015, 277, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Dorn, P.L.; Al-Hallaq, H.A.; Haq, F.; Goldberg, M.; Abe, H.; Hasan, Y.; Chmura, S.J. A prospective study of the utility of magnetic resonance imaging in determining candidacy for partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Godinez, J.; Gombos, E.C.; Chikarmane, S.A.; Griffin, G.K.; Birdwell, R.L. Breast MRI in the evaluation of eligibility for accelerated partial breast irradiation. AJR Am. J. Roentgenol. 2008, 191, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Consul, N.; Jadeja, P.; Kwak, E.; Patel, S.; Wynn, R.; Hershman, D.; Connolly, E.P.; Ha, R. Utility of preoperative breast MRI in patient selection for accelerated partial breast irradiation by different consensus guidelines. Breast J. 2019, 25, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, J.S.; Gombos, E.C.; Raza, S.; Chikarmane, S.A.; Swami, A.; Birdwell, R.L. MR imaging assessment of the breast after breast conservation therapy: Distinguishing benign from malignant lesions. RadioGraphics 2012, 32, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Batumalai, V.; Liney, G.; Delaney, G.P.; Rai, R.; Boxer, M.; Min, M.; Berry, M.; Pham, T.; Phan, P.; Choong, C.; et al. Assessment of MRI image quality for various setup positions used in breast radiotherapy planning. Radiother. Oncol. 2016, 119, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.N.Y.; Ter Beek, L.C.; Loo, C.E.; Winter-Warnars, G.; Lange, C.A.H.; van Loveren, M.; Alderliesten, T.; Sonke, J.J.; Nijkamp, J. Supine Breast MRI Using Respiratory Triggering. Acad. Radiol. 2017, 24, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Pierce, L.J.; Feng, M.; Griffith, K.A.; Jagsi, R.; Boike, T.; Dryden, D.; Gustafson, G.S.; Benedetti, L.; Matuszak, M.M.; Nurushev, T.S.; et al. Recent Time Trends and Predictors of Heart Dose From Breast Radiation Therapy in a Large Quality Consortium of Radiation Oncology Practices. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1154–1161. [Google Scholar] [CrossRef]
- Formenti, S.C.; DeWyngaert, J.K.; Jozsef, G.; Goldberg, J.D. Prone vs supine positioning for breast cancer radiotherapy. JAMA 2012, 308, 861–863. [Google Scholar] [CrossRef]
- Buijsen, J.; Jager, J.J.; Bovendeerd, J.; Voncken, R.; Borger, J.H.; Boersma, L.J.; Murrer, L.H.; Lambin, P. Prone breast irradiation for pendulous breasts. Radiother. Oncol. 2007, 82, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Masini, L.; Caltavuturo, T.; Pisani, C.; Apicella, G.; Negri, E.; Deantonio, L.; Brambilla, M.; Gambaro, G. Prone versus supine position for adjuvant breast radiotherapy: A prospective study in patients with pendulous breasts. Radiat. Oncol. 2013, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Griem, K.L.; Fetherston, P.; Kuznetsova, M.; Foster, G.S.; Shott, S.; Chu, J. Three-dimensional photon dosimetry: A comparison of treatment of the intact breast in the supine and prone position. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; McCormick, B. Prone position breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.D.; Georgian-Smith, D.; Raza, S.; Bussolari, L.; Pawlisz-Hoff, J.; Birdwell, R.L. Positioning in breast MR imaging to optimize image quality. RadioGraphics 2014, 34, E1–E17. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.M.; Luo, W.; Song, J.; Bellon, J.R.; Punglia, R.S.; Wong, J.S.; Killoran, J.H.; Gelman, R.; Harris, J.R. Variability among breast radiation oncologists in delineation of the postsurgical lumpectomy cavity. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1299–1308. [Google Scholar] [CrossRef]
- Major, T.; Gutiérrez, C.; Guix, B.; Mózsa, E.; Hannoun-Levi, J.-M.; Lössl, K.; Niehoff, P.; Resch, A.; van Limbergen, E.; Polgár, C. Interobserver variations of target volume delineation in multicatheter partial breast brachytherapy after open cavity surgery. Brachytherapy 2015, 14, 925–932. [Google Scholar] [CrossRef]
- Petersen, R.P.; Truong, P.T.; Kader, H.A.; Berthelet, E.; Lee, J.C.; Hilts, M.L.; Kader, A.S.; Beckham, W.A.; Olivotto, I.A. Target volume delineation for partial breast radiotherapy planning: Clinical characteristics associated with low interobserver concordance. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 41–48. [Google Scholar] [CrossRef]
- Coles, C.E.; Wilson, C.B.; Cumming, J.; Benson, J.R.; Forouhi, P.; Wilkinson, J.S.; Jena, R.; Wishart, G.C. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: Audit on behalf of the IMPORT Trial Management Group. Eur. J. Surg. Oncol. 2009, 35, 578–582. [Google Scholar] [CrossRef]
- Kirby, A.M.; Yarnold, J.R.; Evans, P.M.; Morgan, V.A.; Schmidt, M.A.; Scurr, E.D.; Desouza, N.M. Tumor bed delineation for partial breast and breast boost radiotherapy planned in the prone position: What does MRI add to X-ray CT localization of titanium clips placed in the excision cavity wall? Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1276–1282. [Google Scholar] [CrossRef]
- Al-Hammadi, N.; Caparrotti, P.; Divakar, S.; Riyas, M.; Chandramouli, S.H.; Hammoud, R.; Hayes, J.; McGarry, M.; Paloor, S.P.; Petric, P. MRI Reduces Variation of Contouring for Boost Clinical Target Volume in Breast Cancer Patients Without Surgical Clips in the Tumour Bed. Radiol. Oncol. 2017, 51, 160–168. [Google Scholar] [CrossRef]
- Jolicoeur, M.; Racine, M.L.; Trop, I.; Hathout, L.; Nguyen, D.; Derashodian, T.; David, S. Localization of the surgical bed using supine magnetic resonance and computed tomography scan fusion for planification of breast interstitial brachytherapy. Radiother. Oncol. 2011, 100, 480–484. [Google Scholar] [CrossRef]
- Jacobson, G.; Zamba, G.; Betts, V.; Muruganandham, M.; Buechler-Price, J. Image-Based Treatment Planning of the Post-Lumpectomy Breast Utilizing CT and 3TMRI. Int. J. Breast Cancer 2011, 2011, 246265. [Google Scholar] [CrossRef] [PubMed]
- den Hartogh, M.D.; van den Bongard, H.J.; Davidson, M.T.; Kotte, A.N.; Verkooijen, H.M.; Philippens, M.E.; van Vulpen, M.; van Asselen, B.; Pignol, J.P. Full-thickness closure in breast-conserving surgery: The impact on radiotherapy target definition for boost and partial breast irradiation. A multimodality image evaluation. Ann. Surg. Oncol. 2014, 21, 3774–3779. [Google Scholar] [CrossRef]
- Pogson, E.M.; Delaney, G.P.; Ahern, V.; Boxer, M.M.; Chan, C.; David, S.; Dimigen, M.; Harvey, J.A.; Koh, E.S.; Lim, K.; et al. Comparison of Magnetic Resonance Imaging and Computed Tomography for Breast Target Volume Delineation in Prone and Supine Positions. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mast, M.; Coerkamp, E.; Heijenbrok, M.; Scholten, A.; Jansen, W.; Kouwenhoven, E.; Nijkamp, J.; de Waard, S.; Petoukhova, A.; Struikmans, H. Target volume delineation in breast conserving radiotherapy: Are co-registered CT and MR images of added value? Radiat. Oncol. 2014, 9, 65. [Google Scholar] [CrossRef]
- Den Hartogh, M.D.; Philippens, M.E.P.; Van Dam, I.E.; Kleynen, C.E.; Tersteeg, R.J.H.A.; Kotte, A.N.T.J.; Van Vulpen, M.; Van Asselen, B.; Van Den Bongard, D.H.J.G. Post-lumpectomy CT-guided tumor bed delineation for breast boost and partial breast irradiation: Can additional pre- and postoperative imaging reduce interobserver variability? Oncol. Lett. 2015, 10, 2795–2801. [Google Scholar] [CrossRef]
- Giezen, M.; Kouwenhoven, E.; Scholten, A.N.; Coerkamp, E.G.; Heijenbrok, M.; Jansen, W.P.; Mast, M.E.; Petoukhova, A.L.; Struikmans, H. MRI- versus CT-based volume delineation of lumpectomy cavity in supine position in breast-conserving therapy: An exploratory study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Smitt, M.C.; Birdwell, R.L.; Goffinet, D.R. Breast Electron Boost Planning: Comparison of CT and US. Radiology 2001, 219, 203–206. [Google Scholar] [CrossRef]
- Hurkmans, C.; Admiraal, M.; van der Sangen, M.; Dijkmans, I. Significance of breast boost volume changes during radiotherapy in relation to current clinical interobserver variations. Radiother. Oncol. 2009, 90, 60–65. [Google Scholar] [CrossRef]
- Whipp, E.C.; Halliwell, M. Magnetic resonance imaging appearances in the postoperative breast: The clinical target volume-tumor and its relationship to the chest wall. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Giezen, M.; Kouwenhoven, E.; Scholten, A.N.; Coerkamp, E.G.; Heijenbrok, M.; Jansen, W.P.; Mast, M.E.; Petoukhova, A.L.; Struikmans, H. Magnetic resonance imaging- versus computed tomography-based target volume delineation of the glandular breast tissue (clinical target volume breast) in breast-conserving therapy: An exploratory study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Dundas, K.; Pogson, E.M.; Batumalai, V.; Delaney, G.P.; Boxer, M.M.; Yap, M.L.; Ahern, V.; Chan, C.; David, S.; Dimigen, M.; et al. The impact of imaging modality (CT vs MRI) and patient position (supine vs prone) on tangential whole breast radiation therapy planning. Pract. Radiat. Oncol. 2018, 8, e87–e97. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Ben-David, M.A.; Moran, J.M.; Marsh, R.B.; Griffith, K.A.; Hayman, J.A.; Pierce, L.J. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hepel, J.T.; Tokita, M.; MacAusland, S.G.; Evans, S.B.; Hiatt, J.R.; Price, L.L.; DiPetrillo, T.; Wazer, D.E. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.C.; van den Bosch, M.A.; Loo, C.E.; Mali, W.P.; Bartelink, H.; Gertenbach, M.; Holland, R.; Peterse, J.L.; Rutgers, E.J.; Gilhuijs, K.G. Precise correlation between MRI and histopathology–Exploring treatment margins for MRI-guided localized breast cancer therapy. Radiother. Oncol. 2010, 97, 225–232. [Google Scholar] [CrossRef]
- Charaghvandi, R.K.; Yoo, S.; van Asselen, B.; Rodrigues, A.; van den Bongard, D.; Horton, J.K. Treatment constraints for single dose external beam preoperative partial breast irradiation in early-stage breast cancer. Clin. Transl. Radiat. Oncol. 2017, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.K.; Blitzblau, R.C.; Yoo, S.; Geradts, J.; Chang, Z.; Baker, J.A.; Georgiade, G.S.; Chen, W.; Siamakpour-Reihani, S.; Wang, C.; et al. Preoperative Single-Fraction Partial Breast Radiation Therapy: A Novel Phase 1, Dose-Escalation Protocol With Radiation Response Biomarkers. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 846–855. [Google Scholar] [CrossRef]
- Blitzblau, R.C.; Arya, R.; Yoo, S.; Baker, J.A.; Chang, Z.; Palta, M.; Duffy, E.; Horton, J.K. A phase 1 trial of preoperative partial breast radiation therapy: Patient selection, target delineation, and dose delivery. Pract. Radiat. Oncol. 2015, 5, e513–e520. [Google Scholar] [CrossRef]
- Bondiau, P.Y.; Courdi, A.; Bahadoran, P.; Chamorey, E.; Queille-Roussel, C.; Lallement, M.; Birtwisle-Peyrottes, I.; Chapellier, C.; Pacquelet-Cheli, S.; Ferrero, J.M. Phase 1 clinical trial of stereotactic body radiation therapy concomitant with neoadjuvant chemotherapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1193–1199. [Google Scholar] [CrossRef]
- Vasmel, J.E.; Charaghvandi, R.K.; Houweling, A.C.; Philippens, M.E.P.; van Asselen, B.; Vreuls, C.P.H.; van Diest, P.J.; van Leeuwen, A.M.G.; van Gorp, J.; Witkamp, A.J.; et al. Tumor Response After Neoadjuvant Magnetic Resonance Guided Single Ablative Dose Partial Breast Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 821–829. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, F.; Bosma, S.C.; van de Vijver, M.J.; Wesseling, J.; Vreeswijk, S.; Rivera, S.; Bourgier, C.; Garbay, J.R.; Foukakis, T.; Lekberg, T.; et al. First results of the preoperative accelerated partial breast irradiation (PAPBI) trial. Radiother. Oncol. 2015, 114, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Kesmodel, S.B.; Bellavance, E.; Drogula, C.; Tkaczuk, K.; Cohen, R.J.; Citron, W.; Morgan, M.; Staats, P.; Feigenberg, S.; et al. Preoperative Accelerated Partial Breast Irradiation for Early-Stage Breast Cancer: Preliminary Results of a Prospective, Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 747–753. [Google Scholar] [CrossRef]
- Charaghvandi, R.K.; den Hartogh, M.D.; van Ommen, A.M.; de Vries, W.J.; Scholten, V.; Moerland, M.A.; Philippens, M.E.; Schokker, R.I.; van Vulpen, M.; van Asselen, B.; et al. MRI-guided single fraction ablative radiotherapy for early-stage breast cancer: A brachytherapy versus volumetric modulated arc therapy dosimetry study. Radiother. Oncol. 2015, 117, 477–482. [Google Scholar] [CrossRef] [PubMed]
- den Hartogh, M.D.; Philippens, M.E.; van Dam, I.E.; Kleynen, C.E.; Tersteeg, R.J.; Pijnappel, R.M.; Kotte, A.N.; Verkooijen, H.M.; van den Bosch, M.A.; van Vulpen, M.; et al. MRI and CT imaging for preoperative target volume delineation in breast-conserving therapy. Radiat. Oncol. 2014, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Vasmel, J.E.; Groot Koerkamp, M.L.; Kirby, A.M.; Russell, N.S.; Shaitelman, S.F.; Vesprini, D.; Anandadas, C.N.; Currey, A.; Keller, B.M.; Braunstein, L.Z.; et al. Consensus on Contouring Primary Breast Tumors on MRI in the Setting of Neoadjuvant Partial Breast Irradiation in Trials. Pract. Radiat. Oncol. 2020, 10, e466–e474. [Google Scholar] [CrossRef] [PubMed]
- Civil, Y.A.; Jonker, L.W.; Groot Koerkamp, M.P.M.; Duvivier, K.M.; de Vries, R.; Oei, A.L.; Slotman, B.J.; van der Velde, S.; van den Bongard, H. Preoperative Partial Breast Irradiation in Patients with Low-Risk Breast Cancer: A Systematic Review of Literature. Ann. Surg. Oncol. 2023, 30, 3263–3279. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Francolini, G.; Di Cataldo, V.; Visani, L.; Becherini, C.; Scoccimarro, E.; Salvestrini, V.; Bellini, C.; Masi, L.; Doro, R.; et al. Preoperative robotic radiosurgery for early breast cancer: Results of the phase II ROCK trial (NCT03520894). Clin. Transl. Radiat. Oncol. 2022, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.; Fyles, A. Establishing the Role of Stereotactic Ablative Body Radiotherapy in Early-Stage Breast Cancer. Int. J. Breast Cancer 2018, 2018, 2734820. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Lagendijk, J.J.; Overweg, J.; Kok, J.G.; Raaijmakers, A.J.; Kerkhof, E.M.; van der Put, R.W.; Meijsing, I.; Crijns, S.P.; Benedosso, F.; et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys. Med. Biol. 2009, 54, N229–N237. [Google Scholar] [CrossRef]
- Crijns, S.P.; Kok, J.G.; Lagendijk, J.J.; Raaymakers, B.W. Towards MRI-guided linear accelerator control: Gating on an MRI accelerator. Phys. Med. Biol. 2011, 56, 4815–4825. [Google Scholar] [CrossRef]
- Bjerre, T.; Crijns, S.; af Rosenschold, P.M.; Aznar, M.; Specht, L.; Larsen, R.; Keall, P. Three-dimensional MRI-linac intra-fraction guidance using multiple orthogonal cine-MRI planes. Phys. Med. Biol. 2013, 58, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Crijns, S.P.; Raaymakers, B.W.; Lagendijk, J.J. Proof of concept of MRI-guided tracked radiation delivery: Tracking one-dimensional motion. Phys. Med. Biol. 2012, 57, 7863–7872. [Google Scholar] [CrossRef] [PubMed]
- van Heijst, T.C.; Philippens, M.E.; Charaghvandi, R.K.; den Hartogh, M.D.; Lagendijk, J.J.; van den Bongard, H.J.; van Asselen, B. Quantification of intra-fraction motion in breast radiotherapy using supine magnetic resonance imaging. Phys. Med. Biol. 2016, 61, 1352–1370. [Google Scholar] [CrossRef]
- Henke, L.E.; Contreras, J.A.; Green, O.L.; Cai, B.; Kim, H.; Roach, M.C.; Olsen, J.R.; Fischer-Valuck, B.; Mullen, D.F.; Kashani, R.; et al. Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A 4.5-Year Clinical Experience. Clin. Oncol. 2018, 30, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Fischer-Valuck, B.W.; Mazur, T.R.; Curcuru, A.; Sona, K.; Kashani, R.; Green, O.; Ochoa, L.; Mutic, S.; Zoberi, I.; et al. Magnetic Resonance Image Guided Radiation Therapy for External Beam Accelerated Partial-Breast Irradiation: Evaluation of Delivered Dose and Intrafractional Cavity Motion. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Pennell, R.; Formenti, S.C. The initial experience of MRI-guided precision prone breast irradiation with daily adaptive planning in treating early stage breast cancer patients. Front. Oncol. 2022, 12, 1048512. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.; Raaymakers, B.W.; van der Meer, S.; Lagendijk, J.J. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: Impact of the surface orientation on the entrance and exit dose due to the transverse magnetic field. Phys. Med. Biol. 2007, 52, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, A.J.; Raaymakers, B.W.; Lagendijk, J.J. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: Dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys. Med. Biol. 2005, 50, 1363–1376. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.; Raaymakers, B.W.; Lagendijk, J.J. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: Dependence on the magnetic field strength. Phys. Med. Biol. 2008, 53, 909–923. [Google Scholar] [CrossRef]
- Park, J.M.; Shin, K.H.; Kim, J.I.; Park, S.Y.; Jeon, S.H.; Choi, N.; Kim, J.H.; Wu, H.G. Air-electron stream interactions during magnetic resonance IGRT: Skin irradiation outside the treatment field during accelerated partial breast irradiation. Strahlenther. Onkol. 2018, 194, 50–59. [Google Scholar] [CrossRef] [PubMed]
- van Heijst, T.C.; den Hartogh, M.D.; Lagendijk, J.J.; van den Bongard, H.J.; van Asselen, B. MR-guided breast radiotherapy: Feasibility and magnetic-field impact on skin dose. Phys. Med. Biol. 2013, 58, 5917–5930. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lim-Reinders, S.; McCann, C.; Ahmad, S.B.; Sahgal, A.; Lee, J.; Keller, B.M. Magnetic field dose effects on different radiation beam geometries for hypofractionated partial breast irradiation. J. Appl. Clin. Med. Phys. 2017, 18, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Thibault, I.; Chiang, A.; Erler, D.; Yeung, L.; Poon, I.; Kim, A.; Keller, B.; Lochray, F.; Jain, S.; Soliman, H.; et al. Predictors of Chest Wall Toxicity after Lung Stereotactic Ablative Radiotherapy. Clin. Oncol. 2016, 28, 28–35. [Google Scholar] [CrossRef]
- Groot Koerkamp, M.L.; Vasmel, J.E.; Russell, N.S.; Shaitelman, S.F.; Anandadas, C.N.; Currey, A.; Vesprini, D.; Keller, B.M.; De-Colle, C.; Han, K.; et al. Optimizing MR-Guided Radiotherapy for Breast Cancer Patients. Front. Oncol. 2020, 10, 1107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).