Navigating Intraductal Papillary Mucinous Neoplasm Management through Fukuoka Consensus vs. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms—A Study on Two European Centers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Slovenian Patients: Results
3.2. Comparison of Slovenian and Serbian Participants
3.3. Patients from Both Centers: Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholten, L.; van Huijgevoort, N.C.M.; van Hooft, J.E.; Besselink, M.G.; Del Chiaro, M. Pancreatic Cystic Neoplasms: Different Types, Different Management, New Guidelines. Visc. Med. 2018, 34, 173–177. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumours of the Pancreas. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, R.; Niedergethmann, M.; Pilarsky, C.; Klöppel, G.; Saeger, H.D. Intraductal Papillary Mucinous Tumors of the Pancreas: Biology, Diagnosis, and Treatment. Oncologist 2010, 15, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Puckett, Y.; Sharma, B.; Kasi, A. Intraductal Papillary Mucinous Cancer of the Pancreas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Machado, N.; al Qadhi, H.; al Wahibi, K. Intraductal Papillary Mucinous Neoplasm of Pancreas. N. Am. J. Med. Sci. 2015, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International Consensus Guidelines 2012 for the Management of IPMN and MCN of the Pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Verbeke, C.; Salvia, R.; Klöppel, G.; Werner, J.; McKay, C.; Friess, H.; Manfredi, R.; Van Cutsem, E.; Löhr, M.; et al. European Experts Consensus Statement on Cystic Tumours of the Pancreas. Dig. Liver Dis. 2013, 45, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, V.; Grubor, N.; Kovac, J.D.; Micev, M.; Milic, N.; Knezevic, D.; Gregoric, P.; Lausevic, Z.; Kerkez, M.; Knezevic, S.; et al. Comparison of Preoperative Evaluation with the Pathological Report in Intraductal Papillary Mucinous Neoplasms: A Single-Center Experience. J. Clin. Med. 2021, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Lee, H.; Park, J.Y.; Kwon, W.; Heo, J.S.; Choi, S.H.; Choi, D.W. Validation of International Consensus Guideline 2012 for Intraductal Papillary Mucinous Neoplasm of Pancreas. Ann. Surg. Treat. Res. 2016, 90, 124. [Google Scholar] [CrossRef] [PubMed]
- Jan, I.-S.; Chang, M.-C.; Yang, C.-Y.; Tien, Y.-W.; Jeng, Y.-M.; Wu, C.-H.; Chen, B.-B.; Chang, Y.-T. Validation of Indications for Surgery of European Evidence-Based Guidelines for Patients with Pancreatic Intraductal Papillary Mucinous Neoplasms. J. Gastrointest. Surg. 2020, 24, 2536–2543. [Google Scholar] [CrossRef]
- van Huijgevoort, N.C.M.; del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Evidence and Guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Krishna, S.G.; Pannala, R. Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics 2023, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jang, J.-Y.; Kang, M.J.; Park, T.; Lee, S.Y.; Jung, W.; Chang, J.; Shin, Y.; Han, Y.; Kim, S.-W. Clinical Implication of Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 for the Prediction of Malignancy in Intraductal Papillary Mucinous Neoplasm of Pancreas. J. Hepatobiliary Pancreat. Sci. 2015, 22, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhen, D.; Xiaoyan, W.; Bin, C.; Ruifeng, W.; Shanyu, Q.; Zhuoran, L.; Kai, S.; Wenming, W.; Aiming, Y.; et al. The Effectiveness of Endoscopic Ultrasonography Findings to Distinguish Benign and Malignant Intraductal Papillary Mucinous Neoplasm. Surg. Endosc. 2023, 37, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Pu, N.; Chen, Q.; Zhang, J.; Yin, H.; Wang, D.; Ji, Y.; Rao, S.; Kuang, T.; Xu, X.; Wu, W.; et al. Circulating Cytokines Allow for Identification of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancer Med. 2023, 12, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Cauvin, T.; Fernandez, B.; Buscail, C.; Marty, M.; Lapuyade, B.; Subtil, C.; Adam, J.-P.; Vendrely, V.; Dabernat, S.; et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas and European Guidelines: Importance of the Surgery Type in the Decision-Making Process. BMC Surg. 2019, 19, 115. [Google Scholar] [CrossRef]
- Heckler, M.; Brieger, L.; Heger, U.; Pausch, T.; Tjaden, C.; Kaiser, J.; Tanaka, M.; Hackert, T.; Michalski, C.W. Predictive Performance of Factors Associated with Malignancy in Intraductal Papillary Mucinous Neoplasia of the Pancreas: Factors Associated with Malignancy in Intraductal Papillary Mucinous Neoplasia of the Pancreas. BJS Open 2018, 2, 13–24. [Google Scholar] [CrossRef]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of high-risk stigmata and worrisome features with advanced neoplasia in intraductal papillary mucinous neoplasms (IPMN): A systematic review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef]
- Lin, T.; Chen, X.; Liu, J.; Cao, Y.; Cui, W.; Wang, Z.; Wang, C.; Chen, X. MRI-Based Pancreatic Atrophy Is Associated With Malignancy or Invasive Carcinoma in Intraductal Papillary Mucinous Neoplasm. Front. Oncol. 2022, 12, 894023. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Yang, C.-Y.; Wu, J.-M.; Kuo, T.-C.; Tien, Y.-W. Utility of the 2006 Sendai and 2012 Fukuoka Guidelines for the Management of Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Single-Center Experience with 138 Surgically Treated Patients. Medicine 2016, 95, e4922. [Google Scholar] [CrossRef]
- Srinivasan, N.; Teo, J.-Y.; Chin, Y.-K.; Hennedige, T.; Tan, D.M.; Low, A.S.; Thng, C.H.; Goh, B.K.P. Systematic Review of the Clinical Utility and Validity of the Sendai and Fukuoka Consensus Guidelines for the Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. HPB 2018, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Friziero, A.; Serafini, S.; Bissoli, S.; Ponzoni, A.; Grego, A.; Grego, E.; Moletta, L. Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. J. Clin. Med. 2020, 9, 2169. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, Y.; Kwon, J.W.; Jun, E.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Yoo, C.; Kim, K.; Jeong, J.H.; et al. Reduced and Normalized Carbohydrate Antigen 19-9 Concentrations after Neoadjuvant Chemotherapy Have Comparable Prognostic Performance in Patients with Borderline Resectable and Locally Advanced Pancreatic Cancer. J. Clin. Med. 2020, 9, 1477. [Google Scholar] [CrossRef] [PubMed]
- Mortoglou, M.; Manić, L.; Buha Djordjevic, A.; Bulat, Z.; Đorđević, V.; Manis, K.; Valle, E.; York, L.; Wallace, D.; Uysal-Onganer, P. Nickel’s Role in Pancreatic Ductal Adenocarcinoma: Potential Involvement of MicroRNAs. Toxics 2022, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Wallace, D.; Matovic, V.; Schweitzer, A.; Oluic, B.; Micic, D.; Djordjevic, V. Cadmium Exposure as a Putative Risk Factor for the Development of Pancreatic Cancer: Three Different Lines of Evidence. BioMed Res. Int. 2017, 2017, 1981837. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, V.R.; Wallace, D.R.; Schweitzer, A.; Boricic, N.; Knezevic, D.; Matic, S.; Grubor, N.; Kerkez, M.; Radenkovic, D.; Bulat, Z.; et al. Environmental Cadmium Exposure and Pancreatic Cancer: Evidence from Case Control, Animal and in Vitro Studies. Environ. Int. 2019, 128, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Froeling, F.; Casolino, R.; Pea, A.; Biankin, A.; Chang, D.; on behalf of Precision-Panc. Molecular Subtyping and Precision Medicine for Pancreatic Cancer. J. Clin. Med. 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Mortoglou, M.; Tabin, Z.K.; Arisan, E.D.; Kocher, H.M.; Uysal-Onganer, P. Non-Coding RNAs in Pancreatic Ductal Adenocarcinoma: New Approaches for Better Diagnosis and Therapy. Transl. Oncol. 2021, 14, 101090. [Google Scholar] [CrossRef]
- Mortoglou, M.; Miralles, F.; Arisan, E.D.; Dart, A.; Jurcevic, S.; Lange, S.; Uysal-Onganer, P. MicroRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1275. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Babaniamansour, S.; Shi, J. Updates in the Diagnosis of Intraductal Neoplasms of the Pancreas. Front. Physiol. 2022, 13, 856803. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fernandez-del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Marie Lennon, A.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef] [PubMed]
| Ljubljana n = 37 | ||
|---|---|---|
| Age, mean ± sd | 70.8 ± 10.9 | |
| Sex, n (%) | Female | 14 (37.8) |
| Male | 23 (62.2) | |
| Location, n (%) | Head | 33 (89.2) |
| Body | 1 (2.7) | |
| Tail | 3 (8.1) | |
| Main duct, n (%) | 12 (32.43) | |
| Branch duct, n (%) | 16 (43.24) | |
| Mixed type, n (%) | 9 (24.3) | |
| Grade, n (%) | Low/moderate | 16 (43.2) |
| High grade | 21 (56.8) | |
| High grade, n (%) | High grade | 3 (14.3) |
| Carcinoma | 18 (85.7) |
| LGD n = 16 | HGD/IC n = 21 | p | |
|---|---|---|---|
| Fukuoka consensus guidelines | |||
| Obstructive jaundice | 3 (18.8) | 13 (61.9) | 0.009 |
| Enhancing solid component > 5 mm | 1 (6.3) | 1 (4.8) | 1.000 |
| Main pancreatic duct 10 mm | 5 (31.3) | 2 (9.5) | 0.202 |
| At least one high-risk stigmata indication for resection | 8 (50) | 14 (66.7) | 0.306 |
| Size, 3 cm | 11 (68.8) | 6 (28.6) | 0.015 |
| Enhancing mural nodule, <5 mm | 2 (12.5) | 0 (0) | 0.180 |
| Thickened and enhancing cyst wall | 3 (18.8) | 0 (0) | 0.072 |
| Main pancreatic duct, 5–9 mm | 5 (31.3) | 15 (71.4) | 0.015 |
| Elevated CA 19-9 | 2 (12.5) | 14 (66.7) | 0.001 |
| Cyst growth rate 5 mm in 2 years | 5 (31.3) | 1 (4.8) | 0.066 |
| Abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | 0 (0) | 0 (0) | NA |
| Lymphadenopathy | 2 (12.5) | 3 (14.3) | 1.000 |
| At least one worrisome feature | 16 (100) | 19 (90.5) | 0.495 |
| EEBGPCNs | |||
| Positive cytology for malignancy/HGD | 0 (0) | 4 (19) | 0.118 |
| Solid mass | 2 (12.5) | 14 (66.7) | 0.001 |
| Jaundice | 3 (18.8) | 13 (61.9) | 0.009 |
| Enhancing mural nodule, >5 mm | 0 (0) | 1 (4.8) | 1.000 |
| MPD dilation, 10 mm | 5 (31.3) | 2 (9.5) | 0.202 |
| At least one absolute indication for resection | 8 (50) | 19 (90.5) | 0.009 |
| Grow rate, 5 mm/year | 5 (31.3) | 1 (4.8) | 0.066 |
| Increased levels of serum CA 19-9, >37 U/mL | 2 (12.5) | 14 (66.7) | 0.001 |
| MPD dilation between 5 and 9.9 mm | 5 (31.3) | 13 (61.9) | 0.065 |
| Cyst diameter, 40 mm | 4 (25) | 4 (19) | 0.705 |
| New-onset DM | 0 (0) | 1 (4.8) | 1.000 |
| Acute pancreatitis | 2 (12.5) | 3 (14.3) | 1.000 |
| Enhancing mural nodule, <5 mm | 2 (12.5) | 0 (0) | 0.180 |
| At least one relative indication for resection | 12 (75) | 19 (90.5) | 0.371 |
| Sn | Sp | PPV | NPV | |
|---|---|---|---|---|
| Fukuoka consensus | ||||
| At least one high-risk stigmata | 66.7% | 50% | 63.6% | 53.3% |
| At least one high-risk stigmata and one worrisome feature | 61.9% | 50% | 61.9% | 50% |
| EEBGPCNs | ||||
| At least one absolute indication | 90.5% | 50% | 70.4% | 80% |
| At least one absolute and one relative indication | 81.0% | 62.5% | 73.9% | 71.4% |
| At least one relative indication | 90.5% | 25.0% | 61.3% | 66.7% |
| Increased levels of serum CA 19-9 | 66.7% | 87.5% | 87.5% | 66.7% |
| Belgrade n = 76 | Ljubljana n = 37 | p | ||
|---|---|---|---|---|
| Age, mean ± sd | 60.8 ± 10.5 | 70.8 ± 10.9 | <0.001 | |
| Sex, n (%) | Female | 36 (47.4) | 14 (37.8) | 0.338 |
| Male | 40 (52.6) | 23 (62.2) | ||
| Location, n (%) | Head | 47 (61.8) | 33 (89.2) | 0.011 |
| Body | 12 (15.8) | 1 (2.7) | ||
| Tail | 7 (9.2) | 3 (8.1) | ||
| Two locations | 10 (13.2) | 0 (0) | ||
| Main duct, n (%) | 12 (15.79) | 12 (32.43) | 0.024 | |
| Branch duct, n (%) | 28 (36.84) | 16 (43.24) | 0.045 | |
| Mixed type, n (%) | 36 (47.37) | 9 (24.3) | 0.060 | |
| Grade, n (%) | Low/moderate | 22 (28.9) | 16 (43.2) | 0.131 |
| High grade | 54 (71.1) | 21 (56.8) | ||
| High grade, n (%) | High grade | 14 (25.9) | 3 (14.3) | 0.280 |
| Carcinoma | 40 (74.1) | 18 (85.7) |
| LGD n = 38 | HGD/IC n = 75 | p | |
|---|---|---|---|
| Fukuoka consensus guidelines | |||
| Obstructive jaundice | 7 (18.4) | 45 (60) | <0.001 |
| Enhancing solid component, >5 mm | 4 (10.5) | 18 (24) | 0.087 |
| Main pancreatic duct, 10 mm | 8 (21.1) | 18 (24) | 0.725 |
| At least one high-risk stigmata indication for resection | 16 (42.1) | 52 (69.3) | 0.005 |
| Size 3 cm | 19 (50) | 41 (54.7) | 0.639 |
| Enhancing mural nodule, <5 mm | 11 (28.9) | 24 (32) | 0.740 |
| Thickened and enhancing cyst wall | 11 (28.9) | 38 (50.7) | 0.028 |
| Main pancreatic duct, 5–9 mm | 22 (57.9) | 52 (69.3) | 0.227 |
| Elevated CA 19-9 | 3 (7.9) | 54 (72) | <0.001 |
| Cyst growth rate, 5 mm in 2 years | 5 (13.2) | 3 (4) | 0.073 |
| Abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | 1 (2.6) | 5 (6.7) | 0.366 |
| Lymphadenopathy | 11 (28.9) | 37 (49.3) | 0.038 |
| At least one worrisome feature | 38 (100) | 73 (97.3) | 0.310 |
| EEBGPCNs | |||
| Positive cytology for malignancy/HGD | 1 (2.6) | 5 (6.8) | 0.351 |
| Solid mass | 8 (21.1) | 54 (72) | <0.001 |
| Jaundice | 7 (18.4) | 42 (56.8) | <0.001 |
| Enhancing mural nodule, >5 mm | 3 (7.9) | 14 (18.9) | 0.124 |
| MPD dilation, 10 mm | 9 (23.7) | 16 (21.6) | 0.804 |
| At least one absolute indication for resection | 20 (52.6) | 64 (85.3) | <0.001 |
| Grow rate, 5 mm/year | 5 (13.2) | 2 (2.7) | 0.029 |
| Increased levels of serum CA 19-9, >37 U/mL | 4 (10.5) | 52 (69.3) | <0.001 |
| MPD dilation between 5 and 9.9 mm | 21 (55.3) | 48 (64) | 0.368 |
| Cyst diameter, 40 mm | 10 (26.3) | 28 (37.3) | 0.242 |
| New-onset DM | 3 (7.9) | 7 (9.3) | 0.799 |
| Acute pancreatitis | 4 (10.5) | 10 (13.3) | 0.669 |
| Enhancing mural nodule, <5 mm | 11 (28.9) | 25 (33.3) | 0.636 |
| At least one relative indication for resection | 33 (86.8) | 71 (94.7) | 0.147 |
| Sn | Sp | PPV | NPV | |
|---|---|---|---|---|
| Fukuoka consensus | ||||
| At least one high-risk stigmata | 69.3% | 57.9% | 76.5% | 48.9% |
| At least one high-risk stigmata and one worrisome feature | 68% | 57.9% | 76.1% | 47.8% |
| EEBGPCNs | ||||
| At least one absolute indication | 85.3% | 47.4% | 76.2% | 62.1% |
| At least one absolute and one relative indication | 82.7% | 55.3% | 78.5% | 61.8% |
| At least one relative indication | 67.3% | 94.7% | 68.3% | 55.7% |
| At least one absolute or one relative indication | 98.6% | 0% | 67% | 0% |
| Increased levels of serum CA 19-9 | 69.3% | 89.5% | 92.9% | 59.6% |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% C.I. for OR | p | OR | 95% C.I. for OR | p | |||
| Lower | Upper | Lower | Upper | |||||
| FCG | ||||||||
| Obstructive jaundice | 6.643 | 2.591 | 17.028 | <0.001 | 6.593 | 2.132 | 20.39 | 0.001 |
| Enhancing solid component, >5 mm | 2.684 | 0.838 | 8.594 | 0.096 | ||||
| Main pancreatic duct, 10 mm | 1.184 | 0.461 | 3.04 | 0.725 | ||||
| Size 3 cm | 1.206 | 0.552 | 2.635 | 0.639 | ||||
| Enhancing mural nodule, <5 mm | 1.155 | 0.492 | 2.709 | 0.74 | ||||
| Thickened and enhancing cyst wall | 2.521 | 1.094 | 5.807 | 0.03 | ||||
| Main pancreatic duct, 5–9 mm | 1.644 | 0.732 | 3.695 | 0.229 | ||||
| Elevated CA 19-9 | 30 | 8.322 | 108.148 | <0.001 | 29.855 | 7.787 | 114.466 | <0.001 |
| Cyst growth rate, 5 mm in 2 years | 0.275 | 0.062 | 1.22 | 0.089 | ||||
| Abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy | 2.643 | 0.298 | 23.466 | 0.383 | ||||
| Lymphadenopathy | 2.39 | 1.037 | 5.506 | 0.041 | ||||
| EEBGPCNs | ||||||||
| Positive cytology for malignancy/HGD | 2.721 | 0.306 | 24.165 | 0.369 | ||||
| Solid mass | 9.643 | 3.81 | 24.406 | <0.001 | 5.132 | 1.591 | 16.552 | 0.006 |
| Jaundice | 5.812 | 2.27 | 14.885 | <0.001 | 2.21 | 0.647 | 7.544 | 0.206 |
| Enhancing mural nodule, >5 mm | 2.722 | 0.731 | 10.137 | 0.135 | ||||
| MPD dilation, 10 mm | 0.889 | 0.351 | 2.254 | 0.804 | ||||
| Grow rate, 5 mm/year | 0.181 | 0.033 | 0.981 | 0.047 | ||||
| Increased levels of serum CA 19-9, >37 U/mL | 19.217 | 6.107 | 60.474 | <0.001 | 15.112 | 4.45 | 51.314 | <0.001 |
| MPD dilation between 5 and 9.9 mm | 1.439 | 0.65 | 3.185 | 0.369 | ||||
| Cyst diameter, 40 mm | 1.668 | 0.706 | 3.943 | 0.244 | ||||
| New-onset DM | 1.201 | 0.292 | 4.932 | 0.799 | ||||
| Acute pancreatitis | 1.308 | 0.382 | 4.481 | 0.669 | ||||
| Enhancing mural nodule, <5 mm | 1.227 | 0.525 | 2.871 | 0.637 | ||||
| Test Result Variable(s) | HGD/IC (vs. LGD) Prediction | ||||
|---|---|---|---|---|---|
| Area under the Curve (AUC) | Std. Error | p | Asymptotic 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||
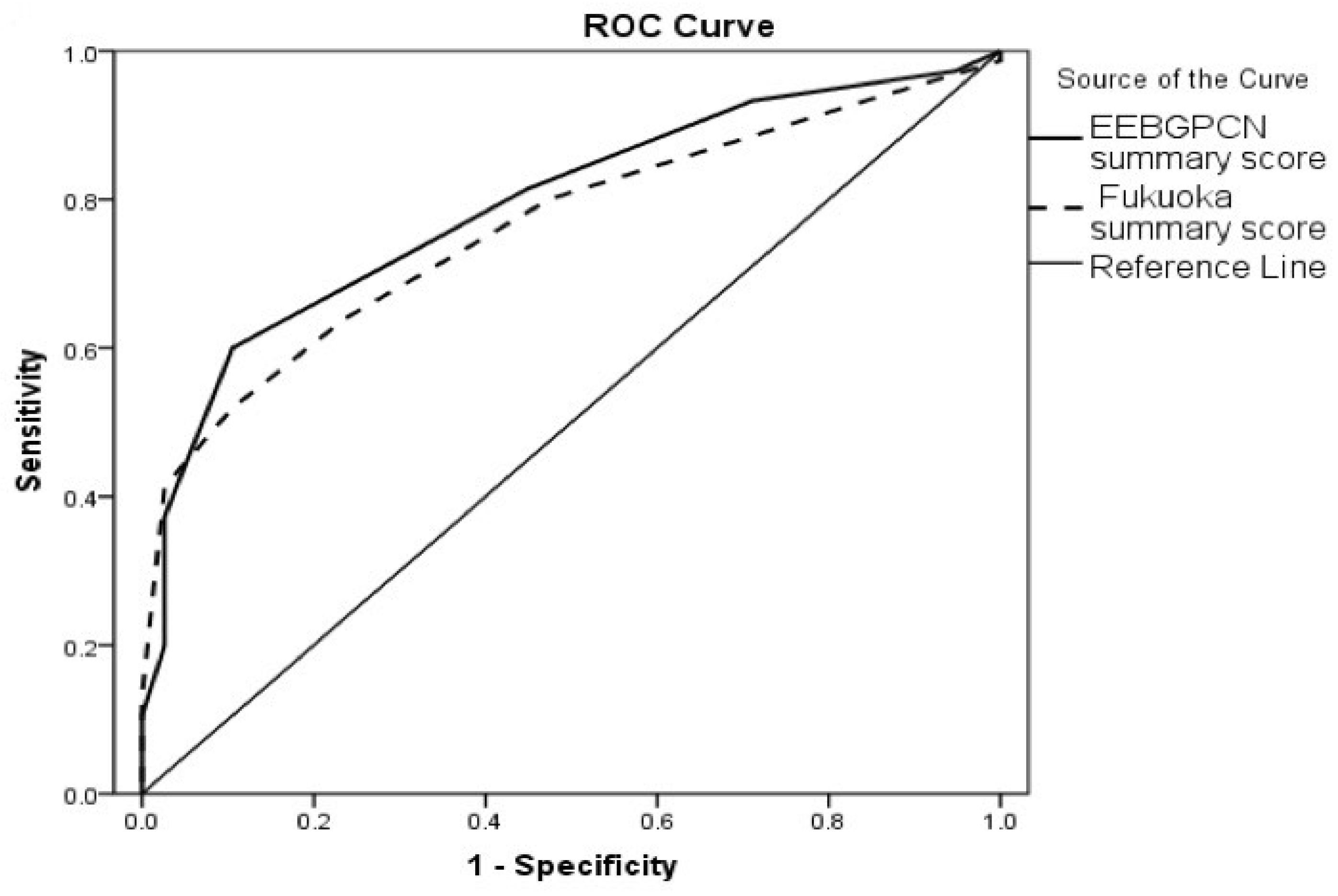
| EEBGPCN summary score | 0.792 | 0.043 | <0.001 | 0.708 | 0.875 |
| Fukuoka summary score | 0.762 | 0.044 | <0.001 | 0.675 | 0.848 |
| Parameter | HGD/IC (vs. LGD) Prediction | IC (vs. HGD) Prediction | ||||
|---|---|---|---|---|---|---|
| Cut-Off Value | Sensitivity (%) | Specificity (%) | Cut-Off Value | Sensitivity (%) | Specificity (%) | |
| EEBGPCN summary score | 3.5 | 60.0 | 89.5 | 3.5 | 66.7 | 66.7 |
| Fukuoka summary score | 4.5 | 52.0 | 89.5 | 2.5 | 87.7 | 47.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjevic, V.; Knezevic, D.; Trotovsek, B.; Tomazic, A.; Petric, M.; Hadzialjevic, B.; Grubor, N.; Djokic, M. Navigating Intraductal Papillary Mucinous Neoplasm Management through Fukuoka Consensus vs. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms—A Study on Two European Centers. Cancers 2024, 16, 2156. https://doi.org/10.3390/cancers16112156
Djordjevic V, Knezevic D, Trotovsek B, Tomazic A, Petric M, Hadzialjevic B, Grubor N, Djokic M. Navigating Intraductal Papillary Mucinous Neoplasm Management through Fukuoka Consensus vs. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms—A Study on Two European Centers. Cancers. 2024; 16(11):2156. https://doi.org/10.3390/cancers16112156
Chicago/Turabian StyleDjordjevic, Vladimir, Djordje Knezevic, Blaz Trotovsek, Ales Tomazic, Miha Petric, Benjamin Hadzialjevic, Nikica Grubor, and Mihajlo Djokic. 2024. "Navigating Intraductal Papillary Mucinous Neoplasm Management through Fukuoka Consensus vs. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms—A Study on Two European Centers" Cancers 16, no. 11: 2156. https://doi.org/10.3390/cancers16112156
APA StyleDjordjevic, V., Knezevic, D., Trotovsek, B., Tomazic, A., Petric, M., Hadzialjevic, B., Grubor, N., & Djokic, M. (2024). Navigating Intraductal Papillary Mucinous Neoplasm Management through Fukuoka Consensus vs. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms—A Study on Two European Centers. Cancers, 16(11), 2156. https://doi.org/10.3390/cancers16112156







