Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
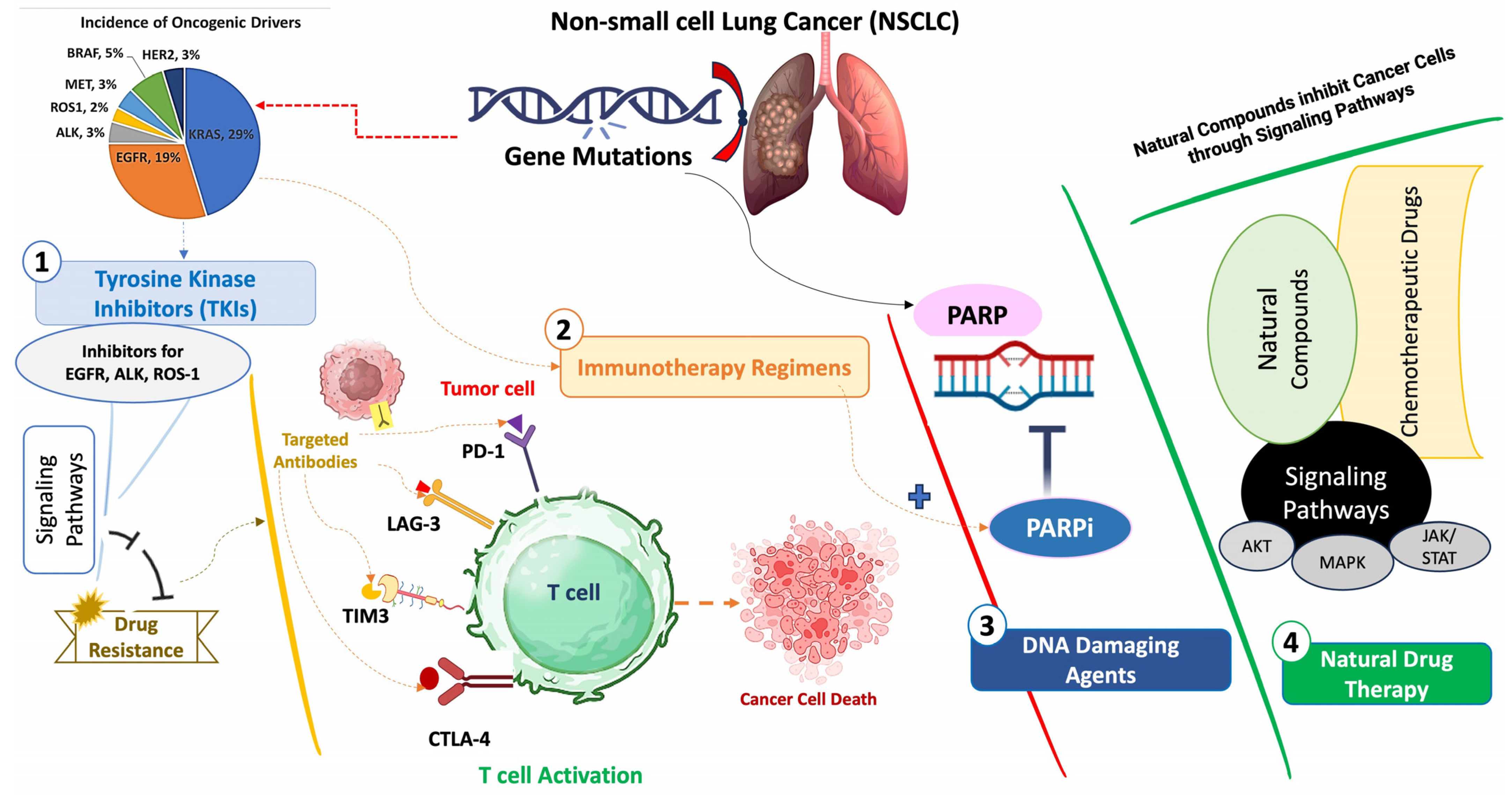
1. Introduction
2. Targeted Therapies
2.1. Epidermal Growth Factor Receptor (EGFR) Inhibitors
2.1.1. First-, Second-, and Third-Line EGFR Inhibitors with Combination Therapy Setting
| Inhibitors for EGFR Rearrangements and Combination Therapy | Starting Dose | Stage of Disease | Previous Treatment | Sample Size (Phase) | Objective Response Rate (ORR) and/or Median Progression-Free Survival (PFS) | References |
|---|---|---|---|---|---|---|
| Exon 19 deletion or L858R point mutation | ||||||
| Gefitinib + Bevacizumab | Gefitinib (250 mg/day) and bevacizumab (15 mg/kg, every 3 weeks) | Stage IIIB or IV | Untreated | 42 patients (phase 2) | ORR: 73.8%, PFS: 12 months | [9] |
| Erlotinib + Bevacizumab | Erlotinib (150 mg/day) and bevacizumab (15 mg/kg) every 21 days | Advanced | Not reported | 160 patients (phase 3) | PFS: 13.8 months | [10] |
| Erlotinib (150 mg/day) and bevacizumab (900.0 mg/cycle)-22 cycles | Stage IIIB or IV | Untreated | 311 patients (phase 3) | ORR: 86.8%, PFS:17.9 months | [11] | |
| Gefitinib + Apatinib | Gefitinib (250 mg/day) and Apatinib (500 mg/day) | Stage IIIB or IV | Not reported | 313 patients (phase 2) | ORR: 77.1%, PFS: 13.7 months | [12] |
| Gefintib + Pemetrexed | Gefintib (250 mg/day) and Pemetrexed (500 mg/m2, 3-weekly) | Stage IV | Untreated | 196 patients (phase 2) | PFS: 16.2 months | [14] |
| Icotinib | (125 mg/day) 3 times per day | Stage IIIB or IV | Untreated | 270 patients (phase 3) | PFS: 11.2 months | [15] |
| Afatinib | 20–40 mg/day | Stage IIIB or IV | Untreated with EGFR inhibitors | 35 patients | ORR: 77.1%, PFS: 13.8 months | [16] |
| Dacomitinib | 45 mg/day | Advanced | Untreated | 227 patients (phase 3) | ORR: 74.9%, PFS: 14.7 months | [17] |
| Exon 19 deletion or L858R point mutation and T790M mutation | ||||||
| Osimertinib | 80 mg/day | Advanced | Untreated | 52 patients in one arm | PFS: 18 months | [22] |
| Osimertinib + Durvalumab | Osimertinib (80 mg/day) and durvalumab (3 or 10 mg/kg), every 2 weeks | Advanced | Part A: pre-treated with EGFR inhibitors and Part B: untreated | 34 patients (phase 1b) | Part A: ORR 43%. Part B: ORR 82% | [18] |
| Rezivertinib | 30 mg | Advanced | Untreated | 153 patients (phase 1) | PFS: 9.7 months | [19] |
| Furmonertinib | 80 mg/day | Advanced | Untreated except 4% of patients | 133 patients with CNS lesions | CNS-ORR: 91% CNS-PFS: 20.8 months | [20] |
| Lazertinib | 240 mg/day | Advanced | Pre-treated with EGFR-TKIs | 78 patients (phase 1/2) | ORR: 55.3%, PFS: 11.1 months | [21] |
2.1.2. Combination of EGFR Inhibitors with Anticancer Drugs Targeting Epigenetic Factors
2.2. Inhibitors for ALK Rearrangements
First, Second-, and Third-Line ALK Inhibitors with Combination Therapy Setting
2.3. Inhibitors for ROS1 Fusion Variants and Combination Therapy
First, Second-, and Third-Line ROS1 Inhibitors with Combination Therapy Setting
| Inhibitors for ROS1 Fusion Variants and Combination Therapy | Starting Dose | Stage of Disease | Previous Treatment | Sample Size (Phase) | Objective Response Rate (ORR) and/or Median Progression-Free Survival (PFS) | References |
|---|---|---|---|---|---|---|
| Repotrectinib | 160 mg/day | meningeal carcinomatosis (G2032R mutated) | Cisplatin/pemetrexed and Crizotinib | Case report | - | [39] |
| Entrectinib | 600 mg/day | Advanced | Not reported | 168 patients | ORR: 68%, 15.7 months | [40] |
| Crizotinib | 250 mg twice per day | Advanced | Not reported | 49 patients (two groups: CD74-ROS1 and non–CD74–ROS1) | ORR: 94.11% (non–CD74-ROS1) and 73.68% (CD74-ROS1), PFS: 17.63 months (non–CD74-ROS1) and 12.63 (CD74-ROS1) | [41] |
| 34 patients (phase 2) | ORR: 88.9%, PFS: 16.8 months | [47] | ||||
| Ensartinib | 225 mg/day | Advanced | Chemotherapy | 59 patients (phase 2) | ORR: 27%, PFS: 4.6 months | [42] |
| Taletrectinib | 600 mg/day | Advanced (G2032R mutated) | Chemotherapy or Crizotinib | 105 patients (phase 2) | ORR: 91.7% (intracranial) | [43] |
| Ceritinib | 750 mg/day | Advanced | Few patients pre-treated with Crizotinib | 32 patients (phase 2) | ORR: 62%, PFS: 19.3 months (crizotinib-naive patients) | [48] |
| Lorlatinib | 100 mg/day | Advanced | CNS radiation | 16 patients (phase 2) | ORR: 87%, PFS: 38.8 months (intracranial) | [49] |
3. Clinical Trial Efficacy of Targeted Therapies in NSCLC
4. PARP Inhibitors: Combination Therapy
5. Immunotherapy Regimens
5.1. Immune Checkpoint Inhibitors (ICIs)
5.2. Immune Checkpoint Co-Inhibitory Receptors
5.3. Targeted Antibodies
6. Nanobodies for Targeted Therapeutic Delivery
7. Natural Drug Therapy: Monotherapy or in Combination
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Yuan, Y.; Eccles, L.; Chitkara, A.; Dalen, J.; Varol, N. Treatment patterns for advanced non-small cell lung cancer in the US: A systematic review of observational studies. Cancer Treat. Res. Commun. 2022, 33, 100648. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Hantschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2022, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Eno, M.S.; Brubaker, J.D.; Campbell, J.E.; De Savi, C.; Guzi, T.J.; Williams, B.D.; Wilson, D.; Wilson, K.; Brooijmans, N.; Kim, J.; et al. Discovery of BLU-945, a Reversible, Potent, and Wild-Type-Sparing Next-Generation EGFR Mutant Inhibitor for Treatment-Resistant Non-Small-Cell Lung Cancer. J. Med. Chem. 2022, 65, 9662–9677. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, E.; Hotta, K.; Nogami, N.; Kuyama, S.; Kishino, D.; Fujii, M.; Kozuki, T.; Tabata, M.; Harada, D.; Chikamori, K.; et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: The Okayama Lung Cancer Study Group Trial 1001. J. Thorac. Oncol. 2015, 10, 486–491. [Google Scholar] [CrossRef]
- Piccirillo, M.C.; Bonanno, L.; Garassino, M.C.; Esposito, G.; Dazzi, C.; Cavanna, L.; Burgio, M.A.; Rosetti, F.; Rizzato, S.; Morgillo, F.; et al. Addition of Bevacizumab to Erlotinib as First-Line Treatment of Patients With EGFR-Mutated Advanced Nonsquamous NSCLC: The BEVERLY Multicenter Randomized Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 1086–1097. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.R.; Cheng, Y.; Liu, Y.P.; Chen, G.Y.; Cui, J.W.; Yang, N.; Song, Y.; Li, X.L.; Lu, S.; et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell 2021, 39, 1279–1291. [Google Scholar] [CrossRef]
- Zhao, H.; Yao, W.; Min, X.; Gu, K.; Yu, G.; Zhang, Z.; Cui, J.; Miao, L.; Zhang, L.; Yuan, X.; et al. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706). J. Thorac. Oncol. 2021, 16, 1533–1546. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Liu, D. EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017, 385, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Cheng, Y.; Murakami, H.; Yang, P.C.; He, J.; Nakagawa, K.; Kang, J.H.; Kim, J.H.; Hozak, R.R.; Nguyen, T.S.; et al. A Randomized Phase 2 Study of Gefitinib with or without Pemetrexed as First-line Treatment in Nonsquamous NSCLC with EGFR Mutation: Final Overall Survival and Biomarker Analysis. J. Thorac. Oncol. 2020, 15, 91–100. [Google Scholar] [CrossRef]
- Shi, Y.K.; Wang, L.; Han, B.H.; Li, W.; Yu, P.; Liu, Y.P.; Ding, C.M.; Song, X.; Ma, Z.Y.; Ren, X.L.; et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): A phase 3, open-label, randomized study. Ann. Oncol. 2017, 28, 2443–2450. [Google Scholar] [CrossRef]
- Iwama, E.; Sakai, K.; Azuma, K.; Harada, T.; Harada, D.; Nosaki, K.; Hotta, K.; Ohyanagi, F.; Kurata, T.; Fukuhara, T.; et al. Monitoring of somatic mutations in circulating cell-free DNA by digital PCR and next-generation sequencing during afatinib treatment in patients with lung adenocarcinoma positive for EGFR activating mutations. Ann. Oncol. 2017, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib with Gefitinib in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; Cho, B.C.; Ou, X.; Walding, A.; Dymond, A.W.; Ren, S.; Cantarini, M.; Janne, P.A. Osimertinib Plus Durvalumab in Patients With EGFR-Mutated, Advanced NSCLC: A Phase 1b, Open-Label, Multicenter Trial. J. Thorac. Oncol. 2022, 17, 718–723. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Y.; Yang, S.; Zhou, J.; Zhang, L.; Chen, G.; Fang, J.; Zhu, B.; Li, X.; Shu, Y.; et al. Safety, Efficacy, and Pharmacokinetics of Rezivertinib (BPI-7711) in Patients with Advanced NSCLC With EGFR T790M Mutation: A Phase 1 Dose-Escalation and Dose-Expansion Study. J. Thorac. Oncol. 2022, 17, 708–717. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Wang, X.; Liu, Y.; Wu, L.; Hao, Y.; Liu, C.; Zhu, S.; Zhang, X.; Li, Y.; et al. Central Nervous System Efficacy of Furmonertinib (AST2818) Versus Gefitinib as First-Line Treatment for EGFR-Mutated NSCLC: Results From the FURLONG Study. J. Thorac. Oncol. 2022, 17, 1297–1305. [Google Scholar] [CrossRef]
- Cho, B.C.; Han, J.Y.; Kim, S.W.; Lee, K.H.; Cho, E.K.; Lee, Y.G.; Kim, D.W.; Kim, J.H.; Lee, G.W.; Lee, J.S.; et al. A Phase 1/2 Study of Lazertinib 240 mg in Patients with Advanced EGFR T790M-Positive NSCLC After Previous EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2022, 17, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Menis, J.; Hasan, B.; Peric, A.; De Maio, E.; Novello, S.; Reck, M.; Berghmans, T.; Wasag, B.; Besse, B.; et al. The APPLE Trial: Feasibility and Activity of AZD9291 (Osimertinib) Treatment on Positive PLasma T790M in EGFR-mutant NSCLC Patients. EORTC 1613. Clin. Lung Cancer 2017, 18, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Witta, S. Histone deacetylase inhibitors in non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, S404–S406. [Google Scholar] [CrossRef] [PubMed]
- Witta, S.E.; Jotte, R.M.; Konduri, K.; Neubauer, M.A.; Spira, A.I.; Ruxer, R.L.; Varella-Garcia, M.; Bunn, P.A., Jr.; Hirsch, F.R. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J. Clin. Oncol. 2012, 30, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Greve, G.; Schiffmann, I.; Pfeifer, D.; Pantic, M.; Schuler, J.; Lubbert, M. The pan-HDAC inhibitor panobinostat acts as a sensitizer for erlotinib activity in EGFR-mutated and -wildtype non-small cell lung cancer cells. BMC Cancer 2015, 15, 947. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Qian, G.; Zong, D.; Fan, S.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.Y. Overcoming acquired resistance of epidermal growth factor receptor-mutant non-small cell lung cancer cells to osimertinib by combining osimertinib with the histone deacetylase inhibitor panobinostat (LBH589). Cancer 2020, 126, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Qian, X.; Zhao, L.; Li, N.; Wu, S.; Chen, B.; Sun, T.; Wang, X. Trichostatin A downregulates bromodomain and extra-terminal proteins to suppress osimertinib resistant non-small cell lung carcinoma. Cancer Cell Int. 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Hase, T.; Shimizu, S.; Ando, M.; Hata, A.; Murakami, H.; Kawakami, T.; Nagase, K.; Yoshimura, K.; Fujiwara, T.; et al. Phase I study of vorinostat with gefitinib in BIM deletion polymorphism/epidermal growth factor receptor mutation double-positive lung cancer. Cancer Sci. 2020, 111, 561–570. [Google Scholar] [CrossRef]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.K.; Sriuranpong, V.; Ho, J.C.M.; Ong, C.K.; Tsai, C.M.; Chung, C.H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef]
- Gadgeel, S.; Peters, S.; Mok, T.; Shaw, A.T.; Kim, D.W.; Ou, S.I.; Perol, M.; Wrona, A.; Novello, S.; Rosell, R.; et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann. Oncol. 2018, 29, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Helland, A.; Oh, I.J.; Migliorino, M.R.; Dziadziuszko, R.; Wrona, A.; de Castro, J.; Mazieres, J.; Griesinger, F.; Chlistalla, M.; et al. Final efficacy and safety data, and exploratory molecular profiling from the phase III ALUR study of alectinib versus chemotherapy in crizotinib-pretreated ALK-positive non-small-cell lung cancer. ESMO Open 2022, 7, 100333. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Choi, Y.; Ahn, M.J. Efficacy and Safety of Lorlatinib in Korean Non-Small-Cell Lung Cancer Patients With ALK or ROS1 Rearrangement Whose Disease Failed to Respond to a Previous Tyrosine Kinase Inhibitor. Clin. Lung Cancer 2019, 20, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, T.E.; Doebele, R.C.; Wang, X.; Gerber, D.E.; Horn, L.; Camidge, D.R. Preliminary Clinical and Molecular Analysis Results from a Single-Arm Phase 2 Trial of Brigatinib in Patients with Disease Progression after Next-Generation ALK Tyrosine Kinase Inhibitors in Advanced ALK+ NSCLC. J. Thorac. Oncol. 2021, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Muzikansky, A.; Kennedy, E.; Kuberski, H.; Stober, L.L.; Wanat, A.C.; Azzoli, C.G.; Lennes, I.; Sequist, L.V.; Dagogo-Jack, I.; et al. Safety and activity of alectinib plus bevacizumab in patients with advanced ALK-rearranged non-small-cell lung cancer: A phase I/II study. ESMO Open 2022, 7, 100342. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Soda, M.; Yamashita, Y.; Ueno, T.; Takashima, J.; Nakajima, T.; Yatabe, Y.; Takeuchi, K.; Hamada, T.; Haruta, H.; et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 2010, 363, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Koivunen, J.; Ogino, A.; Yanagita, M.; Nikiforow, S.; Zheng, W.; Lathan, C.; Marcoux, J.P.; Du, J.; Okuda, K.; et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011, 71, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Metro, G.; Gariazzo, E.; Costabile, S.; Baglivo, S.; Roila, F.; Colamartini, F.; Palumbo, B.; Chiarini, P.; Gori, S.; Conti, A.; et al. Repotrectinib’s Clinical Benefit and Its Brain Penetration in a Patient with Meningeal Carcinomatosis from G2032R-Mutated ROS-1 Positive Non-Small Cell Lung Cancer. Oncol. Ther. 2024, 12, 163–171. [Google Scholar] [CrossRef]
- Drilon, A.; Chiu, C.H.; Fan, Y.; Cho, B.C.; Lu, S.; Ahn, M.J.; Krebs, M.G.; Liu, S.V.; John, T.; Otterson, G.A.; et al. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion-Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100332. [Google Scholar] [CrossRef]
- Li, Z.; Shen, L.; Ding, D.; Huang, J.; Zhang, J.; Chen, Z.; Lu, S. Efficacy of Crizotinib among Different Types of ROS1 Fusion Partners in Patients with ROS1-Rearranged Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Wang, Q.; Cheng, Y.; Liu, X.; Cao, L.; Chen, J.; Dong, X.; Zhou, J.; Fan, Y.; Huang, C.; et al. Safety but Limited Efficacy of Ensartinib in ROS1-Positive NSCLC: A Single-Arm, Multicenter Phase 2 Study. J. Thorac. Oncol. 2021, 16, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Ohe, Y.; Zhou, C.; Choi, C.M.; Yang, N.; Liu, G.; Felip, E.; Perol, M.; Besse, B.; Nieva, J.; et al. TRUST-II: A global phase II study of taletrectinib in ROS1-positive non-small-cell lung cancer and other solid tumors. Future Oncol. 2023, 19, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Chen, T.; Zhang, X.; Shang, G. Current treatment and novel insights regarding ROS1-targeted therapy in malignant tumors. Cancer Med. 2024, 13, e7201. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Schneider, J.L.; Patil, T.; Zhu, V.W.; Goldman, D.A.; Yang, S.R.; Falcon, C.J.; Do, A.; Nie, Y.; Plodkowski, A.J.; et al. Response to Immune Checkpoint Inhibition as Monotherapy or in Combination with Chemotherapy in Metastatic ROS1-Rearranged Lung Cancers. JTO Clin. Res. Rep. 2021, 2, 100187. [Google Scholar] [CrossRef] [PubMed]
- Michels, S.; Massuti, B.; Schildhaus, H.U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohe, C.; Rodriguez-Abreu, D.; Abdulla, D.S.Y.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients with Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Kim, H.R.; Lee, J.S.; Lee, K.H.; Lee, Y.G.; Min, Y.J.; Cho, E.K.; Lee, S.S.; Kim, B.S.; Choi, M.Y.; et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients with Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J. Clin. Oncol. 2017, 35, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.L.; Muzikansky, A.; Lin, J.J.; Krueger, E.A.; Lennes, I.T.; Jacobson, J.O.; Cheng, M.; Heist, R.S.; Piotrowska, Z.; Gainor, J.F.; et al. A Phase 2 Study of Lorlatinib in Patients with ROS1-Rearranged Lung Cancer with Brain-Only Progression on Crizotinib. JTO Clin. Res. Rep. 2022, 3, 100347. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Redman, M.W.; Lilenbaum, R.; Politi, K.; Stinchcombe, T.E.; Horn, L.; Chen, E.H.; Mashru, S.H.; Gettinger, S.N.; Melnick, M.A.; et al. Randomized Trial of Afatinib Plus Cetuximab Versus Afatinib Alone for First-Line Treatment of EGFR-Mutant Non-Small-Cell Lung Cancer: Final Results from SWOG S1403. J. Clin. Oncol. 2020, 38, 4076–4085. [Google Scholar] [CrossRef]
- Goldman, J.W.; Mazieres, J.; Barlesi, F.; Dragnev, K.H.; Koczywas, M.; Goskel, T.; Cortot, A.B.; Girard, N.; Wesseler, C.; Bischoff, H.; et al. A Randomized Phase III Study of Abemaciclib versus Erlotinib in Patients with Stage IV Non-small Cell Lung Cancer with a Detectable KRAS Mutation Who Failed Prior Platinum-Based Therapy: JUNIPER. Front. Oncol. 2020, 10, 578756. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.W.; Perol, M.; Ou, S.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lin, M.C.; Wei, Y.F.; Chang, G.C.; Su, W.C.; Hsia, T.C.; Su, J.; Wang, A.K.; Jen, M.H.; Puri, T.; et al. Efficacy and Tolerability of Ramucirumab Plus Erlotinib in Taiwanese Patients with Untreated, Epidermal Growth Factor Receptor-Mutated, Stage IV Non-small Cell Lung Cancer in the RELAY Study. Target. Oncol. 2023, 18, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Ahn, M.J.; Kang, J.H.; Soo, R.A.; Reungwetwattana, T.; Yang, J.C.; Cicin, I.; Kim, D.W.; Wu, Y.L.; Lu, S.; et al. Lazertinib Versus Gefitinib as First-Line Treatment in Patients with EGFR-Mutated Advanced Non-Small-Cell Lung Cancer: Results From LASER301. J. Clin. Oncol. 2023, 41, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Han, J.Y.; Telaranta-Keerie, A.; Huang, X.; Kohlmann, A.; Hodge, R.; Rukazenkov, Y.; Chmielecki, J.; Espenschied, C.R.; Lefterova, M.; et al. Pan-Tumor Analytical Validation and Osimertinib Clinical Validation in EGFR Mutant Non-Small-Cell Lung Cancer, Supporting the First Next-Generation Sequencing Liquid Biopsy in Vitro Diagnostic. J. Mol. Diagn. 2024, 26, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhou, Q.; Han, B.; Fan, Y.; Shan, L.; Chang, J.; Sun, S.; Fang, J.; Chen, Y.; Sun, J.; et al. NEPTUNE China cohort: First-line durvalumab plus tremelimumab in Chinese patients with metastatic non-small-cell lung cancer. Lung Cancer 2023, 178, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in metastatic non-small cell lung cancer: CheckMate 9LA 2-year patient-reported outcomes. Eur. J. Cancer 2023, 183, 174–187. [Google Scholar] [CrossRef]
- Cho, B.C.; Lee, J.S.; Wu, Y.L.; Cicin, I.; Dols, M.C.; Ahn, M.J.; Cuppens, K.; Veillon, R.; Nadal, E.; Dias, J.M.; et al. Bintrafusp Alfa versus Pembrolizumab in Patients with Treatment-Naive, Programmed Death-Ligand 1-High Advanced NSCLC: A Randomized, Open-Label, Phase 3 Trial. J. Thorac. Oncol. 2023, 18, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.X.; Jiang, T.; Long, J.; Ma, Z.Y.; Lu, A.P.; Cheng, Y.; Cao, D.S. The ups and downs of Poly(ADP-ribose) Polymerase-1 inhibitors in cancer therapy-Current progress and future direction. Eur. J. Med. Chem. 2020, 203, 112570. [Google Scholar] [CrossRef]
- Fu, X.; Li, P.; Zhou, Q.; He, R.; Wang, G.; Zhu, S.; Bagheri, A.; Kupfer, G.; Pei, H.; Li, J. Mechanism of PARP inhibitor resistance and potential overcoming strategies. Genes Dis. 2024, 11, 306–320. [Google Scholar] [CrossRef]
- Juncheng, P.; Joseph, A.; Lafarge, A.; Martins, I.; Obrist, F.; Pol, J.; Saavedra, E.; Li, S.; Sauvat, A.; Cerrato, G.; et al. Cancer cell-autonomous overactivation of PARP1 compromises immunosurveillance in non-small cell lung cancer. J. Immunother. Cancer 2022, 10, e004280. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Bardia, A.; Dvorkin, M.; Galsky, M.D.; Beck, J.T.; Wise, D.R.; Karyakin, O.; Rubovszky, G.; Kislov, N.; Rohrberg, K.; et al. Avelumab Plus Talazoparib in Patients with Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol. 2023, 9, 40–50. [Google Scholar] [CrossRef]
- Clarke, J.M.; Patel, J.D.; Robert, F.; Kio, E.A.; Thara, E.; Ross Camidge, D.; Dunbar, M.; Nuthalapati, S.; Dinh, M.H.; Bach, B.A. Veliparib and nivolumab in combination with platinum doublet chemotherapy in patients with metastatic or advanced non-small cell lung cancer: A phase 1 dose escalation study. Lung Cancer 2021, 161, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, R.M.; Muirhead, G.; Krastev, D.B.; Adam, J.; Morel, D.; Garrido, M.; Lamb, A.; Henon, C.; Dorvault, N.; Rouanne, M.; et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Investig. 2019, 129, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Su, X.; Wang, J.; Zhao, Y.; Tumbath, S.; Kilgore, J.A.; Williams, N.S.; Chen, Y.; Wang, X.; et al. KP372-1-Induced AKT Hyperactivation Blocks DNA Repair to Synergize with PARP Inhibitor Rucaparib via Inhibiting FOXO3a/GADD45alpha Pathway. Front. Oncol. 2022, 12, 976292. [Google Scholar] [CrossRef] [PubMed]
- Yusoh, N.A.; Chia, S.L.; Saad, N.; Ahmad, H.; Gill, M.R. Synergy of ruthenium metallo-intercalator, [Ru(dppz)(2)(PIP)](2+), with PARP inhibitor Olaparib in non-small cell lung cancer cells. Sci. Rep. 2023, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Arrieta, O.; Gimenez-Capitan, A.; Aldeguer, E.; Drozdowskyj, A.; Chaib, I.; Reguart, N.; Garcia-Campelo, R.; Chen, J.H.; Molina-Vila, M.A.; et al. BRCA1 Expression and Outcome in Patients with EGFR-Mutant NSCLC Treated with Gefitinib Alone or in Combination with Olaparib. JTO Clin. Res. Rep. 2021, 2, 100113. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, Y.; Zeng, Z.; Luo, Y.; Jiang, X.; Zhang, J.; Li, J.; Zhang, J.; Gong, Y.; Xie, C. PARP inhibitor niraparib as a radiosensitizer promotes antitumor immunity of radiotherapy in EGFR-mutated non-small cell lung cancer. Clin. Transl. Oncol. 2021, 23, 1827–1837. [Google Scholar] [CrossRef]
- Dominici, C.; Sgarioto, N.; Yu, Z.; Sesma-Sanz, L.; Masson, J.Y.; Richard, S.; Raynal, N.J. Synergistic effects of type I PRMT and PARP inhibitors against non-small cell lung cancer cells. Clin. Epigenet. 2021, 13, 54. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, H.; Li, Y.; Yin, J.; Guo, S.; Lin, S.Y.; McGrail, D.J. PARP inhibitors synergize with gemcitabine by potentiating DNA damage in non-small-cell lung cancer. Int. J. Cancer 2019, 144, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.; Odedra, R.; Wallez, Y.; Wijnhoven, P.W.G.; Hughes, A.M.; Gerrard, J.; Jones, G.N.; Bargh-Dawson, H.; Brown, E.; Young, L.A.; et al. ATR Inhibitor AZD6738 (Ceralasertib) Exerts Antitumor Activity as a Monotherapy and in Combination with Chemotherapy and the PARP Inhibitor Olaparib. Cancer Res. 2022, 82, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Deben, C.; Lardon, F.; Wouters, A.; Op de Beeck, K.; Van den Bossche, J.; Jacobs, J.; Van Der Steen, N.; Peeters, M.; Rolfo, C.; Deschoolmeester, V.; et al. APR-246 (PRIMA-1(MET)) strongly synergizes with AZD2281 (olaparib) induced PARP inhibition to induce apoptosis in non-small cell lung cancer cell lines. Cancer Lett. 2016, 375, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Dey, P.; Ghosh, S.; Sarma, A.; Ghosh, U. Reduction of metastatic potential by inhibiting EGFR/Akt/p38/ERK signaling pathway and epithelial-mesenchymal transition after carbon ion exposure is potentiated by PARP-1 inhibition in non-small-cell lung cancer. BMC Cancer 2019, 19, 829. [Google Scholar] [CrossRef]
- Luo, J.; Dai, X.; Hu, H.; Chen, J.; Zhao, L.; Yang, C.; Sun, J.; Zhang, L.; Wang, Q.; Xu, S.; et al. Fluzoparib increases radiation sensitivity of non-small cell lung cancer (NSCLC) cells without BRCA1/2 mutation, a novel PARP1 inhibitor undergoing clinical trials. J. Cancer Res. Clin. Oncol. 2020, 146, 721–737. [Google Scholar] [CrossRef]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef]
- Kwok, H.H.; Yang, J.; Lam, D.C. Breaking the Invisible Barriers: Unleashing the Full Potential of Immune Checkpoint Inhibitors in Oncogene-Driven Lung Adenocarcinoma. Cancers 2023, 15, 2749. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Chen, S.; Eldredge, J.B.; Schokrpur, S.; Li, D.; Quan, Z.; Chan, J.W.; Cummings, A.L.; Daly, M.E.; Goldman, J.W.; et al. The Role of Chemotherapy Plus Immune Checkpoint Inhibitors in Oncogenic-Driven NSCLC: A University of California Lung Cancer Consortium Retrospective Study. JTO Clin. Res. Rep. 2022, 3, 100427. [Google Scholar] [CrossRef]
- Miyawaki, E.; Murakami, H.; Mori, K.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; Ono, A.; Kenmotsu, H.; et al. PD-L1 expression and response to pembrolizumab in patients with EGFR-mutant non-small cell lung cancer. Jpn. J. Clin. Oncol. 2020, 50, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.J.; Ricciuti, B.; Gainor, J.F.; Kehl, K.L.; Kravets, S.; Dahlberg, S.; Nishino, M.; Sholl, L.M.; Adeni, A.; Subegdjo, S.; et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 2019, 30, 1653–1659. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, W.; Zhou, B.; Wang, S.; Li, N.; Qiu, B.; Lv, F.; Zhao, L.; Li, J.; Shao, K.; et al. Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2022, 17, 909–920. [Google Scholar] [CrossRef]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejias, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lu, S.; Cheng, Y.; Zhou, C.; Wang, J.; Mok, T.; Zhang, L.; Tu, H.Y.; Wu, L.; Feng, J.; et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population with Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J. Thorac. Oncol. 2019, 14, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Szczesna, A.; Ahn, M.J.; Schneider, C.P.; Gonzalez Mella, P.F.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined with Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Laurie, S.A.; Goss, G.D.; Hughes, B.G.M.; Stockler, M.; Tsao, M.S.; Hwang, D.M.; Joubert, P.; Kulkarni, S.; Blais, N.; et al. CCTG BR34: A Randomized Phase 2 Trial of Durvalumab and Tremelimumab with or without Platinum-Based Chemotherapy in Patients with Metastatic NSCLC. J. Thorac. Oncol. 2022, 17, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Novello, S.; Halmos, B.; Felip, E.; Speranza, G.; Hui, R.; Garon, E.B.; Horinouchi, H.; Sugawara, S.; et al. Associations of Tissue Tumor Mutational Burden and Mutational Status with Clinical Outcomes with Pembrolizumab Plus Chemotherapy versus Chemotherapy for Metastatic NSCLC. JTO Clin. Res. Rep. 2023, 4, 100431. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673. [Google Scholar] [CrossRef]
- Kawachi, H.; Yamada, T.; Yoshimura, A.; Morimoto, K.; Iwasaku, M.; Tokuda, S.; Kim, Y.H.; Shimose, T.; Takayama, K. Rationale and design of phase II clinical trial of dual inhibition with ramucirumab and erlotinib in EGFR exon 19 deletion-positive treatment-naive non-small cell lung cancer with high PD-L1 expression (SPIRAL-3D study). Ther. Adv. Med. Oncol. 2023, 15, 17588359231177022. [Google Scholar] [CrossRef]
- Wang, J.; Chi, Y.; Chen, H.; Jia, B.; Zhai, X.; Ma, M.; Li, J.; Zhuo, M. Analysis of the Effcacy and Safety of Amivantamab in Non-small Cell Lung Cancer Patients with EGFR/MET Gene Abnormalities: A Single Center’s Experience. Zhongguo Fei Ai Za Zhi 2022, 25, 493–500. [Google Scholar] [CrossRef]
- Kovalchuk, B.; Berghoff, A.S.; Karreman, M.A.; Frey, K.; Piechutta, M.; Fischer, M.; Grosch, J.; Heiland, S.; Breckwoldt, M.O.; Hilberg, F.; et al. Nintedanib and a bi-specific anti-VEGF/Ang2 nanobody selectively prevent brain metastases of lung adenocarcinoma cells. Clin. Exp. Metastasis 2020, 37, 637–648. [Google Scholar] [CrossRef]
- Tabtimmai, L.; Suphakun, P.; Srisook, P.; Kiriwan, D.; Phanthong, S.; Kiatwuthinon, P.; Chaicumpa, W.; Choowongkomon, K. Cell-penetrable nanobodies (transbodies) that inhibit the tyrosine kinase activity of EGFR leading to the impediment of human lung adenocarcinoma cell motility and survival. J. Cell. Biochem. 2019, 120, 18077–18087. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Nguyen, T.M.; Jayasinghe, M.K.; Gao, C.; Pham, T.T.; Vu, L.T.; Yeo, E.Y.M.; Yap, G.; Wang, L.; Goh, B.C.; et al. Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J. Extracell. Vesicles 2022, 11, e12187. [Google Scholar] [CrossRef]
- Karn, V.; Ahmed, S.; Tsai, L.W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular Vesicle-Based Therapy for COVID-19: Promises, Challenges and Future Prospects. Biomedicines 2021, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Sanchez-Navarro, M.; Rosell, R.; Giralt, E.; Codony-Servat, J. Anti-EGF nanobodies enhance the antitumoral effect of osimertinib and overcome resistance in non-small cell lung cancer (NSCLC) cellular models. Med. Oncol. 2022, 39, 195. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.; Liu, S.; Chen, Q.; Zeng, L.; Chen, X.; Zhang, Q. Targeted nanobody complex enhanced photodynamic therapy for lung cancer by overcoming tumor microenvironment. Cancer Cell Int. 2020, 20, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, J.; Yang, M.; Zhang, H.; Xu, T.; Kan, F.; Zhang, X.; Zhang, S.; Yin, Y.; Yu, F. In vitro and in vivo study on the treatment of non-small cell lung cancer with radionuclide labeled PD-L1 nanobody. J. Cancer Res. Clin. Oncol. 2023, 149, 8429–8442. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yang, Y.; Wang, C.; Zou, J.; Lin, J.; Qiu, L. Immuno-PET imaging of PD-L1 expression in patient-derived lung cancer xenografts with [(68)Ga]Ga-NOTA-Nb109. Quant. Imaging Med. Surg. 2022, 12, 3300–3313. [Google Scholar] [CrossRef]
- Corominas-Faja, B.; Oliveras-Ferraros, C.; Cuyas, E.; Segura-Carretero, A.; Joven, J.; Martin-Castillo, B.; Barrajon-Catalan, E.; Micol, V.; Bosch-Barrera, J.; Menendez, J.A. Stem cell-like ALDH(bright) cellular states in EGFR-mutant non-small cell lung cancer: A novel mechanism of acquired resistance to erlotinib targetable with the natural polyphenol silibinin. Cell Cycle 2013, 12, 3390–3404. [Google Scholar] [CrossRef]
- Baharuddin, P.; Satar, N.; Fakiruddin, K.S.; Zakaria, N.; Lim, M.N.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2016, 35, 13–25. [Google Scholar] [CrossRef]
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 2014, 77, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Bhummaphan, N.; Pongrakhananon, V.; Sritularak, B.; Chanvorachote, P. Cancer Stem Cell-Suppressing Activity of Chrysotoxine, a Bibenzyl from Dendrobium pulchellum. J. Pharmacol. Exp. Ther. 2018, 364, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Li, X.F.; Jin, L.F.; Zhao, Y.; Zhu, G.J.; Shen, W.Z. Dieckol inhibits non-small-cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, Z.J.; Lee, C.J.; Lai, W.H.; Chen, J.C.; Huang, H.C. epsilon-Viniferin and alpha-viniferin alone or in combination induced apoptosis and necrosis in osteosarcoma and non-small cell lung cancer cells. Food Chem. Toxicol. 2021, 158, 112617. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Ren, J.; Wang, Y.; Su, J.Y.; Zhu, Y.M.; Chen, C.G.; Long, W.G.; Jiang, Q.; Li, J. Synergistic killing effect of paclitaxel and honokiol in non-small cell lung cancer cells through paraptosis induction. Cell. Oncol. 2021, 44, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.Q.; Liu, H.; Liu, J.; Hou, W.; Lin, H.S. A multicenter, large-sample, randomized clinical trial on improving the median survival time of advanced non-small cell lung cancer by combination of Ginseng Rg3 and chemotherapy. Zhonghua Zhong Liu Za Zhi 2018, 40, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Hong, M.; Sun, X.Y.; Huang, D.W.; He, D.H.; Chen, Y.F.; Yuan, Y.; Liu, Y.Q. Silybin has therapeutic efficacy against non-small cell lung cancer through targeting of Skp2. Acta Mater. Medica 2022, 1, 302–313. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, C.B.; Yang, B.; Zhou, X. Baicalin Inhibits EMT through PDK1/AKT Signaling in Human Nonsmall Cell Lung Cancer. J. Oncol. 2021, 2021, 4391581. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Cao, D.; Ren, Q.N.; Zhang, S.S.; Zhou, N.N.; Mai, S.J.; Feng, B.; Wang, H.Y. Combination Treatment with Inhibitors of ERK and Autophagy Enhances Antitumor Activity of Betulinic Acid in Non-small-Cell Lung Cancer In Vivo and In Vitro. Front. Pharmacol. 2021, 12, 684243. [Google Scholar] [CrossRef]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; et al. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, D.; Bao, T.T.; Fan, F.T.; Yu, C. The Anticancer Effects of Atractylenolide III Associate with the Downregulation of Jak3/Stat3-Dependent IDO Expression. Front. Pharmacol. 2019, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, K.; Xu, A.; Jiao, P.; Li, H.; Xing, L. The sesquiterpene lactone eupatolide induces apoptosis in non-small cell lung cancer cells by suppressing STAT3 signaling. Environ. Toxicol. Pharmacol. 2021, 81, 103513. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Feng, J.H.; Pham, T.A.; Ma, H.Y.; Ma, M.X.; Song, R.; Shen, W.; Xiong, F.; Zhang, X.Q.; Ye, W.C.; et al. Identification of amentoflavone as a potent highly selective PARP-1 inhibitor and its potentiation on carboplatin in human non-small cell lung cancer. Phytomedicine 2018, 50, 88–98. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Q.; Wang, Y.; Cui, L.; Zhang, W.; Teng, Y.; Yu, P. Synergy between vinorelbine and afatinib in the inhibition of non-small cell lung cancer progression by EGFR and p53 signaling pathways. Biomed. Pharmacother. 2021, 134, 111144. [Google Scholar] [CrossRef]
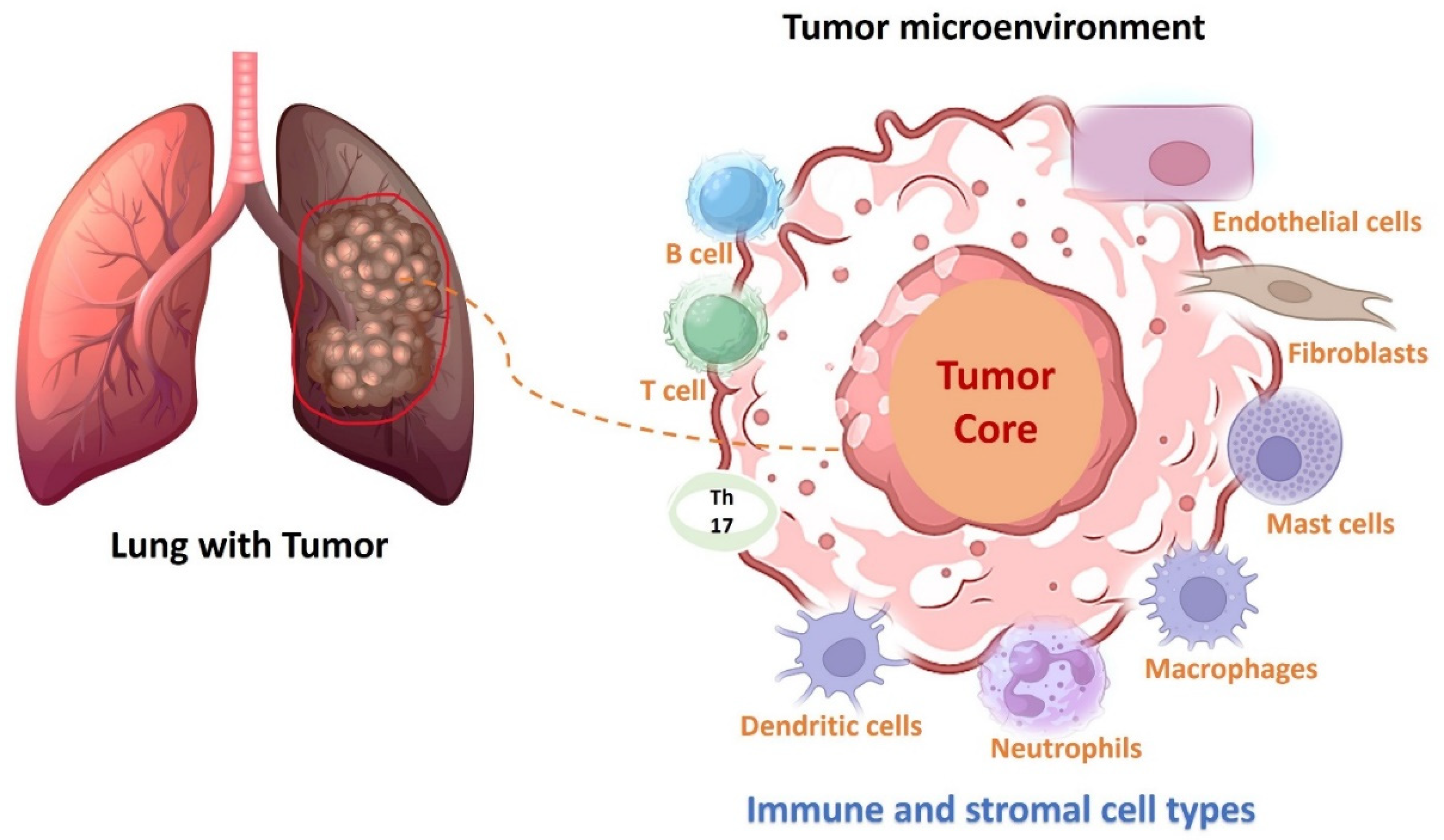
| Inhibitors for ALK Rearrangements and Combination Therapy | Starting Dose | Stage of Disease | Previous Treatment | Sample Size (Phase) | Objective Response Rate (ORR) and/or Median Progression-Free Survival (PFS) | References |
|---|---|---|---|---|---|---|
| Ceritinib | 750 mg/day | Stage IIIB/IV | Untreated | 189 patients (phase 3) | PFS: 16.6 months | [29] |
| Crizotinib | 250 mg/day twice daily | Advanced | Untreated | 104 patients (phase 3) | ORR: 87.5%, PFS: 11.1 months | [30] |
| Alectinib | 600 mg/day twice daily | Advanced | Pre-treated with chemotherapy and crizotinib | 119 patients (phase 3) | ORR: 50.6%, PFS: 10.9 months | [32] |
| Untreated | 152 patients (phase 3) | ORR: 85.7% | [31] | |||
| Lorlatinib | 100 mg/day | Advanced | Pre-treated with 1st and 2nd generation TKIs | 10 patients (phase 1) | ORR: 64%, PFS: 6.5 months | [33] |
| Brigatinib | 90 mg/day | Stage IIIB or IV | Not reported | 20 patients (phase 2) | ORR: 40%, PFS: 7 months | [34] |
| Alectinib and Bevacizumab | Alectinib (600 mg/day) twice daily and bevacizumab (15 mg/kg) every 3 weeks | Advanced | 55% of patients were untreated, and 46% pre-treated with ALK inhibitors | 11 patients (phase I/II) | PFS (3 pre-treated patients): 9.5 months | [35] |
| Identifier: Clinical Study ID Numbers | NSCLC Patients (Any Mutation or Marker Expression) | Number of Baseline Participants | Treatment Arm (Experimental and Comparator Treatment) | Serious Adverse Events | Primary Outcome Measures: Median (Months) [DFS (Disease-Free Survival); OS (Overall Survival); PFS: (Progression-Free Survival)] | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| NEPTUNE: NCT02542293 | EGFR and ALK mutation-positive | 256 (26.9%) | 697 (73.1%) | Durvalumab + Tremelimumab | Global: 47.07% | bTMB ≥ 20 mut/Mb: OS (11.7) |
| SoC Chemotherapy | Global: 28.07% | bTMB ≥ 20 mut/Mb: OS (9.1) | ||||
| MYSTIC: NCT02453282 | EGFR and ALK wild type | 346 (30.9%) | 772 (69.1%) | Durvalumab Monotherapy | 35.50% | PD-L1 (TC ≥ 25%): OS (16.3) |
| Durvalumab + Tremelimumab | 47.98% | PD-L1 (TC ≥ 25%): OS (11.9), PFS (3.9) | ||||
| SoC Chemotherapy | 31.82% | PD-L1 (TC ≥ 25%): OS (12.9), PFS (5.4) | ||||
| PEARL: NCT03003962 | EGFR and ALK mutation-positive with PD-L1 high expression | 132 (19.7%) | 537 (80.3%) | Durvalumab | 39.40% | OS (14.6), PD-L1 (TC ≥ 25%): OS (14.6) |
| Platinum-based SoC | 31.80% | OS (12.8), PD-L1 (TC ≥ 25%): OS (15.0) | ||||
| NCT02438722 | EGFR mutation positive | 112 (66.7%) | 56 (33.3%) | Afatinib dimaleate + Cetuximab | 37.18% | PFS (11.9) |
| Afatinib dimaleate | 35.29% | PFS (13.4) | ||||
| LASER301: NCT04248829 | EGFR mutation-positive | 251 (63.9%) | 142 (36.1%) | Lazertinib | 26.02% | PFS (20.6) |
| Gefitinib | 25.89% | PFS (9.7) | ||||
| RELAY: NCT02411448 | EGFR mutation-positive | 294 (63.5%) | 169 (36.5%) | Ramucirumab + Erlotinib | 29.41% | PFS (19.4) |
| Placebo + Erlotinib | 20.89% | PFS (12.4) | ||||
| AURA3: NCT02151981 | EGFR mutation-positive | 269 (64.2%) | 150 (35.8%) | Osimertinib | 30.11% | PFS (10.1) |
| Platinum-based doublet chemotherapy | 26.47% | PFS (4.4) | ||||
| LEAP-007: NCT03829332 | PD-L1 (TPS) greater than or equal to 1% | 169 (27.1%) | 454 (72.9%) | Pembrolizumab + Lenvatinib | 56.63% | OS (14.1), PFS (6.6) |
| Pembrolizumab + Placebo | 33.97% | OS (16.4). PFS (4.2) | ||||
| NCT03631706 | High PD-L1-tumor expression with no EGFR or ALK mutation | 78 (25.7%) | 226 (74.3%) | M7824 | 59.60% | OS (21.1), PFS (7.0) |
| Pembrolizumab | 40.13% | OS (22.2), PFS (11.1) | ||||
| JUNIPER: NCT02152631 | KRAS mutation | 181 (40%) | 272 (60%) | Abemaciclib | 42.26% | OS (7.4) |
| Erlotinib | 24.57% | OS (7.8) | ||||
| CodeBreak 200: NCT04303780 | KRAS mutation | 141 (40.9%) | 204 (59.1%) | AMG 510 | 53.85% | PFS (5.62) |
| Docetaxel | 44.37% | PFS (4.47) | ||||
| NCT02838420 | ALK mutation-positive | 89 (47.6%) | 98 (52.4%) | Alectinib | 15.20% | - |
| Crizotinib | 25.81% | PFS (11.1) | ||||
| ALEX: NCT02075840 | ALK mutation-positive | 171 (56.4%) | 132 (43.6%) | Alectinib | 28.95% | - |
| Crizotinib | 29.80% | PFS (11.1) | ||||
| NCT03052608 | ALK mutation-positive | 175 (59.1%) | 121 (40.9%) | Lorlatinib monotherapy | 34.23% | - |
| Crizotinib monotherapy | 27.46% | PFS (9.3) | ||||
| CheckMate 9LA: NCT03215706 | - | 215 (29.9%) | 504 (70.1%) | Nivolumab + Ipilimumab + Chemotherapy | 56.70% | OS (14.13) |
| Chemotherapy only | 41.26% | OS (10.74) | ||||
| PEARLS: NCT02504372 | - | 373 (31.7%) | 804 (68.3%) | Pembrolizumab | 24.48% | DFS (53.8), PD-L1 (TPS ≥ 50%): DFS (67.0) |
| Placebo | 15.49% | DFS (43.0), PD-L1 (TPS ≥ 50%): DFS (47.6) | ||||
| POSEIDON: NCT03164616 | - | 243 (24.0%) | 770 (76.0%) | Tremelimumab + Durvalumab + SoC chemotherapy | 44.24% | - |
| Durvalumab + SoC chemotherapy | 40.12% | OS (13.3), PFS (5.5) | ||||
| SoC chemotherapy | 35.14% | OS (11.7), PFS (4.8) | ||||
| PARP Inhibitor and Combination Therapy/Agent | Dosing | Inhibition Effect | Model/Clinical Trial | References |
|---|---|---|---|---|
| Talazoparib + Avelumab (immune checkpoint inhibitor) | Talazoparib (1 mg/day) + Avelumab (800 mg every 2 weeks) | Changes in genes related to homologous recombination | Clinical study | [64] |
| Veliparib + Nivolumab (PD-1) + chemotherapy agents (carboplatin and Paclitaxel) | Veliparib (120 mg twice daily) + Nivolumab (360 mg) + carboplatin (AUC 6 mg/mL·min) + paclitaxel (200 mg/m2) | - | Clinical study | [65] |
| Rucaparib + Olaparib | Rucaparib (25 μM) and Olaparib (40 μM) | Activate cGAS/STING, downstream type I IFN signaling, and CCL5 secretion | Pre-Clinical study | [66] |
| Rucaparib + KP372-1 | Rucaparib (15 µM) and KP372-1 (0.4 µM) | NQO-1-dependent DNA damage | Pre-Clinical study | [67] |
| Olaparib + Ruthenium [Ru(dppz)2(PIP)]2+ | - | DNA Double-strand break (DSB) and level of reactive oxygen species (ROS) increases | Test on zebrafish embryos | [68] |
| Olaparib + Gefitinib (EGFR inhibitor) | - | low mRNA expression of CtIP | Phase 2 clinical trial | [69] |
| Niraparib + Radiation | Niraparib (30 mg/kg) + 8 Gy × 3 radiation | CD8+ T lymphocytes Increase, and the STING/TBK1/IRF3 pathway is activated | In vivo studies on mice | [70] |
| Talazoparib + Type I PRMT inhibitor: MS023 (epigenetic modulator) | Talazoparib (50 nM) and MS023 (2 μM) | DNA Damage (γ-H2AX foci elevated) | Pre-Clinical study | [71] |
| Talazoparib + Olaparib + Gemcitabine (Chemotherapeutic drug) | Gemcitabine (80 mg/kg) + Talazoparib (0.333 mg/kg) | Induces single-strand DNA breaks | Xenograft model | [72] |
| Olaparib + Ceralasertib (ATR inhibitor) | Olaparib (50 mg/kg once daily) + Ceralasertib (12.5 mg/kg twice daily) | Activates ATM-dependent signaling pathway | Xenograft model | [73] |
| Olaparib + APR-246 (PRIMA-1Met) | Olaparib (0–80 μM) and APR-246 (0–40 μM) | ROS production increased, and p53 translocated to the mitochondria | Pre-Clinical study | [74] |
| Olaparib + Carbon ion (12C) radiotherapy | Olaparib (2 μM) + 12C ion (0.5 Gy) | Activation of MMP-2,-9 transcription pathway | Pre-Clinical study | [75] |
| Fluzoparib + Radiotherapy | Fluzoparib (30.84 μM) | Activation of p21 via p53 pathway | Xenograft mouse model | [76] |
| Therapy | Key Biomarker | Overall Survival Rate | References |
|---|---|---|---|
| Pembrolizumab + datopotamab deruxtecan (Dato-DXd) | PD-L1 (50% expression level) | 62% | [80] |
| Pembrolizumab monotherapy | 29.6% | ||
| Pembrolizumab monotherapy | PD-L1 (≥50% expression level) | - | [81] |
| Nivolumab + ipilimumab | PD-L1 (1% expression level) | 62.6% | [82] |
| Chemotherapy | 56.2% | ||
| Pembrolizumab monotherapy | PD-L1 (50% expression level) | 80.2% | [83] |
| Five chemotherapy regimens | 72.4% | ||
| Pembrolizumab monotherapy | PD-L1 (1% expression level) | - | [84] |
| Docetaxel (Chemotherapy) | - | ||
| Nivolumab monotherapy | PD-L1 (more than 5% expression level) | 64% | [85] |
| Docetaxel (Chemotherapy) | - | ||
| Pembrolizumab + pemetrexed (chemotherapy) | PD-L1 (50% expression level) | 69.2% | [86] |
| Placebo combination therapy | 49.4% | ||
| Nivolumab + ipilimumab with two cycles of chemotherapy | PD-L1 | 63% | [87] |
| Chemotherapy | 47% | ||
| Pembrolizumab monotherapy | PD-L1 (50% expression level) | 21.8% | [88] |
| Pembrolizumab monotherapy | PD-L1 (90–100% expression level) | 60% | [89] |
| Sintilimab monotherapy | PD-L1 (1–50% expression level) | 88.5% | [90] |
| Therapeutic Drugs | Potential Function | Signaling Pathway | Assay/Analysis | References |
|---|---|---|---|---|
| Curcumin + cisplatin | Cell cycle arrests (downregulation of cyclin D1), p21 expression increases, and the activation of Apaf1 and caspase-9 | Intrinsic apoptotic pathway | Apoptosis and migration assay | [110] |
| Resveratrol oligomers: α-viniferin + ε-viniferin (Origin: Vitis sp.) | p-AKT expression decreases, and cleaved PARP expression increases | Akt pathway | MTT assay, flow cytometric assay, immunofluorescence assay, colony formation assay, animal experimentation, and TUNEL assay | [114] |
| Bibenzyl: Gigantol (Origin: Dendrobium draconis) | Down-regulation of caveolin-1 (Cav-1), activation of Cdc42 | Cav-1-dependent pathway | Apoptosis and invasion assay and Western blot analysis | [111] |
| Flavonoid: Baicalin | E-cadherin increases and vimentin decreases; p-PDK1 and p-AKT levels decrease | PDK1/AKT signaling pathway | Immunofluorescence, Western blot, and immunohistochemistry assay | [118] |
| Bibenzyl: Chrysotoxine (Origin: Dendrobium draconis) | Suppression of Sox2 | Src-Akt pathway | Spheroids formation assay, WST assay, and Western blot analysis | [112] |
| Vanillin (Origin: Vanilla planifolia) | Downregulation of Oct4 and Nanog | Ubiquitin-proteasomal pathway | Anchorage-independent growth assay, spheroid formation assay, Western blot analysis, ad immunoprecipitation assay | [120] |
| Polyphenol: Silibinin + erlotinib | ALDH cells decrease | KEGG pathway | Microarray, ALDEFLUOR activity assay, and tumor sphere formation assay | [109] |
| Sesquiterpene lactone: Parthenolide (origin: Tanacetum parthenium) | Inhibition of B-Raf and c-Myc | MAPK/Erk pathway | Clone formation assay, flow cytometry, Western blot, and immunohistochemistry assay | [121] |
| Polyphenol: Dieckol, (origin: Ecklonia cava) | Activates E-cadherin | P13K/AKT/mTOR signaling pathway | MTT assay, flow cytometry, and immunoblotting technique | [113] |
| Flavonoid: Silybin (origin: Silybum marianum) | Inhibition of Skp2 and EGF-mediated Akt activation | Skp2/p27 pathway | Cell viability and colony formation assays, cellular thermal shift assays, Western blotting, molecular docking, and animal studies | [117] |
| Lactones: Atractylenolide III (ATLIII) (origin: Atractylodes chinensis) | IFN-γ-induced indoleamin-2,3-dioxygenase-1 (IDO) expression | Jak3 and Stat3 pathway | Flow cytometry, Western blot analysis, immunofluorescence assay, promoter luciferase assay, CHIP assay, site-directed mutations, and in vivo studies | [122] |
| Ginsenoside Rh2 (origin: Ginseng) | Decreased the protein level of matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF), which prevents metastasis | - | Flow cytometry, cell proliferation, wound healing assay, enzyme-linked immunosorbent assay (ELISA), Western blot analysis, RT-qPCR, immunohistochemistry, and animal studies | [123] |
| Pentacyclic triterpenoid: Betulinic acid + ERK inhibitor (U0126, trametinib) + Hydroxychloroquine | Protective Autophagy and activation of mitogen-activated protein kinases | AKT pathway | Cell viability assays, siRNA transfection, flow cytometry, Western blot analysis, mRFP-GFP-LC3 adenovirus transfection, transmission electron microscopy (TEM), and in vivo studies | [119] |
| Biphenolic: Honokiol (origin: Magnolia) combined with Paclitaxel | Induces paraptotic cell death | MAPK pathway | Cell viability assays, clonogenic assay, transmission electron microscopy (TEM), in vivo tumor growth inhibition assay, immunofluorescence staining, siRNA transfection, flow cytometry, Western blot analysis, and measurement of intracellular and mitochondrial Ca2+ | [115] |
| Ginseng Rg3 combined with chemotherapy | Myelosuppression decreased | - | Randomized double-blind trial with III-IV NSCLC 414 patients | [116] |
| Sesquiterpene lactone: Eupatolide (origin: Inula helenium) Eupatolide + Cisplatin and Eupatolide + 5-FU | Suppresses the activation of STAT3 | Stat3 pathway | Cell proliferation and viability, xenograft study, Western blot, qRT-PCR analysis, and cell apoptosis | [124] |
| Biflavonoid: Amentoflavone (origin: Selaginella moellendorffii) Amentoflavone + carboplatin | Inhibits PARP-1; the number of apoptotic cells in tumor tissues increases | Mitochondrial apoptotic pathways | Intracellular PAR assay, PARP-1/2 inhibition assay, tunnel assay, transfection for PARP-1 silencing, Western blot analysis, immunofluorescence histochemistry, and xenograft experiments | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Singh, S.K.; Mishra, M.K.; Singh, S.; Singh, R. Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer. Cancers 2024, 16, 2205. https://doi.org/10.3390/cancers16122205
Kaur P, Singh SK, Mishra MK, Singh S, Singh R. Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer. Cancers. 2024; 16(12):2205. https://doi.org/10.3390/cancers16122205
Chicago/Turabian StyleKaur, Prabhjot, Santosh Kumar Singh, Manoj K. Mishra, Shailesh Singh, and Rajesh Singh. 2024. "Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer" Cancers 16, no. 12: 2205. https://doi.org/10.3390/cancers16122205
APA StyleKaur, P., Singh, S. K., Mishra, M. K., Singh, S., & Singh, R. (2024). Promising Combinatorial Therapeutic Strategies against Non-Small Cell Lung Cancer. Cancers, 16(12), 2205. https://doi.org/10.3390/cancers16122205










